Abstract
Mathematical models show that the induction period of intermittent cancer therapy drives cancer toward resistance
Over the past decade, multiple clinical trials using intermittent therapy, which typically applies on/off treatment cycles after an initial induction period, have been published with mixed but generally unimpressive results. For example, Dooley et al found that intermittent dosing of vemurafenib for BRAF V600W–mutant melanoma produced similar clinical benefit with less toxicity than continuous therapy,1 but Algazi et al,2 found that intermittent therapy with BRAF and MEK inhibitors in BRAF-mutant melanoma did not improve progression-free survival and follow-up analysis suggested a poorer clinical outcome.3 In a large trial, Hussain et al,4 found that intermittent therapy using androgen deprivation therapy (ADT) in men with metastatic castrate-sensitive prostate cancer produced no significant benefit compared with standard dosing. Most recently, Brown et al5 reported a randomized phase II/III trial for patients with metastatic clear cell renal cell cancer comparing cessation versus continuous sunitinib or pazopanib after 24 weeks of therapy on the basis of initial response. Cessation therapy proved to be feasible, safe, cost-effective, and without a significant decline in life expectancy in comparison with continuous drug dosing.
We note that there is an implicit, and often explicit, assumption that the intermittent treatment strategy exploits evolutionary principles to delay the onset of resistance while decreasing accumulated drug doses and reducing toxicity. However, intermittent therapy is quite different from evolution-based on/off treatment strategies (adaptive therapy), which have shown more positive results.6 Both treatment strategies are based on the evolutionarily reasonable hypothesis that modulation of selection pressure from drug treatment will delay the acquisition of resistance while decreasing treatment-induced toxicity, but there are differences in their adherence to fundamental evolutionary principles, which have significant clinical consequences.
To summarize the governing evolutionary dynamics, benefits from intermittent therapy accrue because of the fitness cost incurred by synthesizing, maintaining, and operating the molecular machinery of resistance.7 This investment of resources may be a small fraction of the cell's global energy budget; but, in a substrate-poor environment typically found in tumors, it is sufficient to reduce proliferation.8 In evolutionary theory, fitness and proliferation can be viewed as equivalent, which allows an indirect estimate of the fitness cost by the relative size of the resistant and sensitive populations before treatment.9 That is, if the sensitive population is much larger than the resistant population, it usually indicates that there is a high fitness cost for resistance. Although an exhaustive measure of pretreatment cancer cell subpopulations is usually not feasible, the global ratio of sensitive-to-resistant cells can often be inferred from the initial response to therapy. For example, a large decline in prostate specific antigen (PSA) on initiating ADT indicates that the ratio of sensitive-to-resistant cells is very high with a corresponding large difference in fitness. On the other hand, a minimal response suggests that the fitness difference is small and cannot be exploited using evolution-based strategies.10
Thus, the optimal evolutionary dynamics induced by intermittent therapy is the following sequence: When therapy is applied, the sensitive population declines, whereas the resistant population will remain stable or increase. When therapy is discontinued, all cancer populations will proliferate, but an evolutionary benefit is gained when the treatment-sensitive population, because of its fitness advantage in the absence of treatment, outcompetes and suppresses growth of the resistant cells. As a result, when treatment is restarted, the ratio of size of the sensitive/resistant populations is similar to its pretreatment value allowing a similar clinical response.7,11 Through repeated cycles, proliferation of the resistant cells is suppressed and, therefore, treatment response can be prolonged with reduced treatment toxicity and cost12 during the off cycles.13 Note the evolutionary contrast to continuous application of treatment agents at maximum tolerated dose (MTD), which strongly selects for resistance and, by eliminating potential competitors, tends to accelerate proliferation of the resistant population.
Thus, successful intermittent therapy uses the treatment-sensitive population as a forcing function to delay or prevent growth of the resistant population to a clinically significant size. In other words, although preventing growth of the resistant population is the goal, the primary focus of intermittent therapy is optimizing the size of the sensitive population.
With these principles in mind, the evolutionary flaws in traditional intermittent treatment protocols become apparent. First, intermittent therapy typically begins with an induction period of prolonged drug administration at MTD. In the study by Brown et al5, the induction period was 24 weeks, and in the study of continuous versus intermitted ADT in prostate cancer, induction ADT was administered at MTD for 7 months. Mathematical analysis of this prolonged induction period demonstrates reductions in the size of the treatment-sensitive population so that it is ineffective in its evolutionary role of suppressing proliferation of resistant cells during the off cycle (Fig 1). This evolutionary flaw in the trial design also extends to subsequent cycles in the study protocol. For example, in the prostate cancer intermittent trial, each new treatment cycle arbitrarily required continuous MTD treatment for 7 months.4 By contrast, when treatment is modulated on the basis of PSA, an optimal size for suppression of the resistant population was patient-dependent but generally in the range of 3-4 months.6,11,14
FIG 1.
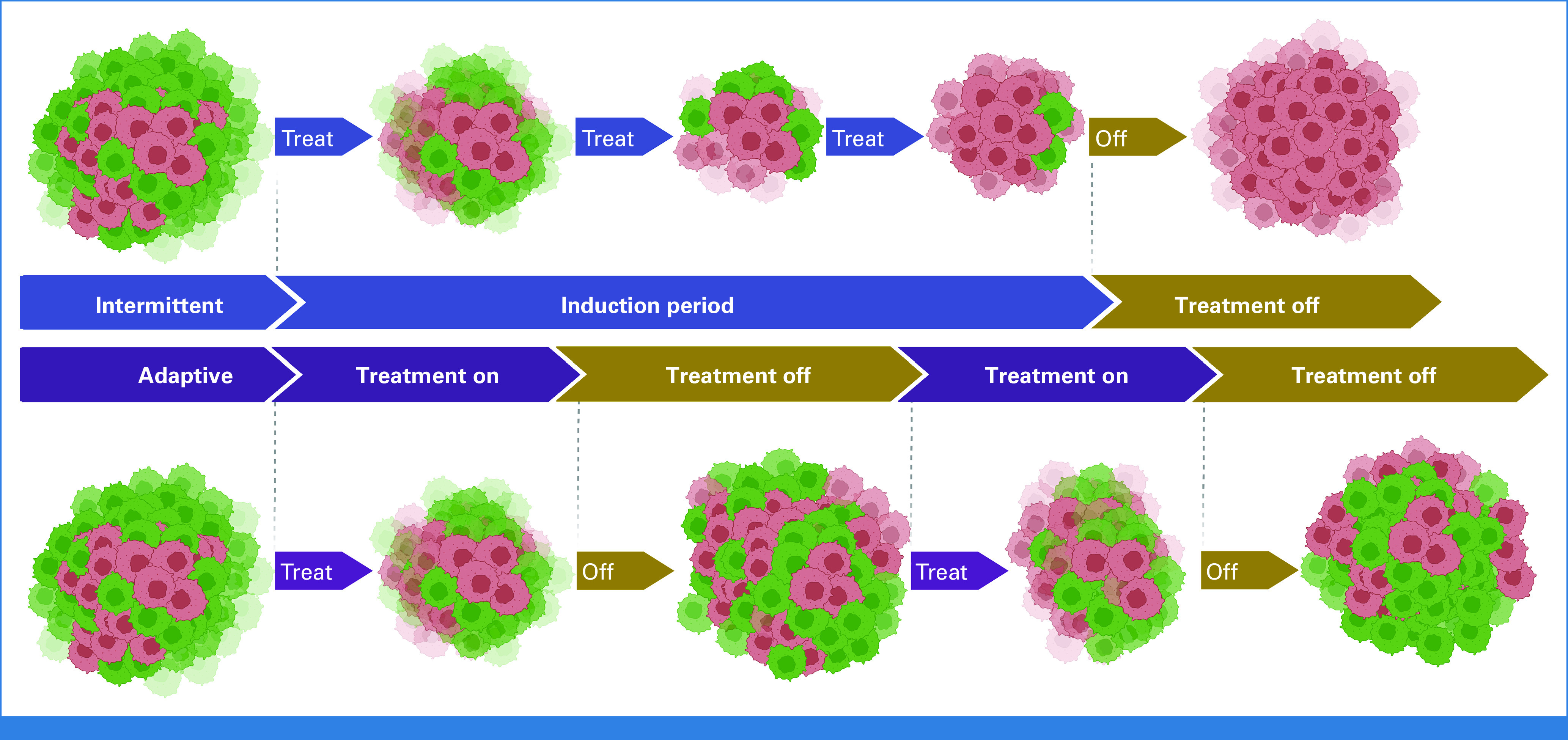
Simplified evolutionary dynamics during intermittent therapy. For the full mathematical model and computer simulations, see the study by Zhang et al.11 Green cells are sensitive to treatment, and red cells are resistant. The top panel demonstrates intermittent therapy that begins with a prolonged induction period. This excessively reduces the frequency of resistant cells at the expense of sensitive ones, rendering the subsequent drug-off period less effective in restoring sensitivity. In the lower panel, we demonstrate that these dynamics can be avoided by using on/off cycles from the start of therapy.
In conclusion, cancers, as complex, nonlinear, dynamical systems, often exhibit nonintuitive responses to therapy. Integrating evolutionary dynamics into cancer requires a clear understanding of first principles and generally benefits from computer simulations to anticipate counter intuitive nonlinearities.15 It is also useful to explicitly define the evolutionary goal of therapy. If the expected outcome is cure, then the evolutionary goal of treatment is extinction of the cancer population,16 a goal that can only be achieved by induction of maximum cancer cell death. However, if cure is not achievable, then the goal of maximal cancer cell killing, while intuitively appealing, is usually evolutionarily unwise because it applies intense selection pressure for resistance and eliminates the resistant population's potential competitors.17 These dynamics, termed competitive release, are well-recognized in, for example, pest management and can accelerate emergence of resistant populations.18 That is, current intermittent therapy protocols reflect divergent and incompatible goals of exploiting evolutionary principles for control while also maximizing cancer cell death. Thus, we suggest that optimizing cancer treatment requires systematic integration of mathematical models to anticipate and steer the cancer's ecoevolutionary dynamics and to make rigorous after-action analyses to improve future outcomes.19
ACKNOWLEDGMENT
The authors gratefully acknowledge support from the Moffitt Center of Excellence for Evolutionary Therapy.
Jad Chahoud
Consulting or Advisory Role: Aveo, DAVA Pharmaceuticals, Pfizer, Exelixis
Jingsong Zhang
Honoraria: Sanofi, AstraZeneca/MedImmune, Bayer, Seagen, Sanofi/Aventis, Pfizer, EMD Serono, Dendreon
Consulting or Advisory Role: AstraZeneca/MedImmune, Bayer, Dendreon, Pfizer, EMD Serono
Speakers' Bureau: Sanofi, AstraZeneca, Seagen, Dendreon
No other potential conflicts of interest were reported.
SUPPORT
Supported by grants U54 CA143970, R01 CA077575, and U01CA232382 to R.A.G., J.B., A.R.A.A., and J.Z.
AUTHOR CONTRIBUTIONS
Financial support: Alexander R.A. Anderson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Evolutionary Dynamics and Intermittent Therapy for Metastatic Cancers
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jad Chahoud
Consulting or Advisory Role: Aveo, DAVA Pharmaceuticals, Pfizer, Exelixis
Jingsong Zhang
Honoraria: Sanofi, AstraZeneca/MedImmune, Bayer, Seagen, Sanofi/Aventis, Pfizer, EMD Serono, Dendreon
Consulting or Advisory Role: AstraZeneca/MedImmune, Bayer, Dendreon, Pfizer, EMD Serono
Speakers' Bureau: Sanofi, AstraZeneca, Seagen, Dendreon
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dooley AJ, Gupta A, Bhattacharyya M, et al. : Intermittent dosing with vemurafenib in BRAF V600E-mutant melanoma: Review of a case series. Ther Adv Med Oncol 6:262-266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algazi AP, Othus M, Daud AI, et al. : Continuous versus intermittent BRAF and MEK inhibition in patients with BRAF-mutated melanoma: A randomized phase 2 trial. Nat Med 26:1564-1568, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Cao M, Mayo de Las Casas C, Oramas J, et al. : Intermittent BRAF inhibition in advanced BRAF mutated melanoma results of a phase II randomized trial. Nat Commun 12:7008, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussain M, Tangen CM, Berry DL, et al. : Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med 368:1314-1325, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JE, Royle KL, Gregory W, et al. : Temporary treatment cessation versus continuation of first-line tyrosine kinase inhibitor in patients with advanced clear cell renal cell carcinoma (STAR): An open-label, non-inferiority, randomised, controlled, phase 2/3 trial. Lancet Oncol 24:213-227, 2023 [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Cunningham J, Brown J, et al. : Evolution-based mathematical models significantly prolong response to abiraterone in metastatic castrate resistant prostate cancer and identify strategies to further improve outcomes. Elife 11:e76284, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatenby RA, Silva AS, Gillies RJ, et al. : Adaptive therapy. Cancer Res 69:4894-4903, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strobl MAR, West J, Viossat Y, et al. : Turnover modulates the need for a cost of resistance in adaptive therapy. Cancer Res 81:1135-1147, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basanta D, Anderson AR: Exploiting ecological principles to better understand cancer progression and treatment. Interface Focus 3:20130020, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady-Nicholls R, Zhang J, Zhang T, et al. : Predicting patient-specific response to adaptive therapy in metastatic castration-resistant prostate cancer using prostate-specific antigen dynamics. Neoplasia 23:851-858, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Cunningham JJ, Brown JS, et al. : Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat Commun 8:1816, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason NT, Burkett JM, Nelson RS, et al. : Budget impact of adaptive abiraterone therapy for castration-resistant prostate cancer. Am Health Drug Benefits 14:15-20, 2021 [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham J, Thuijsman F, Peeters R, et al. : Optimal control to reach eco-evolutionary stability in metastatic castrate-resistant prostate cancer. PLoS One 15:e0243386, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Gallaher J, Cunningham JJ, et al. : A phase 1b adaptive androgen deprivation therapy trial in metastatic castration sensitive prostate cancer. Cancers 14:5225, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham JJ, Brown JS, Gatenby RA, et al. : Optimal control to develop therapeutic strategies for metastatic castrate resistant prostate cancer. J Theor Biol 459:67-78, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Gatenby RA, Artzy-Randrup Y, Epstein T, et al. : Eradicating metastatic cancer and the eco-evolutionary dynamics of anthropocene extinctions. Cancer Res 80:613-623, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatenby RA, Brown JS: Integrating evolutionary dynamics into cancer therapy. Nat Rev Clin Oncol 17:675-686, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Seton-Rogers S: Chemotherapy: Preventing competitive release. Nat Rev Cancer 16:199, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Stankova K, Brown JS, Dalton WS, et al. : Optimizing cancer treatment using game theory: A review. JAMA Oncol 5:96-103, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]


