KEY WORDS: Intracranial pressure, Stenosis, Stenting
Abstract
BACKGROUND:
Medically refractory idiopathic intracranial hypertension (IIH) is frequently treated with venous sinus stenosis stenting with high success rates. Patient selection has been driven almost exclusively by identification of supraphysiological venous pressure gradients across stenotic regions based on theoretical assessment of likelihood of response.
OBJECTIVE:
To explore the possibility of benefit in low venous pressure gradient patients.
METHODS:
Using a single-center, prospectively maintained registry of patients with IIH undergoing venous stenting, we defined treatment groups by gradient pressures of ≤4, 5 to 8, and >8 mmHg based on the most frequently previously published thresholds for stenting. Baseline demographics, clinical, and neuro-ophthalmological outcomes (including optical coherence tomography and Humphrey visual fields) were compared.
RESULTS:
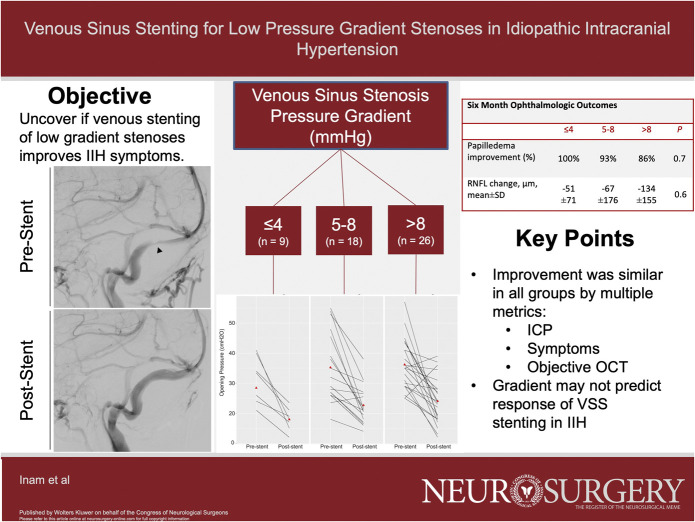
Among 53 patients, the mean age was 32 years and 70% female with a mean body mass index was 36 kg/m2. Baseline characteristics were similar between groups. The mean change in lumbar puncture opening pressure at 6 months poststenting was similar between the 3 groups (≤4, 5-8, and >8 mmHg; 13.4, 12.9, and 12.4 cmH2O, P = .47). Papilledema improvement was observed across groups at 6 months (100, 93, and 86, P = .7) as were all clinical symptoms. The mean changes in optical coherence tomography retinal nerve fiber layer (−30, −54, and −104, P = .5) and mean deviation in Humphrey visual fields (60, 64, and 67, P = .5) at 6 weeks were not significantly different.
CONCLUSION:
Patients with IH with low venous pressure gradient venous sinus stenosis seem to benefit equally from venous stenting compared with their higher gradient counterparts. Re-evaluation of our restrictive criteria for this potentially vision sparing intervention is warranted. Future prospective confirmatory studies are needed.
ABBREVIATIONS:
- HVF
Humphrey visual fields
- IIH
idiopathic intracranial hypertension
- OCT
optical coherence tomography
- RNFL
retinal nerve fiber layer
- VSS
venous sinus stenosis.

Idiopathic intracranial hypertension (IIH) is a progressive and debilitating disease with unclear pathophysiology leading to elevated intracranial pressure. Some authors have postulated that structural disease within arachnoid granulations is the source of the pathology, while others hold that deficient venous outflow, resulting in poor cerebrospinal fluid (CSF) clearance, is the main contributor.1 Increased intracranial pressure is known to cause compression of venous sinus structures.2 By the same token, venous outflow obstruction, like that seen in venous sinus thrombosis, is known to hinder CSF clearance and lead to intracranial hypertension. Growing evidence suggests that venous sinus stenosis (VSS) may play a role as either the inciting event or the secondary propagating lesion in a feedback pressure clearance cycle.1 As such, venous sinus stenting has emerged as a promising treatment for IIH.3-8
Owing to the low prevalence of IIH with concurrent VSS and the heterogeneity of the disease, conducting large-scale studies has proven challenging. An expanding literature of single-center or small multicenter experiences with VSS stenting has provided promising evidence of potential efficacy of this treatment but has also highlighted the immense diversity in patient selection criteria.5,7,9-16 Given the speculative nature of VSS's role in IIH and the physiological evidence of normal superior sagittal sinus to jugular bulb gradients ranging between 0 and 3 mmHg, the field has relied on the presence of supraphysiological gradients across stenotic lesions for selection of optimal candidates.17 Although high thresholds for treatment may theoretically select patients more likely to benefit from stenting, it also limits access to patients with low gradient lesions in whom benefit remains unclear.
In particular, previous published studies have arbitrarily selected gradients ranging from 4 to 21 mmHg as selection criteria to demonstrate clinical benefit.4,6 To the best of our knowledge, only single isolated cases of stenting in low gradient patients have been reported.16 Here, we explore the safety and efficacy of venous sinus stenting as a treatment for IIH in patients with low pressure gradient VSS.
METHODS
Clinical Evaluation
Informed consent for treatment was obtained as per standard clinical practice with care given to highlighting the lack of evidence for benefit of stenting low gradient lesions. This study was initiated and conducted in concordance with our local Institutional Review Board which did not require further patient consent for inclusion given the nature of this study. We conducted a retrospective review of a prospectively maintained database on consecutive patients with IIH and VSS who underwent venous stenting at our institution under our standardized protocol from 2019 to 2020. Screening and follow-up protocols involved clinical evaluation of symptoms, funduscopic examination by an independent expert neuro-ophthalmologist, optical coherence tomography (OCT), Humphrey visual field testing, and lumbar puncture. Symptom questionnaires were performed to evaluate for changes in symptoms at follow-up (resolution, improvement, stability, or worsening) and use of acetazolamide. Magnetic resonance venography was used to screen for VSS, but the diagnosis was confirmed with angiography as described below. Intracranial pressure was determined by lumbar puncture opening pressure performed with fluoroscopy guidance in the lateral decubitus position under local anesthesia, with passive straightening of the lower extremities before recording pressures. Diagnostic cerebral angiography was then performed under local anesthesia with conscious sedation.
Venous Pressure Measurement
All venous sinus pressure measurements and interventional procedures were performed under general anesthesia with 1 exception of a patient undergoing measurements under conscious sedation by personal request. Transfemoral arterial diagnostic cerebral angiograms using a 5Fr diagnostic catheter were performed to confirm venous sinus stenosis and to assist in venous catheter navigation. Femoral venous access was established, and a 7Fr Envoy MPC guide catheter was advanced to the dominant internal jugular vein coaxially. A Phenom 27 microcatheter (Medtronic) was advanced over an Asahi Chikai Ex microwire (Asahi Intercc) to the superior sagittal sinus, and manometry was performed in this location, torcula, transverse sinus (prestenosis and poststenosis), sigmoid sinus, and jugular bulb. Venous gradients were calculated based on differentials from prestenotic to poststenotic measurements. Immediately after stent deployment, pressures were measured in the prestent and poststent segments with gradients calculated across the stent. Treatment groups were defined as pretreatment pressure gradients of ≤4, 5 to 8, and >8 mmHg based on commonly cited thresholds in the literature.
Venous Stenting
Patients with diagnosis of IIH, papilledema, or abnormal OCT or visual field/new acuity deficit with evidence of venous stenosis on noninvasive imaging who then have elevated opening pressure (>20 cmH2O) with a confirmed venous stenosis on angiography were considered eligible for stenting. Degree of stenosis was obtained by trained physicians measuring differential diameter between prestenotic segment and stenotic segment. Exclusion criteria for treatment in our practice include patients with stenosis because of thrombus and stenosis of nondominant sinuses. Patients were pretreated with aspirin and clopidogrel before stenting. Clopidogrel responsiveness was verified, and adjustments were made based on the P2Y12 Reaction Units (PRU). Self-expandable PRECISE PRO RX Stents were deployed at the stenotic segment using the abovementioned guide and microcatheter system. Patients remained on dual antiplatelet therapy for 6 months after stenting, at which time clopidogrel was stopped. Patients then underwent repeat lumbar puncture and diagnostic angiography to assess opening pressure and stent patency, respectively.
Outcomes
Repeat lumbar puncture and diagnostic cerebral angiograms were performed at 6 months after stenting per above protocols. Repeat complete clinical and neuro-ophthalmological examination including optical coherence tomography and Humphrey visual field testing was performed at 6 weeks and 6 months post-treatment in most cases.
Statistical Analysis
Baseline demographics and clinical characteristics were tabulated and compared using descriptive statistics. Comparisons of the means of continuous variables between the 3 pressure gradient groups were performed using 1-way analysis of variance. Any significant results were investigated further with a pairwise comparison of means using the Tukey method. Comparison of proportions of categorical variables was performed using the Fisher exact test, with any significant results further investigated with sequential pairwise comparisons and P values adjusted for multiple measures using the Bonferroni method. P value was considered significance at <0.05. All statistical analysis was performed using Stata v15.1 (StataCorp). There was no extrapolation performed for missing data points.
Data Availability Statement
The data sets analyzed in this study are available from the corresponding author on reasonable request.
RESULTS
A total of 53 patients who underwent VSS stenting were included in the cohort and the median age was 33 ± 10 years, predominantly female (70%), with a mean BMI of 36 kg/m2 (range 22.6-52.9 kg/m2). Headache and visual disturbances were the most common symptoms on presentation (100%), followed by tinnitus (94%) and papilledema (88%). Forty percent of patients had optic nerve atrophy before intervention. The mean baseline venous sinus pressure gradient in the complete cohort was 9.6 mmHg, with a range from 1 to 26 mmHg. The baseline characteristics between low (≤4 mmHg), medium (5-8 mmHg), and high (>8 mmHg) gradient groups were similar (Table 1). Of note, there was a trend toward a higher incidence of arachnoid granulation etiology of stenosis compared with strictures in the lower gradient patients. Representative images of these 2 phenotypes are shown in Figure 1. All data presented in the text below are given as low, intermediate, and then high gradient, with appropriate units as labeled. The baseline prestent lumbar puncture opening pressure showed a trend toward being lower with lower venous pressure gradient but did not reach statistical significance (28 cmH2O vs 35 cmH2O vs 36 cmH2O, P = .3).
TABLE 1.
Comparison of Demographics and Clinical Characteristics According to the Gradient Group
| Variable | ≤4 (n = 9) | 5-8 (n = 18) | >8 (n = 26) | P value |
|---|---|---|---|---|
| Age, y, mean ± SD | 34 ± 10 | 32 ± 10 | 31 ± 10 | 1.0 |
| Female sex, n (%) | 8 (89) | 13 (72) | 16 (62) | .3 |
| BMI, mean ± SD | 32 ± 6 | 37 ± 7 | 36 ± 8 | .3 |
| Baseline symptoms, n (%) | ||||
| Headache | 9/9 (100) | 18/18 (100) | 26/26 (100) | — |
| Tinnitus | 8/9 (88.9) | 17/18 (94.4) | 25/26 (96.2) | .7 |
| Visual disturbance | 9/9 (100) | 18/18 (100) | 26/26 (100) | — |
| Ophthalmological examination | ||||
| Papilledema, n (%)a | 5/6 (83) | 10/10(100) | 13/16 (81) | 1.0 |
| Optic atrophy | 3/9 (33) | 8/18 (44) | 10/26 (38) | .8 |
| Visual field MD, mean ± SD | −3.9 ± 6.3 | −3.3 ± 4.3 | −2.9 ± 2.0 | .9 |
| RNFL, µm, mean ± SD | 119 ± 59 | 146 ± 154 | 208 ± 131 | .4 |
| Prior acetazolamide use, n (%) | 4 (44) | 9 (50) | 15 (58) | .8 |
| Opening pressure, cmH2O, mean ± SD | 28 ± 9 | 35 ± 11 | 36 ± 8 | .1 |
| Venous pressure gradient, mmHg, mean ± SDb | 3 ± 1.1 | 6 ± 1.1 | 14 ± 5.3 | <.0001 |
| Percent stenosis in stented sinus (mean ± SD) | 72 ± 9 | 66 ± 11 | 69 ± 13 | .5 |
| Sinus dominance, n (%)c | .8 | |||
| Dominant | 6/7 (86) | 12/18 (67) | 17/25 (68) | |
| Codominant | 1/7 (14) | 6/18 (33) | 8/25 (32) | |
| Stenosis due to arachnoid granulation, n (%) | 4/9 (44) | 2/18 (11) | 2/26 (8) | .051 |
ANOVA, analysis of variance; BMI, body mass index; RNFL, retinal nerve fiber layer.
Patients with optic atrophy not included in this analysis.
Tukey post hoc comparison shows significant difference between [≤4] vs [>8] and [5 to 8] vs [>8].
All stented sinuses were either dominant or codominant.
Categorical variables analyzed by the Fisher exact test, and continuous variables analyzed by 1-way ANOVA unless otherwise indicated.
FIGURE 1.
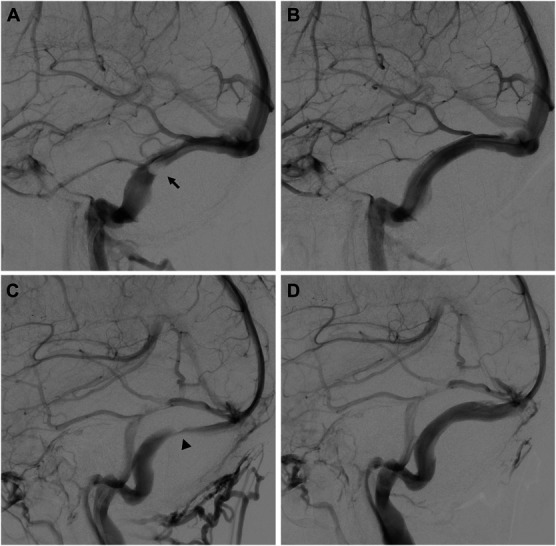
Representative digital subtraction angiography images of A, a prestenting and B, poststenting granulation (arrow) related stenosis as well as C, a prestenting and D, poststenting stricture (arrowhead) related stenosis.
Stent placement was successful in all cases with immediate poststenting gradients being higher in the >8 mmHg group (0.6, 0.6, and 2.2 mmHg, P = .02) despite a higher absolute change (2.4, 5.6, and 12 mmHg, P < .01; Table 2; Figure 2A). However, the mean opening pressure (18, 23, and 24 cmH2O, P = .1) and magnitude of change in opening pressure (13, 13, and 12 cmH2O, P = 1.0) were similar at 6 months between groups (Figure 2B). Resolution of gradient (to physiological ranges 0-3 mmHg) was achieved more often in the lower gradient groups compared with higher gradients; however, this was not statistically significant. The change in intracranial pressure seemed to be clinically impactful across the range of prestent gradients. Headache and tinnitus improved in all patient groups; all low and medium gradient patients improved. Subjective visual disturbances also improved in all groups (100, 83.3, and 92.3%, P = .34).
TABLE 2.
Six-Month Clinical Outcomes According to the Gradient Group
| Variable | ≤4 (n = 9) | 5-8 (n = 18) | >8 (n = 26) | P value |
|---|---|---|---|---|
| Opening pressure, cmH2O, mean ± SDa | 18 ± 4 | 23 ± 6 | 24 ± 8 | .1 |
| Change in opening pressure, cmH2O, mean ± SD | 13 ± 8 | 13 ± 10 | 12 ± 12 | 1.0 |
| Intracranial hypertension resolution, n (%) | 5/7 (71) | 7/17 (41) | 10/24 (42) | .4 |
| Poststent venous pressure gradient, mmHg, mean ± SDa | 0.6 ± 0.5 | 0.6 ± 0.8 | 2 ± 3 | .02 |
| Change in gradient venous pressure gradient, mmHg, mean ± SDb | 2 ± 1 | 6 ± 1 | 12 ± 6 | <.0001 |
| Gradient resolution, n (%) | 9/9 (100) | 18/18 (100) | 20/26 (77) | .047 c |
| Symptoms improvement, n (%) | ||||
| Headache | 9/9 (100) | 18/18 (100) | 23/26 (88) | .3 |
| Tinnitusd | 8/8 (100) | 17/17 (100) | 21/25 (84) | .2 |
| Visual disturbance | 9/9 (100) | 15/18 (83) | 24/26 (92) | .5 |
| Stent restenosis, n (%) | 0 (0) | 0 (0) | 2 (11) | .6 |
ANOVA, analysis of variance.
Tukey post hoc comparison shows significant difference between [5 to 8] vs [>8].
Tukey post hoc comparison shows significant difference between [≤4] vs [>8] and [5 to 8] vs [>8].
Post hoc pairwise comparison with the Fisher exact and Bonferroni correction for multiple analyses is nonsignificant.
Percent of only patients with pretreatment tinnitus.
Categorical variables analyzed by Fisher's exact test, continuous variables analyzed by one-way ANOVA unless otherwise indicated.
Bold values represent statistical significance.
FIGURE 2.
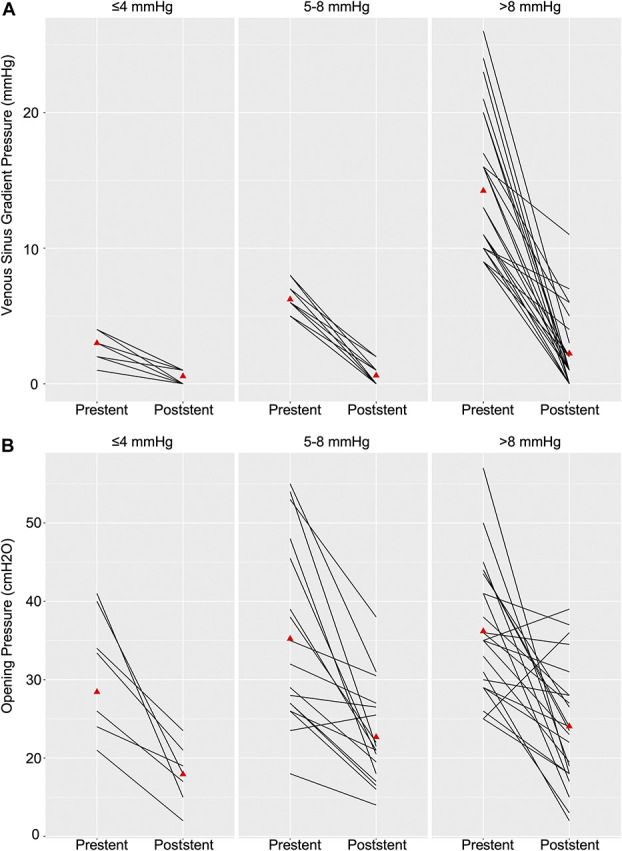
Individual A, venous sinus gradient pressures and B, lumbar puncture opening pressures prestenting and poststenting between the 3 treatment groups. Means represented by (red triangle) some overlapping value and lines.
Ophthalmological data are presented in Table 3. Papilledema seemed to improve in most patients at 6 weeks (100, 87, and 93%, P = .6) and was sustained at 6 months (100, 93, and 86%, P = .7). OCT retinal nerve fiber layer (RNFL) was similar at baseline (119, 146 208 µm, P = .4, Table 1) and also improved at 6 weeks (−30, −54, and −104 µm, P = .5) and 6 months (−51, −67, and −134 µm, P = .6), as measured by mean change, independent of the stented venous gradient. Visual field mean deviation improvement, as measured by Humphrey visual field exam (60, 64, and 67%, P = .5), and visual acuity improvement (100, 91, 83%, P = 1.0) were similar among groups at 6 weeks.
TABLE 3.
Six-Month Ophthalmological Outcomes According to the Gradient Group
| Variable | ≤4 (n = 9) 6 wk | 6 mo |
5-8 (n = 18) 6 wk | 6 mo |
>8 (n = 26) 6 wk | 6 mo |
P value 6 wk | 6 mo |
|---|---|---|---|---|
| Visual acuity improved, n (%) | 5/5 (100) | 1/1 (100) | 10/11 (91) | 9/10 (90) | 10/12 (83) | 8/9 (89) | 1.0 | 0.9 |
| Visual fields mean deviation improved, n (%) | 3/5 (60) | 1/1 (100) | 7/11 (64) | 6/9 (67) | 6/9 (67) | 6/9 (67) | 0.5 | 1.0 |
| Papilledema improvement, n (%) | 8/8 (100) | 8/8 (100) | 13/15 (87) | 14/15 (93) | 22/23 (93) | 18/21 (86) | 0.6 | 0.7 |
| RNFL change, µm, mean ± SDa | −30 ± 47 | −51 ± 71 | −54 ± 150 | −67 ± 176 | −104 ± 119 | −134 ± 155 | 0.5 | 0.6 |
ANOVA, analysis of variance; RNFL, retinal nerve fiber layer.
Analysis performed for 5, 14, and 13 patients per group, respectively.
Categorical variables analyzed by the Fisher exact test, and continuous variables analyzed by 1-way ANOVA unless otherwise indicated.
Of note, there was 1 patient from the high gradient group and 1 patient from the low gradient group that experienced recurrence of symptoms. The high gradient patient was the highest of the group at 18 mmHg with improvement to 11 mmHg poststenting. They had significant improvement for several weeks; there was no evidence of stent failure on worsening of symptoms, and they ultimately required a shunt. A low gradient patient had a delayed recurrence at 18 months and angiography revealed not only a patent stent without a gradient but also showed a new superior sagittal sinus stenosis with a gradient of 1 mmHg, and a new stent was placed.
There were 2 complications in the cohort. One in a patient in the high gradient group who suffered from epistaxis from antithrombotic use. Second one in a low gradient patient with a pelvic hematoma not requiring transfusions or surgical intervention.
DISCUSSION
Venous sinus stenosis stenting for IIH has proven effective for intracranial pressure (ICP) reduction, vision salvage, and clinical symptom improvement in medically refractory patients.18 However, patient selection based on supraphysiological gradient cutoffs has been used in practice based on limited data. The lack of a universally accepted cutoff highlights the concurrent paucity of evidence for a gradient driven exclusion paradigm.
Key Findings
We present a single institutional experience with low gradient VSS stenting. In our cohort, patients with IIH and VSS benefited from stenting independent of the pressure gradient across the stenotic region. Our very low to physiological gradient cohort (≤4 mmHg) had a similar degree of reduction in ICP as compared with the 5 to 8 and >8 mmHg groups. Objective ophthalmological evidence further supported the significance of this reduction, with similar improvement in OCT RNFL, papilledema, visual field mean deviation, and visual acuity across gradient groups. In addition, subjective symptomatic improvement followed this same pattern.
Limitations
Limitations of our study include its small, single-institution, nonrandomized design. In addition, we performed most of our venous pressure measurements (except 1 by patient request in the ≤4 group) under general anesthesia. Although it is clear that anesthetics can affect cerebral venous sinus pressures, the directionality of these effects remains controversial.7,19,20 Venous gradients can also be affected by general anesthesia compared with conscious sedation, although this effect seems to be consistent between patients.19,20 Our clinical approach is to perform a single procedure (measurements and stenting), as opposed to 2 separate procedures, given the added risk of each angiogram. Furthermore, microcatheter diameter and construction could influence pressure measurement accuracy. The Phenom 27 microcatheter (proximal/distal 1.02/0.91 mm) is closest in build to the Marksman 27 (1.1/0.95 mm), which has been shown to be an accurate intracranial pressure monitor, with high performance compared with smaller catheters.21 Thus, using a standardized approach regarding the use of general anesthesia and high performing microcatheter selection decreases the between-patient variability in pressure measurements as much as possible. Given the COVID-19 pandemic, we unfortunately could not perform a standardized follow-up lumbar puncture, angiogram, and clinic visit in a timely fashion for a small number of patients, which resulted in some missing data points as clearly presented in Tables 2 and 3.
Interpretation
Other authors have suggested pressure gradient cutoffs ranging from >4, to ≥8 mmHg, ≥10 mmHg, and even >21 mmHg.4-7,10-14,16 Although the use of pressure gradient as selection criteria is founded in the theoretical logic of hemodynamic significance of stenotic lesions, the paucity of evidence to support this, combined with our findings, suggests this approach may require further evaluation. With approximately a 93% incidence of VSS in IIH and only a 35.4% incidence of venous pressure gradients >8 mmHg among these, there is a large proportion of patients currently excluded from a possible vision-preserving procedure.22,23
Our findings seem somewhat contrary to previous studies that have suggested exclusive benefit at higher venous pressure gradients.16 However, we do not aim to dispute the benefit of stenting high gradient lesions but rather question the proposed lack of benefit in lower gradient cases. First, we know that in some cases CSF removal can result in resolution of VSS and venous pressure gradient.2,24 In other cases, despite ICP normalization with medical treatment, VSS can remain unchanged.25 Altogether this gets to the core of the pathophysiological dilemma of VSS as a primary or secondary event in relation to IIH; the ongoing “chicken or the egg” conundrum. These referenced cases are illustrative of basic principles and suggest that both theories may coexist in a feedback cycle of ICP changes and VSS. Hypothetically, high gradient patients could represent secondary lesions and low gradient patients representing primary lesions or vice versa. In fact, a previous study defining extrinsic (secondary) and intrinsic (primary) appearing lesions found no difference in the reduction of ICP after stenting of high gradient cases.6 This potentially explains our findings that accelerating CSF clearance by improving venous outflow could prove beneficial independent of gradient, given that gradient could represent only an early marker of pathophysiological origin of a lesion. This is contrary to a paradigm of gradient as a continuum of severity of a lesion past a threshold at which stenting becomes beneficial. Taken together, our interpretation is that IIH stenting is not a panacea and gradient is likely to be a treatment effect modifier but may not be a prerequisite for clinical response. However, further research is needed to confirm our findings.
Generalizability
Taken together, our findings suggest that our preconceived notion of the need for a gradient to benefit from VSS stenting should be revisited. Although the small number of cases and nonrandomized nature of this study precludes us from reaching conclusive recommendations, it at least supports the need for further research.
CONCLUSION
This study demonstrates a similar benefit to VSS stenting in low venous pressure gradient patients with IIH, with comparable reductions in ICP, clinical improvement, and ophthalmological response. Because patients with physiological gradients also experienced improvement in ICP and objective ophthalmological criteria after venous sinus stenting, our study calls into question the practice of using an arbitrary pressure gradient to exclude patients from treatment, particularly in an otherwise ideal patient for venous sinus stenting (ie, elevated ICP, visual impairment, and clear evidence of venous sinus stenosis). Larger prospective studies in this previously excluded population are warranted to further evaluate our findings.
Footnotes
CNS Journal Club Podcast and CME Exams available at cns.org/podcasts.
Mehmet Enes Inam and Juan Carlos Martinez-Gutierrez contributed equally to this work.
Contributor Information
Mehmet Enes Inam, Email: Mehmet.Enes.Inam@uth.tmc.edu.
Juan Carlos Martinez-Gutierrez, Email: martinez.juancarlos@gmail.com.
Matthew J. Kole, Email: mkole0209@gmail.com.
Francisco Sanchez, Email: fsanchez@neuroeye.com.
Elvira Lekka, Email: Elvira.Lekka@uth.tmc.edu.
Van Thi Thanh Truong, Email: Van.T.Truong@uth.tmc.edu.
Victor Lopez-Rivera, Email: Victor.LopezRivera@bmc.org.
Faheem G. Sheriff, Email: fsheriff.md@gmail.com.
Laura A. Zima, Email: Laura.A.Zima@uth.tmc.edu.
Claudia Pedroza, Email: Claudia.Pedroza@uth.tmc.edu.
Rosa Tang, Email: rtang@neuroeye.com.
Ore-Ofe Adesina, Email: Ore-Ofeoluwatomi.O.Adesina@uth.tmc.edu.
Allison Engstrom, Email: Allison.C.Engstrom@uth.tmc.edu.
Sunil A. Sheth, Email: Sunil.A.Sheth@uth.tmc.edu.
Funding
This research is supported by Weatherhead Research Fund and Dipaolo Family Research Fund. Dr Sheth discloses NIH funding.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol. 2016;15(1):78-91. [DOI] [PubMed] [Google Scholar]
- 2.Rohr A, Dö L, Stingele R, Buhl R, Alfke K, Jansen O. Reversibility of venous sinus obstruction in idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2007;28(4):656-659. [PMC free article] [PubMed] [Google Scholar]
- 3.Saber H, Lewis W, Sadeghi M, Rajah G, Narayanan S. Stent survival and stent-adjacent stenosis rates following venous sinus stenting for idiopathic intracranial hypertension: a systematic review and meta-analysis. Interv Neurol. 2018;7(6):490-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields LBE, Shields CB, Yao TL, Plato BM, Zhang YP, Dashti SR. Endovascular Treatment for venous sinus stenosis in idiopathic intracranial hypertension: an observational study of clinical indications, surgical technique, and long-term outcomes. World Neurosurg. 2019;121:e165-e171. [DOI] [PubMed] [Google Scholar]
- 5.Dinkin MJ, Patsalides A. Venous sinus stenting in idiopathic intracranial hypertension: results of a prospective trial. J Neuroophthalmol. 2017;37(2):113-121. [DOI] [PubMed] [Google Scholar]
- 6.Patsalides A, Oliveira C, Wilcox J, et al. Venous sinus stenting lowers the intracranial pressure in patients with idiopathic intracranial hypertension. J Neurointerv Surg. 2019;11(2):171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fargen KM, Liu K, Garner RM, Greeneway GP, Wolfe SQ, Crowley RW. Recommendations for the selection and treatment of patients with idiopathic intracranial hypertension for venous sinus stenting. J Neurointerv Surg. 2018;10(12):1203-1208. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Gutierrez JC, Kole MJ, Lopez-Rivera V, et al. Primary balloon angioplasty of venous Sinus stenosis in idiopathic intracranial hypertension. Interv Neuroradiol. Published online ahead of print March 24, 2022. DOI: 10.1177/15910199221089446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumpe DA, Seinfeld J, Huang X, et al. Dural sinus stenting for idiopathic intracranial hypertension: factors associated with hemodynamic failure and management with extended stenting. J Neurointerv Surg. 2017;9(9):867-874. [DOI] [PubMed] [Google Scholar]
- 10.Ducruet AF, Crowley RW, McDougall CG, Albuquerque FC. Long-term patency of venous sinus stents for idiopathic intracranial hypertension. J Neurointerv Surg. 2014;6(3):238-242. [DOI] [PubMed] [Google Scholar]
- 11.Fields JD, Javedani PP, Falardeau J, et al. Dural venous sinus angioplasty and stenting for the treatment of idiopathic intracranial hypertension. J Neurointerv Surg. 2013;5(1):62-68. [DOI] [PubMed] [Google Scholar]
- 12.Asif H, Craven CL, Siddiqui AH, et al. Idiopathic intracranial hypertension: 120-day clinical, radiological, and manometric outcomes after stent insertion into the dural venous sinus. J Neurosurg. 2018;129(3):723-731. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed RM, Wilkinson M, Parker GD, et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. Am J Neuroradiol. 2011;32(8):1408-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radvany MG, Solomon D, Nijjar S, et al. Visual and neurological outcomes following endovascular stenting for pseudotumor cerebri associated with transverse sinus stenosis. J Neuroophthalmol. 2013;33(2):117-122. [DOI] [PubMed] [Google Scholar]
- 15.Elder BD, Rory Goodwin C, Kosztowski TA, et al. Venous sinus stenting is a valuable treatment for fulminant idiopathic intracranial hypertension. J Clin Neurosci. 2015;22(4):685-689. [DOI] [PubMed] [Google Scholar]
- 16.McDougall CM, Ban VS, Beecher J, Pride L, Welch BG. Fifty shades of gradients: does the pressure gradient in venous sinus stenting for idiopathic intracranial hypertension matter? A systematic review. J Neurosurg. 2019;130(3):999-1005. [DOI] [PubMed] [Google Scholar]
- 17.King JO, Mitchell PJ, Thomson KR, Tress BM. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology. 2002;58(1):26-30. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson P, Brinjikji W, Radovanovic I, et al. Venous sinus stenting for idiopathic intracranial hypertension: a systematic review and meta-analysis. J Neurointerv Surg. 2019;11(4):380-385. [DOI] [PubMed] [Google Scholar]
- 19.Fargen KM, Spiotta AM, Hyer M, et al. Comparison of venous sinus manometry gradients obtained while awake and under general anesthesia before venous sinus stenting. J Neurointerv Surg. 2017;9(10):990-993. [DOI] [PubMed] [Google Scholar]
- 20.Raper DMS, Buell TJ, Chen CJ, DIng D, Starke RM, Liu KC. Intracranial venous pressures under conscious sedation and general anesthesia. J Neurointerv Surg. 2017;9(10):986-989. [DOI] [PubMed] [Google Scholar]
- 21.Avery MB, Sambrano S, Khader Eliyas J, Eesa M, Mitha AP. Accuracy and precision of venous pressure measurements of endovascular microcatheters in the setting of dural venous sinus stenosis. J Neurointerv Surg. 2018;10(4):387-390. [DOI] [PubMed] [Google Scholar]
- 22.Levitt MR, Hlubek RJ, Moon K, et al. Incidence and predictors of dural venous sinus pressure gradient in idiopathic intracranial hypertension and non-idiopathic intracranial hypertension headache patients: results from 164 cerebral venograms. J Neurosurg. 2017;126(2):347-353. [DOI] [PubMed] [Google Scholar]
- 23.Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60(9):1418-1424. [DOI] [PubMed] [Google Scholar]
- 24.Buell T, Ding D, Raper D, et al. Resolution of venous pressure gradient in a patient with idiopathic intracranial hypertension after ventriculoperitoneal shunt placement: a proof of secondary cerebral sinovenous stenosis. Surg Neurol Int. 2021;12:14. DOI: 10.25259/SNI_700_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bono F, Giliberto C, Mastrandrea C, et al. Transverse sinus stenoses persist after normalization of the CSF pressure in IIH. Neurology. 2005;65(7):1090-1093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets analyzed in this study are available from the corresponding author on reasonable request.