Abstract
BACKGROUND:
Stereoelectroencephalography (sEEG) facilitates electrical sampling and evaluation of complex deep-seated, dispersed, and multifocal locations. Granger causality (GC), previously used to study seizure networks using interictal data from subdural grids, may help identify the seizure-onset zone from interictal sEEG recordings.
OBJECTIVE:
To examine whether statistical analysis of interictal sEEG helps identify surgical target sites and whether surgical resection of highly ranked nodes correspond to favorable outcomes.
METHODS:
Ten minutes of extraoperative recordings from sequential patients who underwent sEEG evaluation were analyzed (n = 20). GC maps were compared with clinically defined surgical targets using rank order statistics. Outcomes of patients with focal resection/ablation with median follow-up of 3.6 years were classified as favorable (Engel 1, 2) or poor (Engel 3, 4) to assess their relationship with the removal of highly ranked nodes using the Wilcoxon rank-sum test.
RESULTS:
In 12 of 20 cases, the rankings of contacts (based on the sum of outward connection weights) mapped to the seizure-onset zone showed higher causal node connectivity than predicted by chance (P ≤ .02). A very low aggregate probability (P < 10−18, n = 20) suggests that causal node connectivity predicts seizure networks. In 8 of 16 with outcome data, causal connectivity in the resection was significantly greater than in the remaining contacts (P ≤ .05). We found a significant association between favorable outcome and the presence of highly ranked nodes in the resection (P < .05).
CONCLUSION:
Granger analysis can identify seizure foci from interictal sEEG and correlates highly ranked nodes with favorable outcome, potentially informing surgical decision-making without reliance on ictal recordings.
KEY WORDS: Causal connectivity, Epilepsy surgery, Interictal Intracranial EEG, Seizure networks, Stereoelectroencephalography, Surgical planning
ABBREVIATIONS:
- GC
Granger causality
- iEEG
intracranial electroencephalography
- sdDTF
short-time directed transfer function
- sEEG
Stereoelectroencephalography
- SOZ
seizure-onset zone.
Epilepsy arises from aberrant activity in brain networks.1-14 Among the 30% to 40% of epilepsy patients who suffer from pharmacoresistant epilepsy,15,16 surgical intervention for selected patients can offer the possibility of seizure freedom by directly altering the network through removal or disconnection of seizure-generating regions. Focal surgical treatment for intractable seizures should, therefore, logically use data that reveal anomalies in the network to plan an anatomic strategy. In current clinical practice, intracranial electroencephalography (iEEG) obtained over several days with visual analysis of the ictal iEEG recordings is used as the gold standard to localize seizure generators in the epileptogenic network17,18; however, direct demonstration of actual networks derived from iEEG and influential nodes of the network that might indicate anatomic sites causing the seizures have not been routinely incorporated into surgical decision-making.
Granger causality (GC), originally formulated in economics,19 is a well-established statistical algorithm to infer links between data series based on predictability of the future behavior of one variable from the past behavior of one or more other variables.20 The utility of GC has been shown in a number of studies when applied to iEEG recorded with subdural grids (sometimes with sparse depth electrodes), implemented in the time domain and also in the frequency domain by different research groups. Epstein et al21 demonstrated that spectral GC was able to determine the most crucial nodes of the widespread preictal (less than a minute before visible ictal onset) connectivity in the high frequency range (80-250 Hz). However, the clinical utility of this methodology was limited by lengthy required computation time, on the order of 1 hour of processing time per second of iEEG. As a result, major conclusions centered on ictal episodes with interictal data used as an experimental control.21
A different potential approach to extracting useful network descriptions from interictal data was developed by Korzeniewska et al.22 This study applied an algorithm closely related to spectral GC, short-time directed transfer function (sdDTF), to interictal data, and calculated propagation of high frequency (70-175 Hz) causal interactions. The sdDTF method revealed most active nodes in the seizure-onset zone, but the limited number of variables included in the causal network analysis risked providing an incomplete description of causal connectivity.20,22-24
In contrast to the approaches described above, the time domain conditional GC algorithm used in Park and Madsen25 revealed causal connectivity without restricting the number of channels included in the analysis or discriminating causal connectivity at different frequency bands and with a reasonable computation time (a few minutes per 20 seconds of simultaneous recordings from 100 channels). That study focused on the potential practical utility of Granger analysis to extract seizure-onset zone (SOZ) information using interictal baseline recordings from subdural grids and thereby inform clinical decisions, proving that seizure foci and associated areas of resection had statistically very high causal node connectivity. No conclusion about the effect of including high causal nodes in the resection on outcomes was possible due to fairly uniform outcomes.25
Stereoelectroencephalography (sEEG) depth electrode implantation without craniotomy has become increasingly prevalent in North America as an alternative to craniotomy for implanting subdural grids with or without depth electrodes.1,26-29 Although both procedures yield iEEG recordings with high temporal and spatial resolution, sEEG allows greater flexibility in accessing deeply situated foci and investigating epileptic networks with multilobar distribution, which may prompt different trends in patient selection for surgery and hence lead to heterogeneous outcomes. It is, therefore, timely to inquire whether the same statistical approach to the evaluation of network connectivity derived from subdural/depth recordings used previously25 could also be applied to interictal data recorded by sEEG.
In this retrospective study, we tested whether the higher interictal node connectivity area is correlated with the topography of the SOZ as defined by ictal recordings and examined whether the presence of highly ranked nodes in the resection is associated with favorable outcome for postoperative seizure reduction. If such a correlation exists, the results of this study may help optimize surgical decision-making by enabling visualization of causal networks based on analysis of relatively brief periods of interictal baseline sEEG, possibly without reliance on ictal data.
METHODS
Patients
We retrospectively examined interictal iEEG, clinical information, and surgical outcomes from 20 patients with medically refractory focal epilepsy who underwent sEEG electrode implantation. The patient series was consecutive and not selected by outcome or other clinical factors. Demographic information and clinical information are summarized in Table 1 and with more detailed description in Table, Supplemental Digital Content 1, http://links.lww.com/NEU/D245. Our Institutional Review Board approved this retrospective review of iEEG data, clinical information, and postoperative outcomes as a post hoc review which does not require consent. The multidisciplinary epilepsy program at our institution perceived need for intracranial EEG data to optimize surgical planning and by consensus recommended invasive monitoring for each individual patient. The need for better delineation of the SOZ, functional anatomic areas, or both was determined after consideration of data from all available noninvasive diagnostic modalities.
TABLE 1.
Demographic and Clinical Information at the Time of sEEG Monitoring and Subsequent Surgery
| Characteristic | No. (%) (n = 20) |
|---|---|
| Sex | |
| Male | 10 (50) |
| Female | 10 (50) |
| Age at surgery, median, y | 12 |
| <6 | 1 (5) |
| 6-11 | 6 (30) |
| 12-17 | 11 (55) |
| ≥18 | 2 (10) |
| Preoperative MRI findings | |
| Normal | 2 (10) |
| Abnormal/lesional | 12 (60) |
| Nonlocalizing | 2 (10) |
| Possible abnormality but not definitive | 4 (20) |
| Side of surgery | |
| Right | 7 (35) |
| Left | 11 (55) |
| Surgical procedure at the time of sEEG electrodes removal | |
| Focal resection | 10 (50) |
| Focal resection (reresection) | 1 (5) |
| Laser ablation | 6 (30) |
| Subhemispheric disconnection | 1 (5) |
| No intervention | 2 (10) |
| Reasons for no focal resection or ablation at the time of electrodes removal | |
| Proximity of the seizure focus to functional area | 1 (5) |
| Diffuse seizure foci | 1 (5) |
| Independent foci detected from the entirety of all electrodes | 1 (5) |
| Pathologic findings | No. (%) (n= 13a) |
|---|---|
| Focal cortical dysplasia type II | 4 (30.8) |
| Mesial temporal sclerosis | 1 (7.7) |
| Gliosis | 5 (38.4) |
| Tumor/cortex with focal dysplastic features | 1 (7.7) |
| Mild dysplasia/cortex with lymphocytic cuff and subpial necrosis | 1 (7.7) |
| Leptomeningeal inflammation | 1 (7.7) |
sEEG, Stereoelectroencephalography.
Pathology was reviewed in 13 (65%) patients. No pathology was requested in 7 patients (6 patients underwent laser ablation and 1 patient did not undergo resection).
Clinically Identified Seizure-Onset Zones
In this study, we define the SOZ in each case as the subset of contacts of sEEG depth electrodes identified by routine visual analysis of the ictal iEEG data captured during the long-term monitoring period, as recorded in the formal clinical report in the medical record. The SOZ contacts as reported by epileptologists were later used for the statistical analysis (Table 2; n = 20). Calculation of causal connectivity and clinical interpretation of sEEG were each performed by individuals blinded to the other result.
TABLE 2.
Statistical Analysis Using Rank Ordering Approach
| Comparison between causal nodes computed by Granger analysis and seizure-onset zone | Comparison between causal nodes computed by Granger analysis and resection zone | Outcome | |||||
|---|---|---|---|---|---|---|---|
| Patient no. | Rank order sum | Rank order sum by chance | P value | Rank order sum | Rank order sum by chance | P value | Engel scale |
| 8 | 175 | 650 | 1 × 10−6 | 2077 | 2600 | .004 | 1 |
| 12 | 260 | 761 | 8 × 10−5 | 1949 | 2366 | .04 | 3 |
| 10 | 876 | 1269 | 4 × 10−4 | 752 | 1128 | 4 × 10−4 | 1 |
| 1 | 422 | 630 | 7 × 10−4 | 454 | 630 | .004 | 1 |
| 15 | 764 | 1122 | .002 | 3404 | 3300 | .69 | 3 |
| 3 | 219 | 476 | .002 | 401 | 535 | .09 | 4 |
| 4a | 5 | 57 | .003 | n/aa | n/aa | n/aa | 1a |
| 16 | 8598 | 9720 | .006 | 3136 | 3780 | .03 | 1 |
| 7 | 4790 | 5664 | .007 | 1576 | 2016 | .03 | 2 |
| 6 | 1555 | 2150 | .007 | 705 | 935 | .08 | 3 |
| 11 | 951 | 1276 | .01 | 2389 | 2784 | .01 | 3 |
| 17 | 4216 | 4995 | .02 | 777 | 1110 | .05 | 1 |
| 5b | 1143 | 1290 | .10 | 3884 | 3783 | .99b | 1b |
| 9 | 790 | 952 | .10 | 1439 | 1547 | .24 | 1 |
| 19 | 2390 | 2674 | .16 | 1538 | 1712 | .23 | 4 |
| 2 | 274 | 246 | .69 | 501 | 574 | .18 | 1 |
| 14 | 206 | 175 | .70 | 979 | 994 | .45 | n/a |
| 13 | 5121 | 4896 | .82 | 1316 | 1152 | .85 | 3 |
| 20 | 2614 | 2356 | .88 | 671 | 684 | .46 | 3 |
| 18 | 2785 | 2240 | .99 | n/a | n/a | n/a | n/a |
sEEG, Stereoelectroencephalography.
No resection was performed at the time of sEEG depth electrodes removal. The outcome of this patient, Engel 1, is associated with the resection surgery performed a year later through invasive monitoring with grids/strips (see Table, Supplemental Digital Content 1, http://links.lww.com/NEU/D245, for further details).
The surgical procedure for patient 5 included occipital and posterior parietal disconnection, resulting in 89% of the total contacts of all sEEG depth electrodes considered within the resection zone. As a result, the rank order sum was bound to be close to the expected sum and the rank ordering method was not able to properly determine statistical significance. For this reason, the P value computed based on such functional lobectomies was not used for outcome analysis.
Focal Resection/Ablation and Postsurgical Outcomes
Surgery after sEEG is planned jointly by the neurosurgeons and epileptologists based on the iEEG-derived SOZ. At the time of removal of sEEG depth electrodes, focal resection or focal thermal ablation was performed in 17 of 20 patients. Postsurgical outcomes were available in 16 of 17 who had focal epilepsy surgery and were evaluated at a median of 3.6 years (follow-up by an epileptologist [JB] for a period ranging from 2.5 to 4.9 years after surgery) using the modified Engel classification system.30 More detailed information of surgical procedure and outcome is presented in Table, Supplemental Digital Content 1, http://links.lww.com/NEU/D245.
Clinically Identified Resection Zones
Extent of resection was routinely recorded as part of the operative note by the surgeon at or immediately after the surgery, using preoperative and postoperative MRI and computed tomography (CT) image fusion to three dimensionally determine which recording contacts of sEEG depth electrodes were included in the resected tissue. Throughout this report, we define the subset of recording contacts of sEEG depth electrodes encompassing the brain volume resected (based on the operative note and fused postoperative images) the resection zone. The resection zones as reported by neurosurgeons and outcome data were later used for the statistical analysis (Table 2; n = 16). Calculation of causal connectivity and identification of the resection zone were each performed by individuals blinded to the other result.
Data Collection and Granger Causality Analysis
Directional or “causal” connectivity was calculated by applying Granger's statistical approach to 10 minutes of “quiet” (ie, free of interictal epileptiform activity) baseline data recorded extraoperatively at the earliest time available. The GC algorithm is based on linear regression modeling derived from the data. This was accomplished using Granger causality connectivity analysis toolbox v2.9 in MATLAB (the MathWorks, Inc.),31 which allows determination of causal inference among each possible pair of contacts of depth electrode-specific iEEG data streams. The details of the data collection/preprocessing and more technical aspects of GC analysis are described in Supplemental Digital Content 2, http://links.lww.com/NEU/D246.
Statistical Validation Using Rank Order Sum
To determine whether the observed SOZ from ictal recordings correlated with relatively higher node connectivity by Granger analysis, we calculated the rank order of the weighted out-degree of each contact (or equivalently, “causality ranking”) and summed the individual rank orders for all the electrodes in the SOZ set and similarly summed the rank orders of each contact in the resection zone (Table 2). The probability of obtaining a rank order sum at or below the observed value was calculated using the sampling method described previously.25 More details are described in Supplemental Digital Content 2, http://links.lww.com/NEU/D246.
RESULTS
We analyzed 20 interictal baseline recordings, 1 for each subject, using previously reported GC analysis methods.25 Twenty cases sorted by P values are listed on the left panel of Table 2. In 12 of 20 cases, the interictal GC rankings of electrodes that mapped to the SOZ had higher causality than predicted by chance (P ≤ .02). Figure 1A and 1B show statistical resemblance of GC maps to the topography of the SOZ: patient 1 and patient 8 both yielded statistically significant results in the rank order approach (P ≤ .0007 for both cases). The aggregate P value calculated using the Fisher's method of combining multiple independent tests of the same hypothesis is less than 10−18 which means that the probability that the results from all 20 cases could be derived from chance alone is remote. This suggests that the networks highlighted in interictal GC maps correlate with seizure networks.
FIGURE 1.
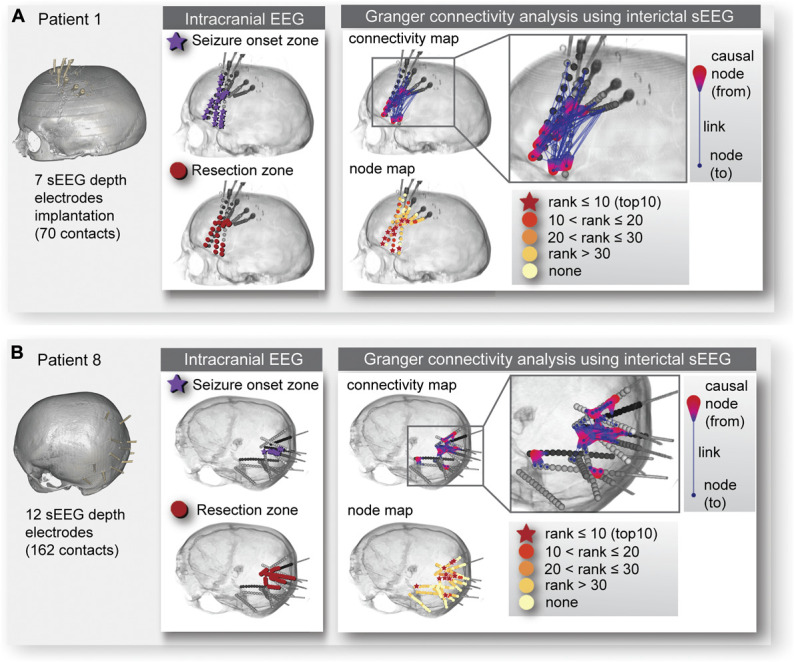
Illustrative cases. As illustrative cases, the seizure-onset zone determined by conventional intracranial electroencephalography visual interpretation, the resection zone, and the causal nodes obtained from Granger causality analysis for A, patient 1 and B, patient 8 are shown. The left panel of A, and B, shows the volume-rendered computed tomography images with implanted sEEG depth electrodes for patient 1 and patient 8, respectively. In the middle panel of A, and B, the seizure-onset zone is indicated by dark purple stars and the resection zone by red dots. The right panel of A, and B, shows the connectivity map and node map for each patient. In the connectivity map, an individual link (blue colored line) connects from the causal node (red colored teardrop shape) to another node (blue colored dot). In the node map, an individual node was evaluated by weighted out-degree (indicating the causal strength) and rank ordered from the most to the least influential. Very highly ranked causal nodes are within the top 10 ranks and those are denoted by red colored stars. The color-coded causal nodes by ranks can be visually compared and also statitically compared (by computing the sum of rank orders) with the seizure-onset zone and the resection zone. EEG, electroencephalography; sEEG, stereoelectroencephalography.
Focal resection or laser ablation was performed in 17 of 20 patients at the time of removal of sEEG depth electrodes. Of those 17, 16 cases with outcome data were used to evaluate statistical significance: P values computed from comparing causality rankings of the resection zone with rankings generated by chance alone are presented on the right panel of Table 2. Lower P values tend to favor the patients with better outcome (Figure 2). To estimate associations between causality in the resection zone and outcome, 16 patients were dichotomized into favorable (Engel 1, 2) and poor (Engel 3, 4) outcome groups. These 2 sets of results are statistically different (Wilcoxon rank-sum, P = .046, 2-tailed), suggesting higher concentration of causality in the resection shown for the favorable outcome.
FIGURE 2.
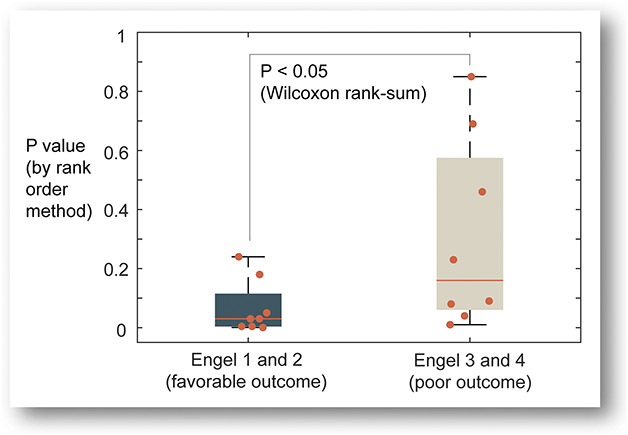
Association between the presence of highly ranked nodes in the resection and favorable surgical outcome. The box plot with individual data points (indicated by solid circles) shows correlation between the presence of high causality (or high-ranked causal nodes) in the resection zone and postoperative outcome, favorable (n = 8) vs poor (n = 8), which were statistically compared using the Wilcoxon rank-sum 2-tailed test with a significance level of 0.05.
DISCUSSION
In this study, we applied Granger analysis to interictal sEEG data obtained from 20 sequential patients and found that the SOZ generally correlated with high weighted out-degree (or high causal connectivity) regions, as seen using the same methodology applied to interictal subdural grid recordings.25 Furthermore, favorable outcome (Engel 1, 2) at a median of 3.6 years also correlated with how well the resection zone captured the brain regions showing high (statistical) causality.
Interictal Granger Connectivity Analysis
For individual cases, we found that interictal baseline sEEG can produce statistical connectivity maps that often indicate the topography of the SOZ. This result is consistent with earlier findings where interictal data (recorded either with subdural grids/depth electrodes13,32-37 or with sEEG electrodes38-42) can identify seizure foci. The primary goal of intracranial electrode implantation is to localize the SOZ in the epileptic network. Our findings suggest that the application of Granger analysis to intracranial recordings facilitates efficient use of interictal data by revealing seizure network properties even before seizures have occurred. However, causality calculations as performed in this study do not definitively delineate the SOZ. They do, however, provide a visual display, like many other current visualization methods (MRI, fMRI, tractography, magnetoencephalography [MEG], etc), which might be helpful in planning a resection.
Over the years, substantial efforts have been made to develop a variety of spectral signal analysis methods that can process multichannel iEEG data43-50 to study epileptic networks and help identify seizure foci.13,21,37 The conditional GC implemented in the time domain is another approach to adding networks to the analysis of multiple streams of iEEG data. We believe this study is the first to report that time domain GC connectivity analysis applied to interictal baseline sEEG data correlates with successful surgical targets, demonstrating that the presence of high (statistical) causality in the resection zone is associated with a favorable outcome. From the perspective of clinical utility, time domain GC has the advantage of reduced data preprocessing steps, assumptions about the data, and computation time compared with other methods formulated in the frequency domain.
Outcome Analysis
One of the notable differences from the earlier study by Park and Madsen25 is that the wider range of clinical outcomes in this study allowed us to explore correlation with surgical outcome. Compared with the previous study where many of the cases were lesional, these patients included a more heterogenous case mix resulting in relatively variable outcomes ranging from Engel 1 to 4. This is in part due to the fact that sEEG depth electrodes can often sample iEEG from a broader range of brain structures than subdural grids.
Patients with better outcomes showed higher causality in the resection compared to those with poorer outcomes. This correlation does not imply causation, and while statistically significant, still requires careful interpretation: When we compare causality in the resection with seizure freedom (Engel 1) and nonseizure freedom (Engel 2-4), this trend did not reach significance.
Limitations of the Study and Future Directions
There are some limitations in this study: (1) GC maps were generated from 10-minute segments of week-long recording sessions. To better evaluate clinical utility in presurgical evaluation, the consistency and robustness of causal networks over time warrants investigation. Granger analysis may be highly variable in its value, in part because the interictal data stream itself may vary with time. Indicators of the degree of confidence expected in any given determination could improve incorporation into the stream of data used in surgical decision-making. This may follow, in part, from a future analysis of variability over time of the network structure revealed by Granger methods and integration of other data modalities into its interpretation. (2) Perhaps the potential future strategy of highest value for interictal analysis would entail real-time analysis of intraoperative data as sEEG depth electrodes were being placed, because this could inform modified strategies for placement of other depth electrodes in real-time and result in a higher-yield final implantation scheme. However, intraoperative data recorded during implantation may include much shorter segments, and the effects of anesthetic agents on this analysis are not yet known. It would be worth investigating the minimum duration of data needed to produce GC maps that similarly predict the SOZ. (3) A larger number of patients with available outcome data would better indicate whether or not interictal sEEG GC analysis can reliably predict seizure outcome after resection and allow us to test the utility of shorter or longer time samples with systematically varied sampling rates. A larger number of cases may also allow us to understand the reason why 12 cases reached significance but 8 did not when compared with seizure-onset zone (left panel of Table 2). Yet uncharacterized features of the seizure networks, such as multifocality, may explain this, but there is no proper way to stratify cases and speculate on potential reasons because of the relatively small number of cases analyzed in this study.
The major utility of this approach is not the prediction of seizure outcome, but as an aid to extracting the most information possible about network interactions from interictal data to aid with surgical decision-making. Further work should include analysis of evolution of interictal network patterns over time and development of algorithms to help define the possible SOZ prospectively from GC network data, potentially supporting the incorporation of network activity measures such as GC into the evaluation of surgical strategies to treat epilepsy. This will include exploration of other network analysis algorithms from the field of graph theory. For example, we are poised to investigate how in-degree correlates with seizure-onset zone, resection zone, and perhaps surgical outcome and compare with findings from using out-degree. Another direction of future studies may include integration of other data streams in a Bayesian synthesis of these analyses with other types of data. An important future direction will be the emerging trend to incorporate structural connectivity (for example, from MRI tractography) into the physiologically demonstrated directional networks.51,52
CONCLUSION
Granger causality analysis applied to sEEG recordings has the potential to help localize ictal networks from relatively brief sampling of interictal baseline data. It is conceivable that GC analysis of sEEG data could eventually aid surgical decision-making. Ultimately, this method may contribute to more efficient and reliable surgical resection and ablation decisions, perhaps even in the absence of ictal events.
Footnotes
Part of the work has been submitted as an abstract at the upcoming 75th American Epilepsy Society Annual Meeting (December 3 to 7, 2021; McCormick Place, Chicago, Illinois).
Supplemental digital content is available for this article at neurosurgeryonline.com.
Scellig S. D. Stone and Eun-Hyoung Park contributed equally to this work.
Contributor Information
Scellig S. D. Stone, Email: Scellig.Stone@childrens.harvard.edu.
Eun-Hyoung Park, Email: Eun-Hyoung.Park@childrens.harvard.edu.
Jeffrey Bolton, Email: jeffrey.bolton@childrens.harvard.edu.
Chellamani Harini, Email: chellamani.harini@childrens.harvard.edu.
Mark H. Libenson, Email: mark.libenson@childrens.harvard.edu.
Alexander Rotenberg, Email: Alexander.Rotenberg@childrens.harvard.edu.
Masanori Takeoka, Email: masanori.takeoka@childrens.harvard.edu.
Melissa Tsuboyama, Email: Melissa.Tsuboyama@childrens.harvard.edu.
Phillip L. Pearl, Email: Phillip.Pearl@childrens.harvard.edu.
Funding
This work was funded by National Institutes of Health (NIH) U01EB023820-01 (Park, Madsen, Pearl). The Boston Children's Hospital (BCH) Intellectual and Developmental Disabilities Research Center (BCH IDDRC, U54HD090255) also supported this study (Pearl).
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplemental Digital Content
Supplemental Digital Content 1. Table. Demographic and clinical information, and surgical outcome.
Supplemental Digital Content 2. Text. Detailed description of methods. A, Intracranial sEEG data, B, Granger causal connectivity analysis, C, Statistical analysis using rank order sum, D, Visualization and summarization of complex causal connectivity network. E, Out-strength centrality measure in directed graph, weighted out-degree. F, Supplementary references.
COMMENT
This manuscript describes the authors' work using Granger causality analysis on interictal data to predict which SEEG electrodes are more likely to be involved in the seizure onset zone. Causality has been used in several studies to look at areas involved in epileptogenic activity, as abnormal pathological activity will drive the electrographic signals of areas of the brain in a manner that manifests as causal linkages. As expected, areas that are unusually strong causal drivers of many areas of the brain correlate to seizure onset areas.
This work, similar to other analyses looking at other interictal markers, has the potential to improve the practice of seizure localization for patients implanted with SEEG. This is particularly important for patients who have infrequent seizures, who may not have seizures recorded during a prolonged intracranial implantation.
Garrett Banks
Guy M. McKhann
New York, New York, USA
REFERENCES
- 1.Bartolomei F, Lagarde S, Wendling F, et al. Defining epileptogenic networks: contribution of SEEG and signal analysis. Epilepsia. 2017;58(7):1131-1147. [DOI] [PubMed] [Google Scholar]
- 2.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676-685. [DOI] [PubMed] [Google Scholar]
- 3.Berg AT, Millichap JJ. The 2010 revised classification of seizures and epilepsy. Continuum (Minneap Minn). 2013;19(3 epilepsy):571-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg AT, Scheffer IE. New concepts in classification of the epilepsies: entering the 21st century. Epilepsia. 2011;52(6):1058-1062. [DOI] [PubMed] [Google Scholar]
- 5.Blumenfeld H. What is a seizure network? Long-range network consequences of focal seizures. In: Scharfman H, Buckmaster P, eds. Issues in Clinical Epileptology: A View from the Bench. Advances in Experimental Medicine and Biology. Vol 813. Springer Dordrecht; 2014:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Englot DJ, Konrad PE, Morgan VL. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia. 2016;57(10):1546-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jehi L. The epileptogenic zone: concept and definition. Epilepsy Curr. 2018;18(1):12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer MA, Cash SS. Epilepsy as a disorder of cortical network organization. Neuroscientist. 2012;18(4):360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson MP. Large scale brain models of epilepsy: dynamics meets connectomics. J Neurol Neurosurg Psychiatry. 2012;83(12):1238-1248. [DOI] [PubMed] [Google Scholar]
- 10.Smith EH, Schevon CA. Toward a Mechanistic understanding of epileptic networks. Curr Neurol Neurosci Rep. 2016;16(11):97. [DOI] [PubMed] [Google Scholar]
- 11.Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43(3):219-227. [DOI] [PubMed] [Google Scholar]
- 12.Wendling F, Chauvel P, Biraben A, Bartolomei F. From intracerebral EEG signals to brain connectivity: identification of epileptogenic networks in partial epilepsy. Front Syst Neurosci. 2010;4:154. DOI: 10.3389/fnsys.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilke C, Worrell G, He B. Graph analysis of epileptogenic networks in human partial epilepsy. Epilepsia. 2011;52(1):84-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaffe RB, Borger P, Megevand P, et al. Physiology of functional and effective networks in epilepsy. Clin Neurophysiol. 2015;126(2):227-236. [DOI] [PubMed] [Google Scholar]
- 15.Engel J, Jr. Approaches to refractory epilepsy. Ann Indian Acad Neurol. 2014;17(suppl 1):S12-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laxer KD, Trinka E, Hirsch LJ, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59-70. [DOI] [PubMed] [Google Scholar]
- 17.Connolly PJ, Spencer DD, Cohen-Gadol AA. A brief history of epilepsy surgery in the United States. In: Lüders H, ed. Textbook of Epilepsy Surgery, 1st ed. Informa Healthcare; 2008:116-117. [Google Scholar]
- 18.Marino AC, Yang GJ, Tyrtova E, et al. Resting state connectivity in neocortical epilepsy: the epilepsy network as a patient-specific biomarker. Clin Neurophysiol. 2019;130(2):280-288. [DOI] [PubMed] [Google Scholar]
- 19.Granger C. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37(3):424-438. [Google Scholar]
- 20.Bressler SL, Seth AK. Wiener-Granger causality: a well established methodology. Neuroimage. 2011;58(2):323-329. [DOI] [PubMed] [Google Scholar]
- 21.Epstein CM, Adhikari BM, Gross R, Willie J, Dhamala M. Application of high-frequency Granger causality to analysis of epileptic seizures and surgical decision making. Epilepsia. 2014;55(12):2038-2047. [DOI] [PubMed] [Google Scholar]
- 22.Korzeniewska A, Cervenka MC, Jouny CC, et al. Ictal propagation of high frequency activity is recapitulated in interictal recordings: effective connectivity of epileptogenic networks recorded with intracranial EEG. Neuroimage. 2014;101:96-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korzeniewska A, Crainiceanu CM, Kuś R, Franaszczuk PJ, Crone NE. Dynamics of event-related causality in brain electrical activity. Hum Brain Mapp. 2008;29(10):1170-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eichler M. A graphical approach for evaluating effective connectivity in neural systems. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):953-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park EH, Madsen JR. Granger causality analysis of interictal iEEG predicts seizure focus and ultimate resection. Neurosurgery. 2018;82(1):99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abou-Al-Shaar H, Brock AA, Kundu B, Englot DJ, Rolston JD. Increased nationwide use of stereoencephalography for intracranial epilepsy electroencephalography recordings. J Clin Neurosci. 2018;53:132-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Englot DJ. Surface or depth: a paradigm shift in invasive epilepsy monitoring. Epilepsy Currents. 2020;20(6):348-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gavvala J, Zafar M, Sinha SR, Kalamangalam G, Schuele S. Stereotactic EEG practices: a survey of United States tertiary referral epilepsy centers. J Clin Neurophysiol. Published online ahead of print November 10, 2020. DOI: 10.1097/wnp.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 29.Joswig H, Lau JC, Abdallat M, et al. Stereoelectroencephalography versus subdural strip electrode implantations: feasibility, complications, and outcomes in 500 intracranial monitoring cases for drug-resistant epilepsy. Neurosurgery. 2020;87(1):E23-E30. [DOI] [PubMed] [Google Scholar]
- 30.Engel J, Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel JJr, ed. Surgical Treatment of the Epilepsies, 2nd ed. Raven Press; 1993:609-621. [Google Scholar]
- 31.Seth AK. A MATLAB toolbox for Granger causal connectivity analysis. J Neurosci Methods. 2010;186(2):262-273. [DOI] [PubMed] [Google Scholar]
- 32.Burns SP, Santaniello S, Yaffe RB, et al. Network dynamics of the brain and influence of the epileptic seizure onset zone. Proc Natl Acad Sci U S A. 2014;111(49):E5321-E5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demuru M, Kalitzin S, Zweiphenning W, et al. The value of intra-operative electrographic biomarkers for tailoring during epilepsy surgery: from group-level to patient-level analysis. Sci Rep. 2020;10(1):14654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geier C, Bialonski S, Elger CE, Lehnertz K. How important is the seizure onset zone for seizure dynamics?. Seizure. 2015;25:160-166. [DOI] [PubMed] [Google Scholar]
- 35.Khambhati AN, Bassett DS, Oommen BS, et al. Recurring functional interactions predict network architecture of interictal and ictal states in neocortical epilepsy. eNeuro. 2017;4(1):ENEURO.0091-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamilia E, Park EH, Percivati S, et al. Surgical resection of ripple onset predicts outcome in pediatric epilepsy. Ann Neurol. 2018;84(3):331-346. [DOI] [PubMed] [Google Scholar]
- 37.Wilke C, van Drongelen W, Kohrman M, He B. Neocortical seizure foci localization by means of a directed transfer function method. Epilepsia. 2010;51(4):564-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antony AR, Alexopoulos AV, González-Martínez JA, et al. Functional connectivity estimated from intracranial EEG predicts surgical outcome in intractable temporal lobe epilepsy. PLoS One. 2013;8(10):e77916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartolomei F, Bettus G, Stam CJ, Guye M. Interictal network properties in mesial temporal lobe epilepsy: a graph theoretical study from intracerebral recordings. Clin Neurophysiol. 2013;124(12):2345-2353. [DOI] [PubMed] [Google Scholar]
- 40.Li YH, Ye XL, Liu QQ, et al. Localization of epileptogenic zone based on graph analysis of stereo-EEG. Epilepsy Res. 2016;128:149-157. [DOI] [PubMed] [Google Scholar]
- 41.Ponten SC, Bartolomei F, Stam CJ. Small-world networks and epilepsy: graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clin Neurophysiol. 2007;118(4):918-927. [DOI] [PubMed] [Google Scholar]
- 42.Yaffe R, Burns S, Gale J, et al. Brain state evolution during seizure and under anesthesia: a network-based analysis of stereotaxic EEG activity in drug-resistant epilepsy patients. 2012 Annu Int Conf IEEE Eng Med Biol Soc. 2012;5158‐5161. DOI: 10.5110.1109/EMBC.2012.6347155. [DOI] [PubMed] [Google Scholar]
- 43.Baccalá LA, Sameshima K. Partial directed coherence: a new concept in neural structure determination. Biol Cybern. 2001;84(6):463-474. [DOI] [PubMed] [Google Scholar]
- 44.Blinowska KJ. Review of the methods of determination of directed connectivity from multichannel data. Med Biol Eng Comput. 2011;49(5):521-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus. 1999;9(2):137-142. [DOI] [PubMed] [Google Scholar]
- 46.Geertsema EE, Visser GH, Velis DN, Claus SP, Zijlmans M, Kalitzin SN. Automated seizure onset zone approximation based on nonharmonic high-frequency oscillations in human interictal intracranial EEGs. Int J Neural Syst. 2015;25(5):1550015. [DOI] [PubMed] [Google Scholar]
- 47.Geweke J. Measures of conditional linear dependence and feedback between time series. J Am Stat Assoc. 1984;79(388):907-915. [Google Scholar]
- 48.Kaminski MJ, Blinowska KJ. A new method of the description of the information flow in the brain structures. Biol Cybern. 1991;65(3):203-210. [DOI] [PubMed] [Google Scholar]
- 49.Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8(4):194-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voytek B, Canolty RT, Shestyuk A, Crone NE, Parvizi J, Knight RT. Shifts in gamma phase-amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Front Hum Neurosci. 2010;4:191. DOI: 10.3389/fnhum.2010.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gleichgerrcht E, Greenblatt AS, Kellermann TS, et al. Patterns of seizure spread in temporal lobe epilepsy are associated with distinct white matter tracts. Epilepsy Res. 2021;171:106571. DOI: 10.1016/j.eplepsyres.2021.106571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Revell AY, Silva AB, Mahesh D, et al. White matter signals reflect information transmission between brain regions during seizures. bioRxiv. Posted online September 17, 2021. DOI: 10.1101/2021.09.15.460549. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Table. Demographic and clinical information, and surgical outcome.
Supplemental Digital Content 2. Text. Detailed description of methods. A, Intracranial sEEG data, B, Granger causal connectivity analysis, C, Statistical analysis using rank order sum, D, Visualization and summarization of complex causal connectivity network. E, Out-strength centrality measure in directed graph, weighted out-degree. F, Supplementary references.


