Abstract
Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) is an immune-mediated inflammatory demyelinating disease of the central nervous system. This study aimed to delineate the clinical manifestations, imaging features, and long-term outcomes in Chinese patients with MOGAD and analyze the recurrence-associated factors. The phenotypic and neuroimaging characteristics of 15 Han Chinese patients with MOGAD were retrospectively analyzed. Demyelinating attacks, MOG antibodies in the cerebrospinal fluid/serum, response to immunotherapy, follow-up outcomes, and recurrence-associated factors were recorded. The median age at disease onset was 34 years (range, 4–65 years). The most common initial presentations included vision loss (10/15, 66.7%) and seizures (5/15, 33.3%). Serum MOG-Ab titers in 14/15 cases were higher than those in the cerebrospinal fluid and were detected in 3/6 relapsed patients. Brain magnetic resonance imaging during acute attacks showed lesions in 10/15 patients (66.7%), mostly in the cortex/subcortical white matter (5/15, 33.3%). Recurrence occurred in 6/15 patients (40.0%); in 4 patients, recurrence occurred shortly after immunotherapy discontinuation. Residual neurological deficits were present in 5/15 patients (33.3%), including visual impairment, incapacitation, cognitive impairment, and speech reduction. Optic neuritis was the most common clinical manifestation of MOGAD. magnetic resonance imaging findings were heterogeneous and the cerebral cortex/subcortical white matter was the most susceptible brain region. Although patients in the acute phase responded well to methylprednisolone pulse therapy, the long-term recurrence rate was high. Consistently detected serum MOG antibodies and inappropriate maintenance immunotherapy may be associated with recurrence, and residual neurological deficits should not be ignored.
Keywords: long-term outcome, magnetic resonance imaging, myelin oligodendrocyte glycoprotein antibody-associated disease, optic neuritis, recurrence
1. Introduction
Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) is an immune-mediated inflammatory demyelinating disease of the central nervous system (CNS).[1] MOGAD is an immune-pathogenetically distinct entity from multiple sclerosis and aquaporin-4 (AQP4)-immunoglobulin (Ig)G-positive neuromyelitis optica spectrum disorder.[2] The clinical and neuroimaging manifestations of MOGAD are heterogeneous, ranging from isolated optic neuritis (ON) or myelitis to multifocal CNS demyelination, often in the form of acute disseminated encephalomyelitis or cortical encephalitis. Genetic and environmental factors may be important determinants of clinical phenotypes and disease progression.[3] Although no age group is exempt, the median age of onset is within the fourth decade of life.[4] Approximately 50% of patients have a relapsing course,[5] and residual disability develops in 50% to 80% of patients.[4]
The clinical features, long-term outcomes, and associated influencing factors of MOGAD in China are unclear. Most patients in these studies were children[4,6,7] or cases of MOGAD with ON.[8,9] Thus, this study aimed to delineate the clinical manifestations, imaging features, and long-term outcomes in Chinese patients (especially for adults) with MOGAD and analyze the factors associated with disease recurrence.
2. Methods
The study was approved by the Medical Ethics Committee of Liuzhou People Hospital (No. KY2022-006-1). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Informed consent was obtained from the patients or legal guardians.
2.1. Aim and study design
Considering the high recurrence and disability rates of MOGAD, we analyzed the disease characteristics in 15 patients in southern China, with the aim of improving its management efficacy. This study retrospectively analyzed the data of 15 patients newly diagnosed with MOGAD at the (blinded for review) between January 2016 and January 2020.
2.2. MOGAD criteria
We analyzed the demographic and clinic characteristics of each patient including sex, age, clinical manifestations, serum and cerebrospinal fluid (CSF) antibodies, electroencephalogram, neuroimaging, treatment, and long-term outcomes.
MOGAD was diagnosed according to the following diagnostic criteria[1]: serum MOG-IgG-positive, detected by cell-based assay with full-length human MOG as the target antigen; clinical manifestations, including one or more of the following: ON, including chronic relapsing inflammatory ON; transverse myelitis; encephalitis or meningoencephalitis; or brain stem encephalitis; magnetic resonance imaging (MRI) or electrophysiology findings (visually evoked potential of isolated ON) associated with CNS demyelination; and other diagnoses that could explain the illness were ruled out.
Criteria for MOGAD recurrence were defined as follows: worsening of the preexisting clinical symptoms or new-onset of neurological symptoms or signs that appear after the acute phase; neuroimaging may show new-onset responsible lesions; ineffective immunotherapy; and the condition is difficult to explain by other etiologies.
2.3. MOG-antibodies detection method
MOG antibodies (MOG-Abs) and AQP4 antibodies were detected by a cell-based assay (Guangzhou Jinyu Medical Laboratory, Guangzhou, China). We previously confirmed that the detection method for MOG-Abs is reliable.[10,11] The cutoff value for being MOG positive in the commercial assay was a titer of 1:10.
2.4. Immunotherapy
Immunotherapy included the following modalities:
Methylprednisolone pulse therapy (MPPT): Adult patients were administered intravenous methylprednisolone (MP) sodium succinate (Pfizer Manufacturing Belgium NV, Puurs-Sint-Amands, Belgium) 1 g/day for 5 days, followed by 0.5 g/day for 3 days and 0.25 g/day for 3 days thereafter, before switching to oral steroid hormones (MP; Tianyao Pharmaceutical Co., LTD., Tianjin, China; 0.8 mg/kg/day) or prednisone acetate (PED; Xianju Pharmaceutical Co., Ltd., Zhejiang, China; 1 mg/kg/day). This dose was gradually reduced by 1 tablet per week (MP, 4 mg per tablet; PED, 5 mg per tablet) until the patient received 2 tablets per day. This dose was maintained for 4 months. Pediatric patients received intravenous MP (20~30 mg//kg/day) for 3 to 5 days and were then tapered to oral prednisone acetate (1 mg/kg, similar to the adult patients who were tapered off within 3–6 months).
Intravenous gamma globulin (Taibang Biological Products Co., Ltd., Guizhou, China; 0.4 g/kg/day) for 5 days.
Other immunotherapies included oral azathioprine (Shanghai Xinyi Pharmaceutical Co., Ltd., Shanghai, China), mycophenolate mofetil (MMF; Zhongmei Huadong Pharmaceutical Co., Ltd., Hangzhou, Zhejiang, China), and methotrexate (Shanghai Xinyi Pharmaceutical Co., Ltd).
3. Results
The median age at disease onset was 34 years (range, 4–65 years). Eight patients were male and 7 were female (Table 1).
Table 1.
Demographics, clinical features, main therapy, and long-term follow-up outcomes of Cases with MOG-antibody-associated disorders.
| Age/Sex | Prodromal symptoms | Neurological symptoms and signs | MOG-Ab in CSF/ Serum |
CSF*,† analysis | EEG | MRI | Acute therapy | Maintenance therapy‡,§ | Relapses | Long-term follow-up outcomes | VEP or OCT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 52 Y/F | Headaches | Limb weakness, unsteady gait, positive Romberg sign, dysarthria, dysphagia | 1:10/1:10; Serum MOG-Ab 1:32 in the relapse |
CSF protein 0.61 g/L | Mild abnormality | Hyperintense signal on T2WI/FLAIR/ADC and hypointense signal on T1WI in the brainstem, left cerebellum, right temporal lobe, corpus callosum; the hyperintense MRI signal weakened 15 d later. optic nerve MRI: (−) | MPPT and high-dose IVIG | Low-dose MP and AZA for 30 Ms | 4 Ms after discharge | Symptoms were relieved at 30 Ms | None |
| Case 2 | 48 Y/M | Fever | Acute cognitive impairment, slow response, slight neck rigidity, increased muscle tone in the extremities | Negative/1:10 | No abnormality | Roughly normal EEG | Initial MRI showed hyperintense signal on T2WI/FLAIR and hypointense signal on T1WI in the brainstem, hypothalamus, and hippocampus. One mo later, the hyperintense MRI FLAIR signal in the brainstem disappeared | MPPT and high-dose IVIG | MP 48 mg/d, tapered off to 16 mg/d, maintained for 1 Y | None | Cognitive impairment, slow response, and needs assistance with activities of daily living at 25 Ms | None |
| Case 3 | 27 Y/F | None | Repeated impaired vision and hypomnesis, convulsions, increased muscle tone in extremities; positive bilateral ataxia signs | Negative/1:10 | No abnormality | Mild abnormality | Hyperintense signal on T2WI/FLAIR and hypointense signal on T1WI at the bilateral cerebellar hemispheres, frontotemporal, and left parietal lobes, without diffusion restriction | MPPT | MP 40 mg/d, tapered off, low dose for 6 Ms | Cognitive decline after MP therapy discontinuation, however improved after rehospitalization | Incapacitation and poor verbal ability at 26 Ms | None |
| Case 4 | 29 Y/M | Headache and fever | Impaired vision in right eye, convulsions |
Negative/1:32; Serum MOG-Ab 1:32 in the 2nd relapse | No abnormality | Roughly normal EEG | MRI showed hyperintense signal on FLAIR in a sulcus in the right frontotemporal lobe, and right optic nerve thickening with uniform enhancement | MPPT | Low-dose PED for 6 Ms, initial methotrexate 15 mg/W, followed by 12.5 mg/d after the 2nd relapse | One mo after PED discontinuation, and again after 4 Ms | Improved vision at 27 Ms | None |
| Case 5 | 29 Y/M | Low-grade fever and headache | Impaired vision, convulsions, slight neck rigidity, disappearance of pupillary light reflex in the right eye |
Negative/1:32 | CSF WBC, 264 × 106/L; protein 0.954 g/L | Mild abnormality | Initial MRI showed hyperintense signal on FLAIR in the corpus callosum; 3 Ms later, the hyperintense MRI signal nearly disappeared | MPPT | Low-dose PED for 6 Ms, MMF for 8 Ms | None | Normal vision and daily activity at 32 Ms | None |
| Case 6 | 43 Y/M | Coughs, nasal obstruction | Decreased vision in the right eye, weakness and numbness of the lower limbs | 1:32/1:100 | CSF pressure 180 mmH2O, protein 804.6 mg/L | Mild abnormality | Initial brain, optic nerve, and cervicothoracic spine MRI showed hyperintense signal on T2WI and equisignal on T1WI at C3–4. Thoracic spine MRI: (−); 6 Ms later, the hyperintense MRI signal at C3–4 disappeared | MPPT | PED 60 mg/d, tapered off, then low dose for 18 Ms; AZA 100 mg/d after the 1st relapse, and maintained for 25 Ms | 3 Ms after the 1st episode, and again after another 3 Ms | Poor right-eye vision at 36 Ms | VEP at the 1st episode showed right optic nerve damage |
| Case 7 | 65 Y/F | None | Impaired vision in both eyes, droopy right upper eyelid, sluggish, diminished pupillary light reflex in the right eye with impaired adduction | Negative/1:32 | CSF pressure 200 mmH2O, protein 1009.6 mg/L | Mild abnormality | MRI 1 mo after onset showed a small syringomyelia and encephalanalosis; cervical spine MRI: (−) | MPPT | Initial PED 55 mg/d, tapered off, then low dose for 18 Ms with AZA for 44 Ms | None | Impaired right-eye vision at 44 Ms | 16 Ms later, VEP showed amplitude reduction in right eye. OCT showed thinning of PRNFL in left eye |
| Case 8 | 35 Y/F | None | Repeated impaired vision in the right eye, convulsions | Negative/1:32 | No abnormality | No abnormality | Brain and optic nerve MRI showed slightly thickened right optic nerve | MPPT and MMF | Low-dose MP for 10 Ms, MMF for 33 Ms | 2 Ms after MP discontinuation | Slightly decreased vision at 33 Ms | None |
| Case 9 | 4 Y/F | None | Decreased vision in both eyes with right gaze palsy | Negative/1:100; serum MOG-Ab: 1:100 in the 1st relapse | CSF protein 212.1 mg/L | No abnormality | Initial MRI showed multiple intracranial foci. Cervicothoracic spine MRI: (−). Two yr later MRI showed hyperintense signal on T2WI and hypointense signal on T1WI at the bilateral frontotemporal lobes; 8 Ms later, MRI showed that the foci nearly disappeared | MPPT | MP tapered off, followed by low-dose MP for 1 yr; long-term maintenance with AZA 50 mg/d | 7 Ms after initial hospitalization, and 2nd 3 Ms after immunotherapy discontinuation | Improved vision 64 Ms after the 1st onset | None |
| Case 10 | 16 Y/F | Low-grade fever, general fatigue | Raving, drowsiness, disappearance of pharyngeal reflex | Negative/1:10 | CSF protein 352.3 mg/L | Mild-to-moderate abnormality | Initial MRI showed abnormal signals in the medulla oblongata; 6 Ms later, MRI showed abnormal signals in the area postrema, left basal ganglia, and around the third ventricle; 30 Ms later, MRI showed softening foci in the basal ganglia and area postrema | MPPT and AZA | Initial PED 60 mg/d, tapered off, low dose for 24 Ms and AZA for 48 Ms | None | Resumed good general condition at 48 Ms | None |
| Case 11 | 41 Y/F | None | Dizziness, walk unsteadily, positive Romberg sign, left sided positive finger- nose test |
Negative/1:10 | No abnormality | No abnormality | Brain and cervicothoracic spine MRI: (−). Optic nerve MRI showed bilateral optic nerve swelling. | MPPT | Without immunotherapy | None | Freedom of movement at 40 Ms | None |
| Case 12 | 27 Y/M | Cold symptoms | Limb numbness, decreased muscle strength in lower extremities, increased muscle tone in the extremities, bilateral positive Babinski sign | 1:10/1:32 | CSF WBC 82 × 106/L | Not done | MRI showed hyperintensity on T2WI and isointense signal on T1WI at C3–6. Brain and thoracic spine MRI: (−) | MPPT | Initial PED 60 mg/d, tapered off, low dose for 6 Ms; MMF 0.5 g/d for 18 Ms | None | Return to normal life and work at 29 Ms | None |
| Case13 | 22 Y/M | None | Left eye swelling with impaired vision, limited outreach in both eyes |
Negative/1:10 | No abnormality | Mild-to-moderate abnormality | Brain, optic nerve, and cervicothoracic spine MRI: (−) | MPPT | Initial PED 60 mg/d, tapered off, low dose for 1 yr | None | Near complete vision recovery at 37 Ms | None |
| Case 14 | 6 Y/M | None | Decreased vision in both eyes | Negative/1:10 | No abnormality | Not done | Brain MRI: (−) | MPPT | Initial PED 60 mg/d, tapered off, low dose for 6 Ms | None | Return to normal vision at 36 Ms | None |
| Case 15 | 32 Y/M | None | Decreased vision in both eyes, visual field defect in the right eye | Negative/1:32 | No abnormality | Not done | Brain, optic nerve, and cervicothoracic spine MRI: (−) | MPPT | Initial PED 60 mg/d, tapered off, low dose for 9 Ms, MMF 1 g/d for 11 Ms | None | Good vision recovery at 24 Ms without affecting normal work | VEP: P100 latency with moderate amplitude reduction in right eye, and severe amplitude reduction in left eye. 1 yr later, OCT: thinning of PRNFL in both eyes |
(−) = no abnormality, ADC = apparent diffusion coefficient, AZA = Azathioprine, C = cervical vertebra, CSF = cerebrospinal fluid, EEG = Electroencephalogram, F = female, FLAIR = fluid-attenuated inversion-recovery, IVIG = intravenous immunoglobulin, M = male, MMF = mycophenolate mofetil, MOG = myelin oligodendrocyte glycoprotein, MOG-Ab = myelin oligodendrocyte glycoprotein antibody, MP = methylprednisolone, MPPT = methylprednisolone pulse treatment, MRI = magnetic resonance imaging, Ms = months, OCT = optical coherence tomography, PED = prednisone, PRNFL = peripapillary retinal nerve fiber layer, VEP = Visual evoked potential, W = week, WBC = white blood cell, WI = weighted imaging, Y = year, Yrs = years.
CSF protein reference range: 150–450 mg/L.
CSF WBC reference range: 0–8 × 106/L.
Tapered off, decrease of prednisone by 5 mg/wk or methylprednisolone by 4 mg/wk.
Low dose, 5–10 mg/d prednisone or 4 mg/d methylprednisolone.
Seven patients had a history of prodromal infection. The most common clinical symptoms were impaired vision (10/15, 66.7%), epilepsy (5/15, 33.3%), and limb weakness or numbness (4/15, 26.7%). Mental abnormality, walking instability, and cognitive decline were observed in 2, 2, and 1 patient, respectively. Four of the 10 patients with impaired vision (40%) also experienced oculomotor nerve paralysis, and 5 (50%) had concurrent impaired vision in both eyes. One patient felt eye distension and pain, and another had visual field loss. MOGAD patients with disease relapse were more prone to recurrence of visual impairment (5/6, 83.3%).
3.1. Laboratory findings
MOG-Abs were detected in the serum and CSF of all patients. The CSF pressure was elevated (reference range: <180 mmH2O) in 2/15 cases. CSF analysis showed an increased white blood cell count in 2/15 patients and increased protein concentrations in 4/15 cases. Normal CSF sugar and chloride contents were noted. Three of the 15 cases were positive for CSF MOG-Ab. The serum MOG-Ab titers in 14 cases were higher than in the CSF. AQP4 antibody was detected in 2 cases (MOG-Ab was identified as the responsible antibody). Serum MOG-Abs remained detectable in 3/6 relapsed patients, and the titer was almost the same as that at the initial presentation.
3.2. Electroencephalogram finding
Electroencephalogram was performed in 12/15 patients, showing no abnormalities in 5, mild abnormalities in 6, and mild-to-moderate abnormalities in 1 patient. Four patients had seizures, with 2 showing spikes/sharp waves in their electroencephalograms, while the other 2 had normal electroencephalograms.
3.3. Neuroimaging findings
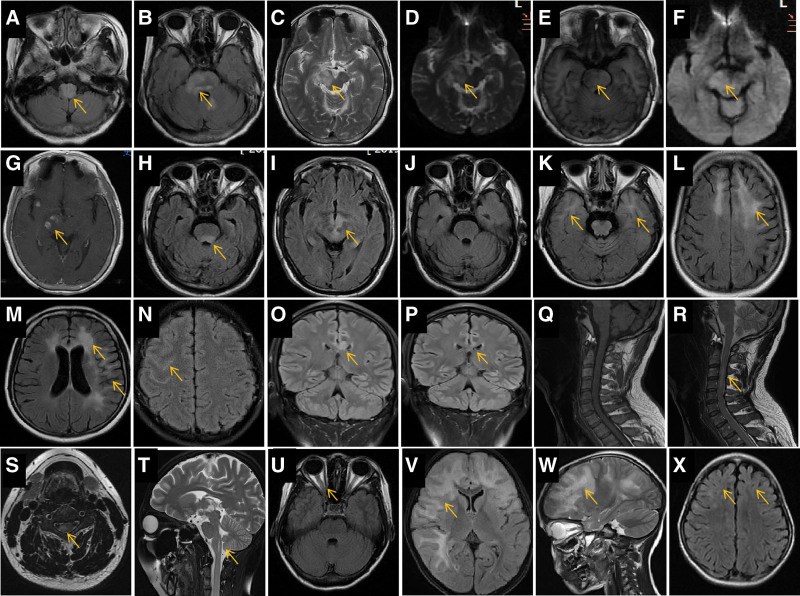
Brain, cervical-thoracic vertebra, and orbital MRIs were performed for 15, 10, and 7 patients, respectively. Acute lesions were widely distributed, including in the medulla oblongata (Fig. 1A), dorsal or lateral pons (Fig. 1B, E, H), cerebral peduncle and midbrain (Fig. 1C, D, F, G), areas surrounding the third ventricle (Fig. 1I), bilateral frontal lobe (Fig. 1K and L), bilateral temporal lobe (Fig. 1M), basal ganglia (Fig. 1K), corpus callosum (Fig. 1O), cervical spinal cord (Fig. 1P–T), and optic nerve (Fig. 1U). The most common lesion locations in the brain were the cortical and subcortical white matter (5/15, 33.3%), limbic lobe (4/15, 26.7%), brainstem (4/15, 26.7%), optic nerve (3/15, 20%), cervical medulla (2/10, 20%), and corpus callosum (1/15, 6.7%).
Figure 1.
Characteristics and lesion evolution in brain magnetic resonance imaging (MRI) of representative cases. •Patient 1. Initial brain MRI showing hyperintense regions in the bilateral medulla oblongata (A, arrow), pons (B, arrow), right pedunculus cerebri, and right midbrain (C, arrow) on fluid-attenuated inversion recovery (FLAIR, A and B, arrows), T2-weighted imaging (WI, C, arrow), and apparent diffusion coefficient (D, arrow), hypointense on T1WI (E, arrow), and without diffusion restriction on diffusion-weighted imaging (DWI, F). Several spot-like enhanced lesions are noted in the right pedunculus cerebri (G, arrow). •Patient 2. Initial MRI showing hyperintense regions in the bilateral dorsal pons (H, arrow), midbrain, and areas around the third ventricle (I, arrow) on FLAIR. The hyperintense signal in the brainstem FLAIR disappeared 1 mo later (J). •Patient 3. FLAIR MRI showing a hyperintense signal in the bilateral temporal poles (K, arrow), frontal white matter regions (L, arrow), anterior horns of the lateral ventricle (M, arrow), and cortical and subcortical regions in the left temporal lobe (M, arrow). •Patient 4. FLAIR MRI showing a hyperintense signal in the right frontotemporal lobe sulcus (N, arrow). •Patient 5. Initial FLAIR MRI showing a hyperintense signal in the corpus callosum body (O, arrow); the hyperintense signal nearly disappeared on MRI 3 mo later (P, arrow). •Patient 6. Initial sagittal T1WI MRI of the cervical spinal cord showing isointense signal (Q, arrow) and short, segmental hyperintense signal in the sagittal (R, arrow) and axial (S, arrow) T2WI. •Patient 7. MRI 1 mo after onset showing a small syringomyelia (T, arrow). •Patient 8. Brain MRI showing slight thickening of the right optic nerve (U, arrow). •Patient 9. MRI showing a large hyperintense area on T2WI in the bilateral frontotemporal lobes, mimicking a tumor (V and W, arrow); MRI performed 8 mo later showed that the abnormal signals nearly disappeared, with slight brain atrophy (X, arrows).
The MOGAD lesions appeared hyperintense on T2-weighted imaging (WI; Fig. 1C), fluid-attenuated inversion recovery (Fig. 1A and B), and apparent diffusion coefficient (Fig. 1D), isointense or slightly hyperintense on diffusion-WI (Fig. 1F), and hypointense on T1WI (Fig. 1E). Some lesions showed partial nodular enhancement (Fig. 1G) or no enhancement at all.
The acute lesions had many forms, including single or multiple (Fig. 1K and L), symmetric or asymmetric (Fig. 1N and O), patchy, or irregularly shaped. Large lesions appeared similar to tumors (Fig. 1V and W), with the invasion of several lobes, while small lesions were occasionally confined to the sulci in the frontal lobe (Fig. 1N). A diseased optic nerve had the potential to become thick (Fig. 1U).
In the spinal cord, short-segmental lesions were relatively common (Fig. 1R) and axial MRI showed patchy unilateral lesions (Fig. 1S). The abnormal signal within the lesions was evidently reduced or had even completely disappeared after immunotherapy (Fig. 1J), and slight brain atrophy was observed at the original lesion sites on follow-up MRI (Fig. 1X).
3.4. Treatment and outcomes
MPPT was provided as acute therapy to 14 of 15 patients during hospitalization. Of these, 2 also received high-dose intravenous immunoglobulin (IVIG), 1 received oral azathioprine, and 1 received oral MMF. Active immunotherapy alleviated their symptoms.
Maintenance therapy after discharge comprised oral PED in 9 patients, among whom 3 received oral PED alone, 3 received azathioprine, 2 received MMF, and 1 received methotrexate. Five patients received oral MP after discharge, of which 2 received oral MP alone, 1 received azathioprine, and 1 received MMF. One patient received purely symptomatic treatment.
3.5. Long-term follow-up findings
The median time from the initial onset to the last follow-up assessment was 34.6 months (range, 24–64 months). Nine symptom recurrences in 6 patients were noted (40%). Of these, 4 recurrences occurred shortly after the steroid hormone was discontinued. Residual neurological deficits were evident in 5 of the 15 patients (33.3%), and manifested as visual impairment in 4, incapacitation in 2, cognitive impairment in one, and speech reduction in 1 patient.
4. Discussion
Our findings revealed that ON is the most common clinical manifestation of the first episode and in recurring MOGAD in Chinese patients. MOGAD presented highly heterogeneous MRI findings. The cerebral cortex and subcortical white matter were the most susceptible brain regions, followed by the limbic lobe and optic nerve. Patients in the acute phase responded well to MPPT; however, the long-term recurrence rate was high, and one in every 3 patients had residual neurological deficits.
4.1. Initial clinical manifestations of MOGAD
The current results indicate that ON was the most common manifestation of MOGAD, followed by epilepsy and limb weakness/numbness. Cognitive decline, psychiatric disorders, slurred speech, and unsteady gait may also occur. The disease course can be monophasic or relapsing, with subsequent relapses most commonly involving the optic nerve.[1] Functional blindness in one or both eyes was noted during at least 1 ON episode in approximately 70% of the patients.[12] Half of our patients had impaired vision with simultaneous presentation in both eyes. MOGAD typically presents with isolated ON (55%, bilateral in almost half of these cases), transverse myelitis (18%), or acute disseminated encephalomyelitis-like (18%).[2] Both children in our study had visual impairment as the initial symptom. Visual loss is reportedly common in children with MOGAD.[7] Isolated ON was the most frequent clinical presentation in both children and adults, and acute disseminated encephalomyelitis syndrome was more frequent in children than adults.[13] One study[6] reported that, at the onset, ADEM was the most common clinical phenotype in children, followed by isolated ON or NMOSD. Notably, the clinical presentation of MOGAD changes with age.[14] Impaired vision in our patients was often accompanied by oculomotor nerve paralysis; 1 patient felt eye distension and pain and another experienced visual field loss. Patients with ON often complain of ocular, ocular rotation, or orbital pain or acute loss of vision in one or both eyes, visual field defects, changes in color vision, or decreased contrast sensitivity during the acute phase.[5] Optical coherence tomography in our patients showed obvious thinning of the peripapillary retinal nerve fiber layer after the acute episode, which may be the cause of the impaired vision. Patients with MOG-associated ON show varying degrees of atrophy of the peripapillary retinal nerve fiber layer and macular ganglion cell-inner plexiform layer 6 months after disease onset,[8] which could be the reason for the observed residual visual deficit. Seizures and encephalopathy occur frequently in patients with MOGAD and are often associated with cortical and subcortical brain lesions.[15]
4.2. Relapsing clinical manifestations of MOGAD
Our results showed that patients with MOGAD had a high recurrence rate (40%) and that 4 of the 9 (44.4%) relapses occurred shortly after discontinuing steroid hormone treatment. A French nationwide adult cohort study[16] showed that the probability of encountering the first relapse after 2 and 5 years was 44.8% and 61.8%, respectively, indicating that longer observation time is associated with higher recurrence rates. Treatment failure leading to the rapid development of disability and flare-ups after steroid withdrawal have been noted in many patients.[12] Our results showed that relapsed patients were prone to experience visual impairment recurrence. A high degree of vigilance is required for patients with MOGAD presenting recurrent ON. Cognitive decline, limb weakness, and autonomic nervous disorders can present in relapses of MOGAD, according to the results of this study.
4.3. Analysis of CSF and MOG-Abs
CSF analysis showed low specificity. The white blood cell and protein content in the CSF increased in a few cases. The MOG-Ab positive rate and titer in the CSF were lower than those in the serum, possibly because the MOG-Abs were derived from peripheral immune cells.[17] Thus, blood serum is the preferred sample type, while CSF antibody testing can provide supplementary information.[18] One study[12] revealed that serum MOG-Ab titers were higher during attacks than during remission and declined at follow-up treatment, indicating that serum titers depended on disease activity and treatment. Our study showed that the serum MOG-Abs remained detectable in most relapsed patients, and the titer was almost the same as that at the initial presentation. The recurrence of MOGAD episodes in many children was associated with persistently high MOG-ab titers.[19] Evidently, rechecking serum MOG-Abs is very important in the assessment of disease recurrence.
4.4. Neuroimaging features
MRI findings of MOGAD displayed a high degree of heterogeneity, and lesions often improved after immunotherapy. Acute lesions were widely distributed. These findings enrich our understanding of the MOGAD neuroimaging spectrum in a Chinese population. We found that intracranial lesions in the cortex/subcortical white matter were the most common, followed by the limbic lobe, brainstem, optic nerve, and cervical spinal cord. One study found cortical lesions in 16.3% of patients.[16] Our findings suggest that the lesion location among Chinese patients with MOGAD is not entirely consistent with that of other reports, possibly owing to racial differences. Cortical gray/juxtacortical white matter lesions on brain MRI could help distinguish MOGAD from AQP4-positive neuromyelitis optica spectrum disorder.[20] Patients with MOGAD can have fluffy brainstem lesions, often located in the pons or adjacent to the fourth ventricle.[21] The corpus callosum, internal capsule, brainstem, and cerebellum can also be affected.[22] Short-segmental lesions were relatively common in the spinal cord. The above-mentioned MRI phenotypes were observed in our study; however, some previous studies described spinal cord H-sign as an indicator of MOGAD diagnosis,[5,23] which was not observed in our study.
The typical manifestations of optic nerve involvement in MOGADON are obvious thickening of the optic nerve with blurred edges and obvious and uniform enhancement.[5] Our results revealed a low positive rate in optic nerve MRI, however, this does not rule out the absence of optic nerve damage. VEP in our study could often detect optic nerve damage (VEP presented delayed latency in P100 wave or amplitude reduction). The degree of amplitude reduction is related to the severity of optic nerve involvement. Hence, the results of VEP have important implications for the location of nerve damage.
4.5. Treatment
Depending on the disease stage, treatment for MOGAD can be divided into acute and maintenance phase therapies. Intravenous MP is the first-line treatment during the acute phase,[1] Second-line therapies, including IVIG and plasmapheresis, are prescribed when the response to intravenous MP is insufficient.[24] Our results showed that MPPT was effective for all patients. Acute attacks in MOGAD appeared to be very responsive to high-dose steroids and plasma exchange,[25] and IVIG may be considered during the refractory cases.
The expert consensus[18] recommends that low-dose prednisone be maintained for 6 months after the initial episode. Most patients in this study received steroid hormones for at least half a year after discharge. However, follow-up showed that 40% had relapsed, often soon after immunotherapy cessation, possibly due to the rapid withdrawal. Some patients with MOGAD might become dependent on steroid hormones. Therefore, the tapering schedule should be slow and can be combined with immunosuppressants.[5] Azathioprine can potentially reduce the recurrence of MOGAD, especially when combined with low-dose steroid hormones.[15,26] The choice and duration of the drugs used to prevent relapse are inconclusive.[7] Relapses often occur during steroid weaning or soon after, suggesting that a longer initial treatment was required.[14] Our study suggested that MOG-Abs, consistently detected during follow-up, could be one of the reasons for relapse. Longitudinal serologic evaluations of MOG-Ab could help predict the disease course and indicate when immunotherapy should be considered.[27]
Determination of whether long-term immunotherapy is necessary for patients with a first MOGAD episode should be based on the lesion location, disease severity, and the MOG-Ab titer.[5] Azathioprine, MMF, rituximab, and particularly IVIG were associated with a reduced relapse frequency,[28] comprising the 4 first-line treatments for recurrence prevention.[24] After the first relapse, maintenance treatment should be initiated to prevent further recurrences and permanent sequelae.[24] In case of further relapse despite maintenance treatment, the consensus group recommends treatment escalation with rituximab[1] or IVIG, followed by their combination, and ultimately adding oral maintenance steroids.[24] Attack-prevention treatments lack class-I data, and empiric maintenance treatment is generally reserved for relapsing cases or patients with severe residual disabilities.[25]
4.6. Prognosis
Our findings revealed that 33.3% of the patients had visible residual neurological deficits, including visual impairment, activity limitations, cognitive impairment, and speech reduction. A previous study reported that 47% of the patients presented with permanent disabilities, such as impaired vision, restricted activity, and bladder, bowel, and erectile dysfunction.[2] Another study indicated that MOGAD resulted in a significant disability in 40% of the patients (mean follow-up, 75.0 ± 46.5 months), with severe visual impairment (36%) and markedly impaired ambulation as the most common long-term sequelae.[12] MOGAD-ON attacks often entail residual symptoms of optic nerve damage. Residual symptoms accumulate gradually after multiple relapses and impaired vision becomes increasingly severe. Severe vision loss is associated with more recurrences.[29] Recurrence of diseased eyes is an independent risk factor for poor visual prognosis.[9] Thus, it is essential to maximize visual recovery and minimize recurrences of OA.[30] Future research is required to determine the optimal long-term disease management strategy.[4]
4.7. Limitations
Only a few cases were included in this study, reflecting the clinical phenotypes and prognosis of patients (mainly adults) with MOGAD in southern China. However, our results yield valuable information for disease management. Large, prospective studies are required to validate and expand on our findings.
5. Conclusion
Our findings enrich the understanding of the clinical phenotypes and neuroimaging spectrum of MOGAD in a Chinese population (especially for adults). The long-term recurrence rate was high. Serum MOG-Abs were consistently detected, and the titers remained stable through recurrence, suggesting that MOGAD recurrence may be associated with inappropriate maintenance immunotherapy. Residual neurological deficits and other sequelae of MOGAD should not be ignored. Our findings provide a basis for larger, multicenter studies to better understand the disease and its management.
Acknowledgments
We would like to thank the “Double-First Class” Application Characteristic Discipline of Hunan Province (Pharmaceutical Science) for its support.
Author contributions
Formal analysis: Wei Zeng, Lu Yu, Jiarui Wu.
Investigation: Fang Wang, Xudong Liu, Shuqun Ren, Daxue Zhang, Baorong Lian.
Methodology: Fang Wang, Xudong Liu, Shuqun Ren, Daxue Zhang, Baorong Lian, Minghua Hu, Liming Cao.
Supervision: Minghua Hu, Liming Cao.
Writing – original draft: Wei Zeng, Lu Yu, Jiarui Wu.
Writing – review & editing: Minghua Hu, Liming Cao.
Abbreviations:
- CNS
- central nervous system
- CSF
- cerebrospinal fluid
- IVIG
- high-dose intravenous immunoglobulin
- MOG
- Myelin oligodendrocyte glycoprotein
- MOG-abs
- MOG antibodies
- MOGAD
- antibody-associated disease
- MP
- methylprednisolone
- MPPT
- methylprednisolone pulse therapy
- MRI
- magnetic resonance imaging
- ON
- optic neuritis
- WI
- weighted imaging
WZ, LY, JW, LC and MH contributed equally to this work.
This work was supported by the health commission of the Guangxi Zhuang autonomous region (No. Z-B20221318), Liuzhou People Hospital (No. lry202104), and the scientific research projects of the Health Commission of Hunan Province (No. 202203075030).
Written consent for publication was obtained.
Approval was obtained from the Medical Ethics Committee of Liuzhou People Hospital (No. KY2022-006-1). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Informed consent was obtained from legal guardians.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Zeng W, Yu L, Wu J, Wang F, Liu X, Ren S, Zhang D, Lian B, Hu M, Cao L. Clinical characteristics and long-term follow-up outcomes of myelin oligodendrocyte glycoprotein antibody-associated disease in Han Chinese participants. Medicine 2023;102:40(e35391).
Contributor Information
Wei Zeng, Email: zw1120060345@163.com.
Lu Yu, Email: yuyu9126@163.com.
Jiarui Wu, Email: a20130947@163.com.
Fang Wang, Email: wangfang001001@sina.com.
Xudong Liu, Email: 1550484375@qq.com.
Shuqun Ren, Email: 1692682924@qq.com.
Daxue Zhang, Email: 2661861224@qq.com.
Baorong Lian, Email: 157237126@qq.com.
Minghua Hu, Email: minghuahucsmu@163.com.
References
- [1].Wynford-Thomas R, Jacob A, Tomassini V. Neurological update: MOG antibody disease. J Neurol. 2019;266:1280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease:a UK study. Brain. 2017;140:3128–38. Erratum in:Brain.2018;141:e31. [DOI] [PubMed] [Google Scholar]
- [3].Liu J, Mori M, Zimmermann H, et al. Anti-MOG antibody- associated disorders: differences in clinical profiles and prognosis in Japan and Germany. J Neurol Neurosurg Psychiatry. 2020:jnnp-2020-324422. [DOI] [PubMed] [Google Scholar]
- [4].Zhou J, Lu X, Zhang Y, et al. Follow-up study on Chinese children with relapsing MOG-IgG-associated central nervous system demyelination. Mult Scler Relat Disord. 2018;28:44–10. [DOI] [PubMed] [Google Scholar]
- [5].Qiu W, Xu Y. Chinese expert consensus on diagnosis and treatment of anti-myelin oligodendrocyte glycoprotein-IgG associated disorders. Chin J Neuroimmunol & Neurol. 2020;27:86–95. [Google Scholar]
- [6].Li X, Wu W, Hou C, et al. Pediatric myelin oligodendrocyte glycoprotein antibody-associated disease in southern China: analysis of 93 cases. Front Immunol. 2023;14:1162647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xu M, Guo H, He Y, et al. Clinical and imaging features of 13 children with myelin oligodendrocyte glycoprotein antibody- associated encephalomyelitis. Chin J Appl Clin Pediatr. 2019;34:997–1001. [Chinese]. [Google Scholar]
- [8].Song H, Wei S. Clinical characteristics of myelin oligodendrocyte glycoprotein antibody- positive optic neuritis. Chin J Ophthalmol. 2019;55:174–9. [Chinese]. [DOI] [PubMed] [Google Scholar]
- [9].Liu X, Cui S, Peng J, et al. Analysis of the clinical feature and prognosis of the NMOSD-ON and MOGAD-ON with thyroid antibody. Chin J Neuroimmunol Neurol 2022;29:467–475. 494. [Chinese]. [Google Scholar]
- [10].Zeng W, Cao L, Zheng J, et al. Clinical characteristics and long-term follow-up of seven cases of anti-GABABR encephalitis in patients of Han Chinese descent. Neurol Sci. 2020;41:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zeng W, Cao L, Zheng J, et al. Clinical characteristics and long-term prognosis of relapsing anti-N-methyl-D-aspartate receptor encephalitis: a retrospective, multicenter, self-controlled study. Neurol Sci. 2021;42:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cobo-Calvo A, Ruiz A, Rollot F, et al. Clinical features and risk of relapse in children and adults with myelin oligodendrocyte glycoprotein antibody-associated disease. Ann Neurol. 2021;89:30–41. [DOI] [PubMed] [Google Scholar]
- [14].Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15:89–102. [DOI] [PubMed] [Google Scholar]
- [15].Zhong X, Zhou Y, Chang Y, et al. Seizure and myelin oligodendrocyte glycoprotein antibody-associated encephalomyelitis in a retrospective cohort of chinese patients. Front Neurol. 2019;10:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cobo-Calvo A, Ruiz A, Maillart E, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults. Neurology. 2018;90:e1858–69. [DOI] [PubMed] [Google Scholar]
- [17].Wang J, Sui RX, Miao Q, et al. S4B-5 effect of fasudil on remyelination following the cuprizone-induced demyelination in male C57BL/6 Mice. Acta Neuropharmacologica 2018;8:74–6. [Google Scholar]
- [18].Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. Nervenarzt. 2018; 89:1388–1399. [German]. [DOI] [PubMed] [Google Scholar]
- [19].Hennes EM, Baumann M, Lechner C, et al. MOG spectrum disorders and role of MOG-antibodies in clinical practice. Neuropediatrics. 2018;49:3–11. [DOI] [PubMed] [Google Scholar]
- [20].Salama S, Khan M, Shanechi A, et al. MRI differences between MOG antibody disease and AQP4 NMOSD. Mult Scler. 2020;26:1854–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jurynczyk M, Geraldes R, Probert F, et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain 2017;140: 617–627. [DOI] [PubMed] [Google Scholar]
- [22].Chen C, Liu C, Fang L, et al. Different magnetic resonance imaging features between MOG antibody-and AQP4 antibody- mediated disease: a Chinese cohort study. J Neurol Sci. 2019;405:116430. [DOI] [PubMed] [Google Scholar]
- [23].Zhangbao BJZ, Zhou L, Wang L, et al. Clinical characteristics of myelin oligodendrocyte glycoprotein antibody associated myelitis. Zhonghua Yi Xue Za Zhi. 2020;100:334–8. [Chinese]. [DOI] [PubMed] [Google Scholar]
- [24].Bruijstens AL, Wendel EM, Lechner C, et al. E.U. paediatric MOG consortium consensus: part 5-Treatment of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatr Neurol. 2020;29:41–53. [DOI] [PubMed] [Google Scholar]
- [25].Sechi E, Cacciaguerra L, Chen JJ, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): a review of clinical and MRI features, diagnosis, and management. Front Neurol. 2022;13:885218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhou Y, Huang Q, Lu T, et al. Azathioprine therapy in a case of pediatric multiple sclerosis that was seropositive for MOG-IgG. J Clin Neurosci. 2017;38:71–3. [DOI] [PubMed] [Google Scholar]
- [27].López-Chiriboga AS, Majed M, Fryer J, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-associated disorders. JAMA Neurol. 2018;75:1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hacohen Y, Wong YY, Lechner C, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody- associated disease. JAMA Neurol. 2018;75:478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang M, Wu Y, Song H, et al. Vision prognosis and associated factors of optic neuritis in dependence of glial autoimmune antibodies. Am J Ophthalmol. 2022;239:11–25. [DOI] [PubMed] [Google Scholar]
- [30].Saitakis G, Chwalisz BK. Treatment and relapse prevention of typical and atypical optic neuritis. Int J Mol Sci. 2022;23:9769. [DOI] [PMC free article] [PubMed] [Google Scholar]