Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
Patients with Kirsten rat sarcoma viral oncogene homolog (KRAS)–mutated non–small-cell lung cancer (NSCLC) and untreated CNS metastases have a worse prognosis than similar patients without KRAS mutations. Adagrasib has previously demonstrated CNS penetration preclinically and cerebral spinal fluid penetration clinically. We evaluated adagrasib in patients with KRASG12C-mutated NSCLC and untreated CNS metastases from the KRYSTAL-1 trial (ClinicalTrials.gov identifier: NCT03785249; phase Ib cohort), in which adagrasib 600 mg was administered orally, twice daily. Study outcomes included the safety and clinical activity (intracranial [IC] and systemic) by blinded independent central review. Twenty-five patients with KRASG12C-mutated NSCLC and untreated CNS metastases were enrolled and evaluated (median follow-up, 13.7 months); 19 patients were radiographically evaluable for IC activity. Safety was consistent with previous reports of adagrasib, with grade 3 treatment-related adverse events (TRAEs) in 10 patients (40%) and one grade 4 (4%) and no grade 5 TRAEs. The most common CNS-specific TRAEs included dysgeusia (24%) and dizziness (20%). Adagrasib demonstrated an IC objective response rate of 42%, disease control rate of 90%, progression-free survival of 5.4 months, and median overall survival of 11.4 months. Adagrasib is the first KRASG12C inhibitor to prospectively demonstrate IC activity in patients with KRASG12C-mutated NSCLC and untreated CNS metastases, supporting further investigation in this population.
INTRODUCTION
The Kirsten rat sarcoma viral oncogene homolog (KRAS) is mutated in approximately 25% of non–small-cell lung cancer (NSCLC) cases,1-3 with the glycine-to-cysteine mutation at codon 12 (KRASG12C) occurring in approximately 14% of adenocarcinomas.4 CNS metastases are observed at diagnosis in 27%-42% of patients with KRASG12C-mutated NSCLC.5-8 Patients with KRAS-mutated NSCLC and CNS metastases have a worse prognosis and higher rates of CNS failure compared with patients without KRAS mutations.9-11
Adagrasib (MRTX849), a KRASG12C inhibitor that selectively and irreversibly binds the switch II pocket of KRASG12C,12 has demonstrated CNS penetration, intracranial (IC) tumor regression, and increased survival in multiple preclinical models.13 Preliminary clinical data have similarly shown cerebral spinal fluid penetration and regression of CNS lesions by imaging.13
KRYSTAL-1 (ClinicalTrials.gov identifier: NCT03785249) is an ongoing trial of adagrasib in patients with advanced KRASG12C-mutated solid tumors.14 Here, we report data from a phase Ib cohort evaluating adagrasib monotherapy in patients with NSCLC and untreated CNS metastases.
METHODS
Patients with KRASG12C-mutated NSCLC with untreated CNS metastases received adagrasib monotherapy at the recommended phase II dose (600 mg twice a day administered orally [capsule, fasted state]). Study oversight and methodology are described in the Data Supplement ([Appendix 1.1 and 1.2], online only).
Patients
All patients had KRASG12C-mutated NSCLC; neurologically stable, asymptomatic, untreated CNS metastases; and Eastern Cooperative Oncology Group performance status of 0 or 1. Full eligibility criteria are provided in the Data Supplement (Appendix 1.3).
Objectives and End Points
Primary objectives were to characterize the safety and IC clinical activity of adagrasib. Safety assessments included documentation of adverse events (AEs), laboratory abnormalities, dose interruptions, and treatment discontinuations. CNS efficacy was evaluated by blinded independent central review (BICR) using modified CNS RECIST v1.115 and modified Response Assessment in Neuro-Oncology Brain Metastases (mRANO-BM)16 criteria; with both criteria, measurable lesions had a size ≥5 mm. Systemic clinical activity was evaluated according to RECIST v1.1 per BICR. Overall survival (OS) and 1-year survival rate were evaluated. Full details are provided in the Data Supplement (Appendix 1.4).
Statistical Considerations
The safety population was defined as all patients who received ≥1 dose of the study drug. The clinical activity evaluable population included all patients who received ≥1 dose and had disease assessments at baseline and on-study. Statistical analyses and study assessments are described in the Data Supplement (Appendix 1.5 and 1.6).
RESULTS
Patients
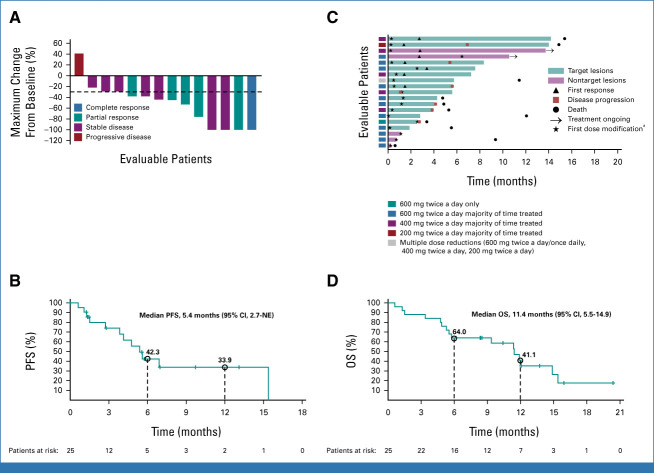
As of August 1, 2022, 25 patients with NSCLC were enrolled, and 19 were evaluable for IC activity by CNS RECIST v1.1, of whom 14 had target lesions and five patients had nontarget lesions only (Data Supplement [Appendix Fig 1]). One additional patient did not have measurable/evaluable CNS metastases at baseline using CNS RECIST v1.1 but was evaluable for systemic activity and IC activity using mRANO-BM criteria. The median follow-up was 13.7 months (95% CI, 8.5 to not evaluable). The median age was 66 years, and the median number of previous systemic therapies was 1 (Table 1).
TABLE 1.
Patient Demographics and Clinical Characteristics
Safety
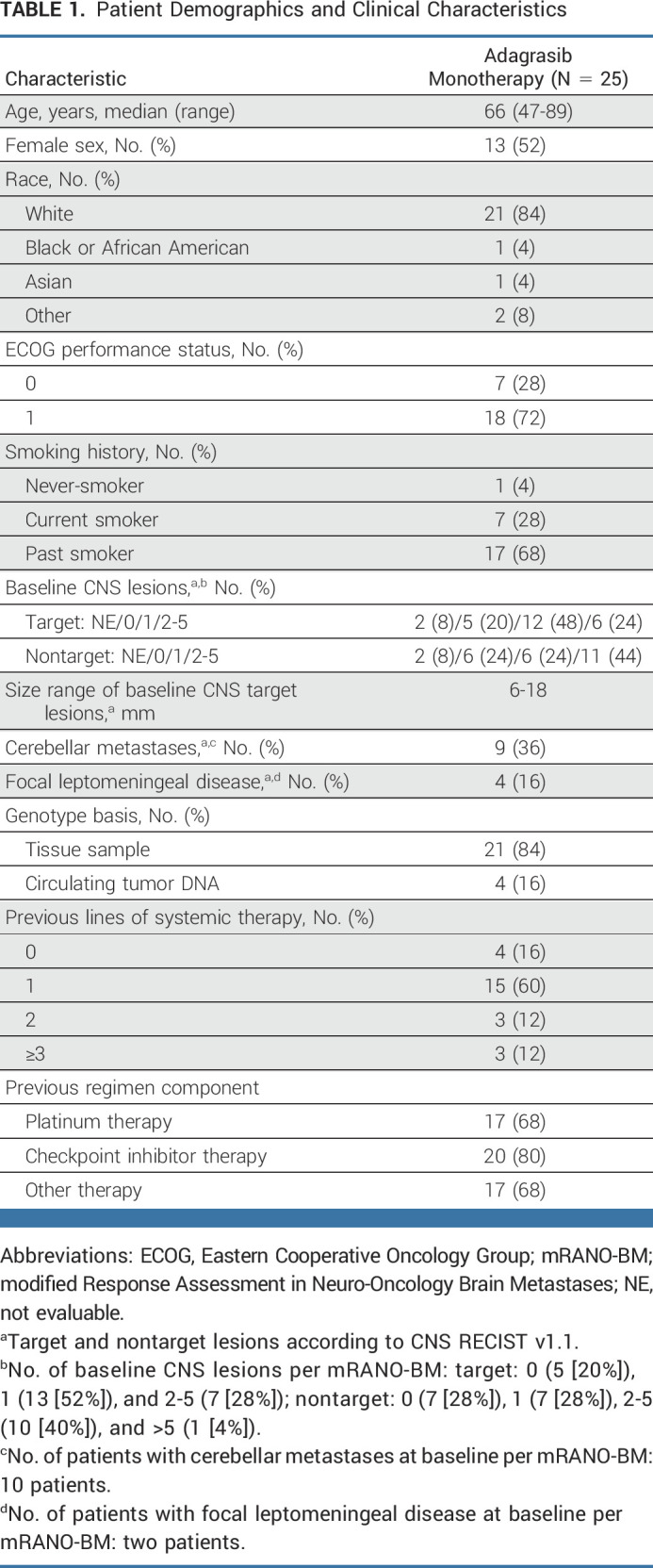
Treatment-related AEs (TRAEs) of any grade occurred in 25 patients (100%); 10 patients (40%) experienced a grade 3 TRAE (Table 2). The most common TRAEs were nausea, diarrhea, vomiting, increased alanine aminotransferase, increased aspartate aminotransferase, and fatigue. One patient experienced a grade 4 TRAE (neutropenia); no grade 5 TRAEs were reported. TRAEs led to dose modifications in 15 patients (60%; dose reduction in eight patients [32%], dose interruption in 14 patients [56%]); two patients (8%) discontinued treatment (grade 3 acute pancreatitis and grade 2 fatigue).
TABLE 2.
Summary of TRAEs (N = 25)
CNS-specific TRAEs, categorized as nervous system disorders, included dysgeusia (24%), dizziness (20%), ataxia (8%), and aphasia, confused state, encephalopathy, headache, and insomnia (each 4%). The majority of CNS-specific TRAEs were grade 1-2; four patients experienced grade 3 CNS-specific TRAEs (dizziness, n = 3; encephalopathy, n = 1), and there were no grade ≥4 CNS-related events (Table 2). Treatment-emergent AEs are summarized in the Data Supplement (Appendix Table 1).
Clinical Outcomes
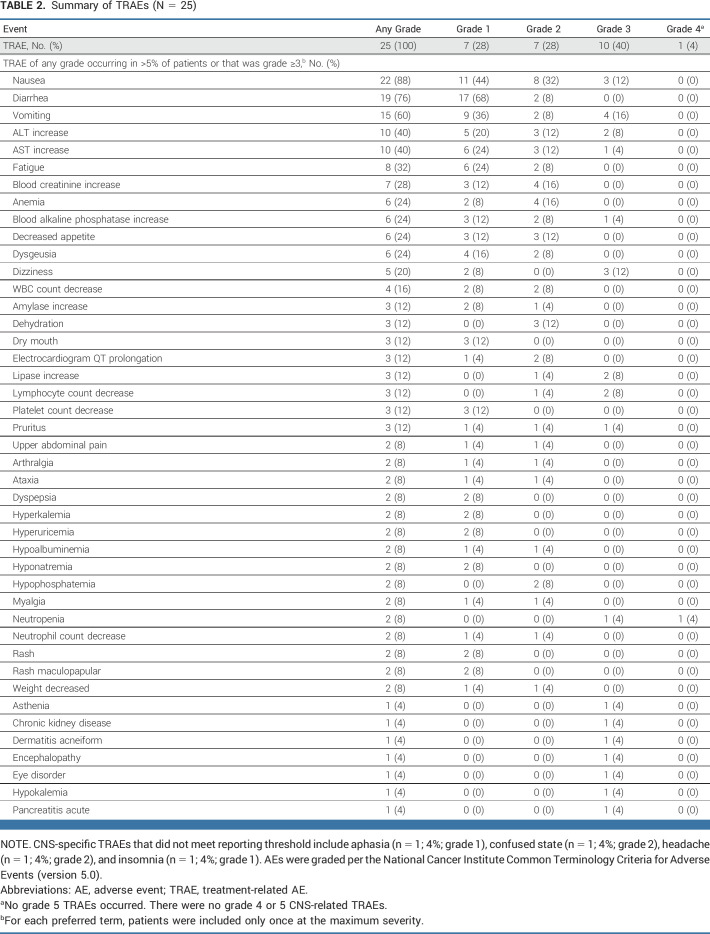
The confirmed IC objective response rate (ORR) per RECIST v1.1 criteria was 42% (95% CI, 20.3 to 66.5; three patients had complete IC response and five patients partial IC response; Data Supplement [Appendix Table 2]; Fig 1A). IC responses by comutational status are shown in the Data Supplement (Appendix Table 3). The IC disease control rate (DCR) was 90%. The median IC progression-free survival (PFS) was 5.4 months (12-month PFS, 33.9%; Fig 1B). The median time to response was 2.1 months; the median IC duration of response (DOR) was 12.7 months, with treatment ongoing for >10 months in two patients still in response (Fig 1C). Of eight patients with confirmed IC response, four (50%) recorded their first response at the second on-study scan. All patients with an IC response had a dose modification during treatment. The CNS failure rate was 37% (7 of 19); two patients experienced CNS progression only. Among 20 patients evaluable for IC activity per mRANO-BM, the confirmed IC ORR was 35% and IC DCR was 85%. Additional IC efficacy outcomes per mRANO-BM are shown in the Data Supplement (Appendix Table 2).
FIG 1.
Efficacy outcomes for evaluable patients per CNS RECIST v1.1 (n = 19). (A) Waterfall plot of maximum percent tumor change from baseline (only patients with target lesions are shown). (B) Kaplan-Meier graphical representation of intracranial PFS. (C) Swimmer plot showing individual duration of treatment, time to first dose modification, response, clinical outcome, and most commonly administered dose at data cutoff. (D) Kaplan-Meier graphical representation of OS for the full analysis set (N = 25). aTime to first dose modification due to any cause, including missed dose, AE, or others. For patients who had a dose modification (reduction or interruption) after initiation of treatment because of an AE, adagrasib could be restarted at the 600 mg twice a day dose following resolution of AEs if deemed appropriate. AE, adverse event; NE, not evaluable; OS, overall survival; PFS, progression-free survival.
The systemic ORR was 30%, with a median DOR of 5.6 months and a median PFS of 5.3 months (Data Supplement [Appendix Table 2 and Fig 2]). The concordance rate between systemic and IC (per CNS RECIST v1.1) disease control was 79% (Data Supplement [Appendix Table 4]). The median OS was 11.4 months (12-month OS, 41.1%; Fig 1D).
DISCUSSION
Patients with KRAS-mutated NSCLC and untreated CNS metastases have limited treatment options and poor clinical outcomes.9-11 Radiation therapy has been the historical standard of care, but recent interest has focused on targeted drugs with CNS penetration (eg, epidermal growth factor receptor or anaplastic lymphoma kinase inhibitors).17,18 This approach avoids adverse effects of radiation such as necrosis and cognitive impairment.19,20
The results presented here are the first prospective data for a KRASG12C inhibitor in patients with NSCLC and untreated CNS metastases. Adagrasib 600 mg twice a day achieved promising IC clinical activity, a high concordance rate between IC and systemic activity (79%), and a low rate of CNS failure (37%). These results provide proof-of-concept for adagrasib's ability to penetrate the CNS and provide CNS activity. The safety and tolerability profile seen in this cohort, with the most common CNS-specific TRAEs reported being dysgeusia and dizziness, was broadly consistent with previous reports from the KRYSTAL-1 trial.14,21
No other KRASG12C inhibitor has previously demonstrated IC activity in a prospective clinical trial, although case studies22-24 and post hoc analyses from registrational data sets13,25 have demonstrated IC responses with sotorasib and adagrasib. However, the post hoc analyses from both sotorasib and adagrasib required adequately treated CNS metastases including radiation before enrollment, leading to an inability to fully interpret the IC activity of the KRASG12C inhibitor.13,25
Limitations of this study include its exploratory nature, limited follow-up period, small evaluable patient population, and exclusion of patients with poor performance status. Additionally, a full analysis of subsequent treatment was unavailable. Therefore, further analyses are required to elucidate the optimal timing and sequencing of adagrasib in relation to radiotherapy. Additional data generation efforts are ongoing in the KRYSTAL program across different lines of therapy in patients with NSCLC, including those with untreated CNS metastases.
In conclusion, adagrasib is the first KRASG12C inhibitor to prospectively demonstrate IC activity, including ongoing responses, in patients with KRASG12C-mutated NSCLC and untreated CNS metastases. Adagrasib had a manageable safety profile with few CNS-specific TRAEs. These findings support continued clinical development of adagrasib for patients with KRASG12C-mutated NSCLC.
ACKNOWLEDGMENT
We thank the participating patients and their families and the clinical study teams and investigators. We also thank Skye Sully and Aditya Shetty from Mirati Therapeutics, Inc for their support in data delivery and analysis. Third-party medical writing assistance, under the direction of the authors, was provided by Rebecca Benatan, BSc, of Ashfield MedComms, an Inizio company, and was funded by Mirati Therapeutics, Inc.
Marcelo V. Negrao
Consulting or Advisory Role: Mirati Therapeutics, Merck, Genentech, Novartis
Research Funding: Mirati Therapeutics (Inst), AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Checkmate Pharmaceuticals (Inst), Genentech (Inst), Alaunos Therapeutics (Inst)
Other Relationship: Ziopharm Oncology, Apothecom, Ashfield Healthcare
Alexander I. Spira
Leadership: Next Oncology (Inst)
Stock and Other Ownership Interests: Lilly
Honoraria: CytomX Therapeutics, AstraZeneca/MedImmune, Merck, Takeda, Amgen, Janssen Oncology, Novartis, Bristol Myers Squibb, Bayer
Consulting or Advisory Role: Array BioPharma (Inst), Incyte, Amgen, Novartis, AstraZeneca/MedImmune (Inst), Mirati Therapeutics, Gritstone Bio, Jazz Pharmaceuticals, Merck (Inst), Bristol Myers Squibb (Inst), Takeda, Janssen Research & Development, Mersana, Blueprint Medicines (Inst), Daiichi Sankyo/Astra Zeneca, Regeneron, Lilly, Black Diamond Therapeutics, Sanofi
Research Funding: Roche (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), MedImmune (Inst), Novartis (Inst), Newlink Genetics (Inst), Incyte (Inst), AbbVie (Inst), Ignyta (Inst), LAM Therapeutics (Inst), Trovagene (Inst), Takeda (Inst), Macrogenics (Inst), CytomX Therapeutics (Inst), LAM Therapeutics, Astex Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Loxo (Inst), Arch Therapeutics (Inst), Gritstone Bio (Inst), Plexxikon (Inst), Amgen (Inst), Daiichi Sankyo (Inst), ADC Therapeutics (Inst), Janssen Oncology (Inst), Mirati Therapeutics (Inst), Rubius Therapeutics (Inst), Synthekine (Inst), Mersana (Inst), Blueprint Medicines (Inst), Regeneron, Alkermes (Inst), Revolution Medicines (Inst), Medikine (Inst), Black Diamond Therapeutics (Inst), BluPrint Oncology (Inst), Nalo Therapeutics (Inst)
Rebecca S. Heist
Consulting or Advisory Role: Novartis, Daichii Sankyo, EMD Serono/Merck, AbbVie, Sanofi, Lilly, Regeneron, Claim Therapeutics
Research Funding: AbbVie (Inst), Novartis (Inst), Roche (Inst), Mirati Therapeutics (Inst), Exelixis (Inst), Corvus Pharmaceuticals (Inst), Daiichi Sankyo (Inst), Agios (Inst), Pfizer (Inst), Lilly (Inst), Turning Point Therapeutics (Inst), Erasca, Inc (Inst), Mythic Therapeutics (Inst)
Pasi A. Jänne
Stock and Other Ownership Interests: Gatekeeper Pharmaceuticals, Loxo
Consulting or Advisory Role: Pfizer, Boehringer Ingelheim, AstraZeneca, Merrimack, Chugai Pharma, Roche/Genentech, Loxo, Mirati Therapeutics, Araxes Pharma, Ignyta, Lilly, Takeda, Novartis, Biocartis, Voronoi Health Analytics, SFJ Pharmaceuticals Group, Sanofi, Daiichi Sankyo, Silicon Therapeutics, Nuvalent, Inc, Eisai, Bayer, Syndax, AbbVie, Allorion Therapeutics, Accutar Biotech, Transcenta, Monte Rosa Therapeutics, Scorpion Therapeutics, Merus, Frontier Medicines, Hongyun Biotech, Duality Biologics
Research Funding: AstraZeneca, Astellas Pharma, Daiichi Sankyo, Lilly, Boehringer Ingelheim, Puma Biotechnology, Takeda, Revolution Medicines
Patents, Royalties, Other Intellectual Property: I am a co-inventor on a DFCI owned patent on EGFR mutations licensed to Lab Corp. I receive post-marketing royalties from this invention
Jose M. Pacheco
Consulting or Advisory Role: Silverback Therapeutics
Jared Weiss
Stock and Other Ownership Interests: Nektar, Vesselon, Achilles Therapeutics, Nuvalent, Inc, Lyell Immunopharma, Enfuego Therapeutics, Vertex
Consulting or Advisory Role: AstraZeneca, EMD Serono, Genentech, G1 Therapeutics, Jounce Therapeutics, AbbVie, Nanobiotix, Regeneron, Genmab, BeiGene, Merck, Sanofi/Aventis
Research Funding: Merck (Inst), AstraZeneca/MedImmune (Inst), G1 Therapeutics (Inst), Immunicum (Inst), Loxo/Lilly (Inst), Mirati Therapeutics (Inst), Sumitomo Dainippon Pharma Oncology (Inst), Boehringer Ingelheim (Inst), PDS Biotechnology (Inst)
Travel, Accommodations, Expenses: Mirati Therapeutics
Shirish M. Gadgeel
Honoraria: Merck
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Bristol Myers Squibb, Takeda, Daiichi Sankyo, Novartis, Blueprint Medicines, Lilly, Pfizer, Janssen Oncology, Mirati Therapeutics, Merck, Eisai, Gilead Sciences, GlaxoSmithKline
Research Funding: Merck, Genentech/Roche (Inst), Merck (Inst), Blueprint Medicines (Inst), Astellas Pharma (Inst), Daiichi Sankyo (Inst), I-Mab (Inst), Nektar (Inst), AstraZeneca, AstraZeneca (Inst), Pfizer (Inst), Amgen (Inst), Turning Point Therapeutics (Inst), Regeneron (Inst), Mirati Therapeutics (Inst), Janssen Oncology (Inst), BioMed Valley Discoveries (Inst), Ymabs Therapeutics Inc (Inst), Calithera Biosciences (Inst), InventisBio (Inst), Dragonfly Therapeutics (Inst), eFFECTOR Therapeutics (Inst), Elevation Oncology (Inst), Erasca, Inc (Inst), Helsinn Therapeutics (Inst), Incyte (Inst), Numab (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: Mirati Therapeutics
Other Relationship: AstraZeneca
Karen Velastegui
Employment: Mirati Therapeutics, Pfizer
Stock and Other Ownership Interests: Mirati Therapeutics, Arena Pharma
Wenjing Yang
Employment: Mirati Therapeutics
Hirak Der-Torossian
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Travel, Accommodations, Expenses: Mirati Therapeutics
James G. Christensen
Employment: Mirati Therapeutics
Leadership: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Consulting or Advisory Role: BridgeBio Pharma
Patents, Royalties, Other Intellectual Property: Multiple patents in last 2 years while employed at Mirati Therapeutics covering discovery of KRAS, LSD1, and EZH2 inhibitors (Inst)
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Navire, Pfizer, Regeneron, Medscape, Takeda
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2022 ASCO Annual Meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by Mirati Therapeutics, Inc.
CLINICAL TRIAL INFORMATION
NCT03785249 (KRYSTAL-1)
DATA SHARING STATEMENT
At Mirati Therapeutics, we are committed to patient care, advancing scientific understanding, and enabling the scientific community to learn from and build on the research we have undertaken. To that end, we will honor legitimate requests for our clinical trial data from qualified researchers and investigators for conducting methodologically sound research. We will share study-level clinical trial data, clinical study reports, study protocols, and statistical analysis plans from clinical trials for which results have been posted on ClinicalTrials.gov for products and indications approved by regulators in the United States and/or European Union. Sharing is subject to protection of patient privacy and respect for the patient's informed consent. In general, data will be made available for specific requests approximately 24 months after clinical trial completion from our in-scope interventional trials. For additional information on proposals with regard to data sharing collaborations with Mirati, please email us at medinfo@mirati.com.
AUTHOR CONTRIBUTIONS
Conception and design: Marcelo V. Negrao, Alexander I. Spira, Jose M. Pacheco, Hirak Der-Torossian, James G. Christensen, Joshua K. Sabari
Provision of study materials or patients: Marcelo V. Negrao, Alexander I. Spira, Rebecca S. Heist, Pasi A. Jänne, Jose M. Pacheco, Jared Weiss, Shirish M. Gadgeel, Karen Velastegui, Joshua K. Sabari
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Intracranial Efficacy of Adagrasib in Patients From the KRYSTAL-1 Trial With KRASG12C–Mutated Non–Small-Cell Lung Cancer Who Have Untreated CNS Metastases
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Marcelo V. Negrao
Consulting or Advisory Role: Mirati Therapeutics, Merck, Genentech, Novartis
Research Funding: Mirati Therapeutics (Inst), AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Checkmate Pharmaceuticals (Inst), Genentech (Inst), Alaunos Therapeutics (Inst)
Other Relationship: Ziopharm Oncology, Apothecom, Ashfield Healthcare
Alexander I. Spira
Leadership: Next Oncology (Inst)
Stock and Other Ownership Interests: Lilly
Honoraria: CytomX Therapeutics, AstraZeneca/MedImmune, Merck, Takeda, Amgen, Janssen Oncology, Novartis, Bristol Myers Squibb, Bayer
Consulting or Advisory Role: Array BioPharma (Inst), Incyte, Amgen, Novartis, AstraZeneca/MedImmune (Inst), Mirati Therapeutics, Gritstone Bio, Jazz Pharmaceuticals, Merck (Inst), Bristol Myers Squibb (Inst), Takeda, Janssen Research & Development, Mersana, Blueprint Medicines (Inst), Daiichi Sankyo/Astra Zeneca, Regeneron, Lilly, Black Diamond Therapeutics, Sanofi
Research Funding: Roche (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), MedImmune (Inst), Novartis (Inst), Newlink Genetics (Inst), Incyte (Inst), AbbVie (Inst), Ignyta (Inst), LAM Therapeutics (Inst), Trovagene (Inst), Takeda (Inst), Macrogenics (Inst), CytomX Therapeutics (Inst), LAM Therapeutics, Astex Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Loxo (Inst), Arch Therapeutics (Inst), Gritstone Bio (Inst), Plexxikon (Inst), Amgen (Inst), Daiichi Sankyo (Inst), ADC Therapeutics (Inst), Janssen Oncology (Inst), Mirati Therapeutics (Inst), Rubius Therapeutics (Inst), Synthekine (Inst), Mersana (Inst), Blueprint Medicines (Inst), Regeneron, Alkermes (Inst), Revolution Medicines (Inst), Medikine (Inst), Black Diamond Therapeutics (Inst), BluPrint Oncology (Inst), Nalo Therapeutics (Inst)
Rebecca S. Heist
Consulting or Advisory Role: Novartis, Daichii Sankyo, EMD Serono/Merck, AbbVie, Sanofi, Lilly, Regeneron, Claim Therapeutics
Research Funding: AbbVie (Inst), Novartis (Inst), Roche (Inst), Mirati Therapeutics (Inst), Exelixis (Inst), Corvus Pharmaceuticals (Inst), Daiichi Sankyo (Inst), Agios (Inst), Pfizer (Inst), Lilly (Inst), Turning Point Therapeutics (Inst), Erasca, Inc (Inst), Mythic Therapeutics (Inst)
Pasi A. Jänne
Stock and Other Ownership Interests: Gatekeeper Pharmaceuticals, Loxo
Consulting or Advisory Role: Pfizer, Boehringer Ingelheim, AstraZeneca, Merrimack, Chugai Pharma, Roche/Genentech, Loxo, Mirati Therapeutics, Araxes Pharma, Ignyta, Lilly, Takeda, Novartis, Biocartis, Voronoi Health Analytics, SFJ Pharmaceuticals Group, Sanofi, Daiichi Sankyo, Silicon Therapeutics, Nuvalent, Inc, Eisai, Bayer, Syndax, AbbVie, Allorion Therapeutics, Accutar Biotech, Transcenta, Monte Rosa Therapeutics, Scorpion Therapeutics, Merus, Frontier Medicines, Hongyun Biotech, Duality Biologics
Research Funding: AstraZeneca, Astellas Pharma, Daiichi Sankyo, Lilly, Boehringer Ingelheim, Puma Biotechnology, Takeda, Revolution Medicines
Patents, Royalties, Other Intellectual Property: I am a co-inventor on a DFCI owned patent on EGFR mutations licensed to Lab Corp. I receive post-marketing royalties from this invention
Jose M. Pacheco
Consulting or Advisory Role: Silverback Therapeutics
Jared Weiss
Stock and Other Ownership Interests: Nektar, Vesselon, Achilles Therapeutics, Nuvalent, Inc, Lyell Immunopharma, Enfuego Therapeutics, Vertex
Consulting or Advisory Role: AstraZeneca, EMD Serono, Genentech, G1 Therapeutics, Jounce Therapeutics, AbbVie, Nanobiotix, Regeneron, Genmab, BeiGene, Merck, Sanofi/Aventis
Research Funding: Merck (Inst), AstraZeneca/MedImmune (Inst), G1 Therapeutics (Inst), Immunicum (Inst), Loxo/Lilly (Inst), Mirati Therapeutics (Inst), Sumitomo Dainippon Pharma Oncology (Inst), Boehringer Ingelheim (Inst), PDS Biotechnology (Inst)
Travel, Accommodations, Expenses: Mirati Therapeutics
Shirish M. Gadgeel
Honoraria: Merck
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Bristol Myers Squibb, Takeda, Daiichi Sankyo, Novartis, Blueprint Medicines, Lilly, Pfizer, Janssen Oncology, Mirati Therapeutics, Merck, Eisai, Gilead Sciences, GlaxoSmithKline
Research Funding: Merck, Genentech/Roche (Inst), Merck (Inst), Blueprint Medicines (Inst), Astellas Pharma (Inst), Daiichi Sankyo (Inst), I-Mab (Inst), Nektar (Inst), AstraZeneca, AstraZeneca (Inst), Pfizer (Inst), Amgen (Inst), Turning Point Therapeutics (Inst), Regeneron (Inst), Mirati Therapeutics (Inst), Janssen Oncology (Inst), BioMed Valley Discoveries (Inst), Ymabs Therapeutics Inc (Inst), Calithera Biosciences (Inst), InventisBio (Inst), Dragonfly Therapeutics (Inst), eFFECTOR Therapeutics (Inst), Elevation Oncology (Inst), Erasca, Inc (Inst), Helsinn Therapeutics (Inst), Incyte (Inst), Numab (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: Mirati Therapeutics
Other Relationship: AstraZeneca
Karen Velastegui
Employment: Mirati Therapeutics, Pfizer
Stock and Other Ownership Interests: Mirati Therapeutics, Arena Pharma
Wenjing Yang
Employment: Mirati Therapeutics
Hirak Der-Torossian
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Travel, Accommodations, Expenses: Mirati Therapeutics
James G. Christensen
Employment: Mirati Therapeutics
Leadership: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Consulting or Advisory Role: BridgeBio Pharma
Patents, Royalties, Other Intellectual Property: Multiple patents in last 2 years while employed at Mirati Therapeutics covering discovery of KRAS, LSD1, and EZH2 inhibitors (Inst)
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Navire, Pfizer, Regeneron, Medscape, Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1.Liu P, Wang Y, Li X: Targeting the untargetable KRAS in cancer therapy. Acta Pharm Sin B 9:871-879, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pakkala S, Ramalingam SS: Personalized therapy for lung cancer: Striking a moving target. JCI Insight 3:e120858, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheffler M, Ihle MA, Hein R, et al. : K-ras mutation subtypes in NSCLC and associated co-occuring mutations in other oncogenic pathways. J Thorac Oncol 14:606-616, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Nassar AH, Adib E, Kwiatkowski DJ: Distribution of KRAS (G12C) somatic mutations across race, sex, and cancer type. N Engl J Med 384:185-187, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Cui W, Franchini F, Alexander M, et al. : Real world outcomes in KRAS G12C mutation positive non-small cell lung cancer. Lung Cancer 146:310-317, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Wu MY, Zhang EW, Strickland MR, et al. : Clinical and imaging features of non-small cell lung cancer with G12C KRAS mutation. Cancers 13:3572, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebastian M, Eberhardt WEE, Hoffknecht P, et al. : KRAS G12C-mutated advanced non-small cell lung cancer: A real-world cohort from the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315). Lung Cancer 154:51-61, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Spira AI, Tu H, Aggarwal S, et al. : A retrospective observational study of the natural history of advanced non-small-cell lung cancer in patients with KRAS p.G12C mutated or wild-type disease. Lung Cancer 159:1-9, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Tomasini P, Serdjebi C, Khobta N, et al. : EGFR and KRAS mutations predict the incidence and outcome of brain metastases in non-small cell lung cancer. Int J Mol Sci 17:2132, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh NR, Likhacheva A, Pinnix C, et al. : Prognostic significance of EGFR and KRAS mutations in NSCLC patients with brain metastases treated with radiosurgery. J Radiosurg SBRT 3:171-178, 2015 [PMC free article] [PubMed] [Google Scholar]
- 11.Lauko A, Kotecha R, Barnett A, et al. : Impact of KRAS mutation status on the efficacy of immunotherapy in lung cancer brain metastases. Sci Rep 11:18174, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fell JB, Fischer JP, Baer BR, et al. : Identification of the clinical development candidate MRTX849, a covalent KRAS(G12C) inhibitor for the treatment of cancer. J Med Chem 63:6679-6693, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Sabari JK, Velcheti V, Shimizu K, et al. : Activity of adagrasib (MRTX849) in brain metastases: Preclinical models and clinical data from patients with KRASG12C-mutant non-small cell lung cancer. Clin Cancer Res 28:3318-3328, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jänne PA, Riely GJ, Gadgeel SM, et al. : Adagrasib in non–small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med 387:120-131, 2022 [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Lin NU, Lee EQ, Aoyama H, et al. : Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol 16:e270-e278, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Singer L, Kumthekar P: Updates on molecular targeted therapies for intraparenchymal CNS metastases. Cancers 14:17, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Lorenzo R, Ahluwalia MS: Targeted therapy of brain metastases: Latest evidence and clinical implications. Ther Adv Med Oncol 9:781-796, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loganadane G, Dhermain F, Louvel G, et al. : Brain radiation necrosis: Current management with a focus on non-small cell lung cancer patients. Front Oncol 8:336, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makale MT, McDonald CR, Hattangadi-Gluth JA, et al. : Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol 13:52-64, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ou SI, Janne PA, Leal TA, et al. : First-in-human phase I/IB dose-finding study of adagrasib (MRTX849) in patients with advanced KRAS(G12C) solid tumors (KRYSTAL-1). J Clin Oncol 40:2530-2538, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koster K-L, Appenzeller C, Lauber A, et al. : Sotorasib shows intracranial activity in patients with KRAS G12C-mutated adenocarcinoma of the lung and untreated active brain metastases. Case Rep Oncol 15:720-725, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh J, Marks JA, Alzeer AH, et al. : Remarkable intracranial response to sotorasib in a patient with KRASG12C-mutated lung adenocarcinoma and untreated brain metastates: A case report. JTO Clin Res Rep 3:100428, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunimasa K, Tamiya M, Inoue T, et al. : Rapid response to sotorasib of a patient with KRAS G12C-mutated lung cancer with cancer-associated disseminated intravascular coagulation: A case report. JTO Clin Res Rep 4:100442, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramalingam S, Skoulidis F, Govindan R, et al. : P52.03 efficacy of sotorasib in KRAS p.G12C-mutated NSCLC with stable brain metastases: A post-hoc analysis of CodeBreaK 100. J Thorac Oncol 16:S1123, 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
At Mirati Therapeutics, we are committed to patient care, advancing scientific understanding, and enabling the scientific community to learn from and build on the research we have undertaken. To that end, we will honor legitimate requests for our clinical trial data from qualified researchers and investigators for conducting methodologically sound research. We will share study-level clinical trial data, clinical study reports, study protocols, and statistical analysis plans from clinical trials for which results have been posted on ClinicalTrials.gov for products and indications approved by regulators in the United States and/or European Union. Sharing is subject to protection of patient privacy and respect for the patient's informed consent. In general, data will be made available for specific requests approximately 24 months after clinical trial completion from our in-scope interventional trials. For additional information on proposals with regard to data sharing collaborations with Mirati, please email us at medinfo@mirati.com.