Abstract
Introduction:
Scrub typhus (ST) is a neglected tropical disease of serious concern in Nepal. This systematic review aims to describe the burden of disease, clinical presentation, and complications of ST infection in Nepal.
Methods:
A systematic search of PubMed, EMBASE, Google Scholar, and national databases was conducted for any literature published in English between January 2000 and January 2023. Any type of study design (observational studies, case series, and interventional studies) that reported laboratory-confirmed ST and was conducted in Nepal among patients of all age groups was included. The seroprevalence of ST among acute undifferentiated febrile illness (AUFI) cases, geographical distribution, monthly distribution, clinical presentations, complications, and treatment were assessed by the study.
Result:
A total of 15 studies with 10, 977 participants were included in the review. The seroprevalence of ST among the AUFI cases in Nepal was 19.31%. Young people at or below 20 years of age were mostly affected. The maximum number of cases were reported from Bagmati province (59.46%) and in the month of August (26.33%). Fever, headache, cough, shortness of breath, nausea, and abdominal pain were the clinical characteristics in decreasing order of occurrence. The most common complication was acute kidney injury, followed by respiratory problems, cardiac issues, and neurological manifestations. The case fatality rate of ST in Nepal was 2.56%.
Conclusion:
The authors findings showed a significant burden of ST among AUFI cases in Nepal. Improved surveillance, general public awareness, and early detection post-calamities could help reduce the disease burden and improve patient outcomes.
Keywords: complications, epidemiology, Nepal, Orientia tsutsugamushi, outcomes, scrub typhus
Introduction
Highlights
The seroprevalence of scrub typhus (ST) among the acute undifferentiated febrile illness cases in Nepal was 19.31%.
The young population of 20 years or less in Nepal are mostly affected by ST infection.
The highest number of ST cases was observed in Bagmati Province in August.
Acute kidney injury is the most commonly reported complication of ST.
Scrub typhus (ST) is a neglected tropical disease caused by the organism Orientia tsutsugamushi (O. tsutsugamushi). ST is transmitted to humans through the bite of an infected larva of a Leptotrombidium mite known as a chigger. The causative organism is maintained by transovarial transmission in trombiculid mites1,2. The disease is characterized by a wide range of clinical manifestations, including fever, headache, myalgia, lymphadenopathy, and rash3. The presence of an eschar is an important finding for the diagnosis of ST, but it is variably present4,5. The disease can also lead to severe complications, such as myocarditis, meningitis, acute respiratory distress syndrome (ARDS), and multi-organ failure6,7. Without treatment, ST infection can have a mortality rate of up to 70.0%3.
Although the global prevalence is not exactly known, it is considered a significant public health concern in the Tsutsugamushi triangle in the Asia-Pacific Region8. The Tsutsugamushi triangle, also known as the ‘ST triangle,’ refers to an area in Asia that is endemic for the infection. The three points of the triangle are Japan in the north, Australia in the south, and Pakistan in the west (Fig. 1)9. Studies have shown that ST is highly endemic in many areas of the Tsutsugamushi triangle, with prevalence rates ranging from 1.1 to 73% depending on the region and population studied8. Even though this region has the highest burden of disease, ST is also reported in Africa, France, the Middle East, and South America.
Figure 1.

Map of the world showing the Tsutsugamushi triangle.
Nepal is a country located in Southeast Asia and falls inside the Tsutsugamushi triangle. However, ST cases were not reported to the Epidemiology and Disease Control Division (EDCD) of the Ministry of Health and Population before 2014 in the country10. After the mega-earthquake in April 2015 in Nepal, two outbreaks in nine districts were officially reported, with a total of 150 cases and eight deaths11. As per the annual health reports of Nepal, the cases of ST have been increasing in frequency and distribution throughout the country since its first outbreak. In 2019, 1610 people were infected, which increased to 2001 in 2021. The highest number of cases (738, 36.9%) in 2021 were reported from the far western province of Nepal called Sudurpaschim12. Despite the significant burden and several outbreaks of ST in Nepal, there is a lack of comprehensive systematic review to assess the burden of disease in the country. An epidemiological study on an important public health problem like ST can establish strong evidence for the policymakers in Nepal to tackle the disease burden. Therefore, the aim of this systematic review is to calculate the seroprevalence of ST, describe the clinical presentations, diagnosis, treatment, and complications of the disease in Nepal.
Methods
Ethical compliance and research registration
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines13 (Supplemental Digital Content 1, http://links.lww.com/MS9/A231). The PRISMA checklist was utilized to guide the study and is available in the Supplementary File, Supplemental Digital Content 1, http://links.lww.com/MS9/A231). The AMSTAR2 (A Measurement Tool to Assess systematic Reviews 2) checklist (Supplemental Digital Content 2, http://links.lww.com/MS9/A232) was used to assess the quality of our review, which showed our review being of moderate quality. The details of the AMSTAR2 checklist are provided in the Supplementary File (Supplemental Digital Content 2, http://links.lww.com/MS9/A232).
Literature search strategy
The medical databases, PubMed, EMBASE, Google Scholar, and national databases, were searched for any literature published between January 2000 and January 2023 using a search strategy. Boolean logic was used for conducting a database search, and the Boolean search operators ‘AND’ and ‘OR’ were used to link search terms. The search terms included ‘Burden’, ‘Epidemiology’, ‘Scrub typhus’, ‘Orientia tsutsugamushi’, ‘Rickettsia’, and ‘Nepal’. The comprehensive search strategy used in the literature review has been provided in the Supplementary File. For an advanced PubMed search, the medical subject headings (MeSH) database was used to determine corresponding MeSH terms for the literature search. Similarly, for an advanced Embase search, corresponding Emtree terms were used for the literature search. All of the identified studies were then imported into the EndNote library, and duplicates were removed accordingly. A subsequent manual check was also performed, with the removal of the remaining duplicates, wherever applicable. Two reviewers independently reviewed the titles and abstracts for all the identified references for inclusion and verified them with another reviewer.
Eligibility criteria
Any type of study design (observational studies, case series, and interventional studies) that reported laboratory-confirmed ST and was conducted in Nepal among patients of any age groups was included. Studies on diagnostic evaluation and epidemiological factors associated with ST were also included. However, case series with fewer than five cases, reviews, meta-analyses, letters, commentaries and editorials were excluded from the analysis. Further, the articles with insufficient information, irretrievable full texts, and those that did not meet the eligibility criteria were also excluded.
Data extraction and data synthesis
The data extraction was performed independently by two reviewers using a standardized data extraction form in the Microsoft Excel 2019 spreadsheet program. The data collected from the studies were the study ID (first author, year of publication), study design, study site, sample size, mean age of patients, sex distribution of patients in the study, geographical and temporal distribution of cases, diagnostic tests or criteria used, number of acute undifferentiated febrile illness (AUFI) cases, number of ST cases, and number of deaths. The data on the number of deaths were further utilized to calculate the case fatality rate (CFR). A systematic narrative synthesis was performed to summarize the included studies in the text and tables. All statistical analyses were performed with STATA version 16.0 (StataCorp, College Station, Texas, USA).
Quality assessment
The quality of studies was evaluated with respect to bias using the Newcastle–Ottawa Scale (https://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf). The scale was used to assess study quality under three major headings: selection, comparability, and exposure. Studies with scores of five or more qualified for inclusion, and studies with more than seven were labeled high-quality studies. The quality of each included study was independently assessed by the two reviewers. The final decision was reached with the help of a third reviewer who mediated in situations of disagreement.
Results
Study selection and study characteristics
A total of 1,559 studies were identified after the initial search of the databases. After the screening of titles and abstracts, 57 potentially relevant papers were retrieved for detailed assessment, and 15 studies14–28 with a total sample size of 10 977 were included in the review based on the inclusion and exclusion criteria. The details of the selection of the eligible studies are included in the PRISMA diagram (Fig. 2).
Figure 2.
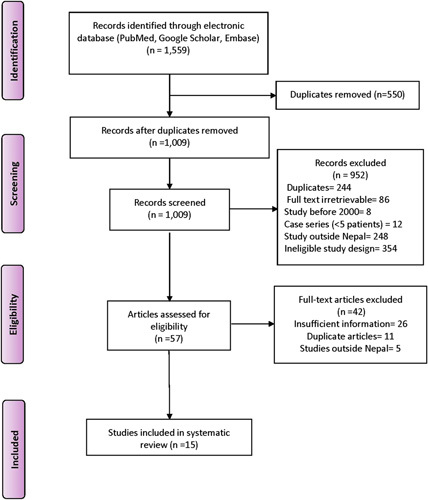
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
The details of 15 studies included in this systematic review are provided in Table 1. All the studies were observational and hospital-based. The sample size of the studies ranged from 20 to 2070. A total of nine studies (n=10, 350) described the seroprevalence of ST among AUFI cases. The rest six studies (n=627) took diagnosed cases of ST as a sample population. The total number of individuals studied in this review was 2,750 laboratory-confirmed ST cases.
Table 1.
Characteristics of the included studies.
| References | Study design | Study site | Sample size | AUFI patients | Number of ST cases | Male:Female ratio | Age (mean±SD) (years) |
|---|---|---|---|---|---|---|---|
| Sedhain and Bhattarai 14 | Prospective, cross-sectional | CMCTH, Chitwan | 1398 | 1398 | 502 | 0.79 | 30.37±18.81 |
| Mishra et al.15 | Prospective, cross-sectional | NMCTH, Birgunj | 52 | NA | 52 | 1.89 | 8.3 |
| Gurung et al.18 | Case series | Aaruchanaute PHC, Gorkha | 23 | NA | 23 | 0.92 | NA |
| Thapa et al.16 | Prospective, cross-sectional | CMCTH, Chitwan | 1797 | 1797 | 524 | 0.67 | NA |
| Bhandari et al.20 | Cross-sectional | COMSTH, Chitwan | 1024 | 1024 | 55 | 0.83 | 9.2±3.9 |
| Madhup et al.17 | Retrospective, cross-sectional | Dhulikhel Hospital, Kavre | 784 | 784 | 133 | 0.82 | 36.8±19.4 |
| Gautam et al.28 | Cross-sectional | Hospitals of central Nepal | 1585 | 1585 | 358 | 0.63 | NA |
| Dhimal et al.19 | Cross-sectional | NPHL, Kathmandu | 401 | NA | 401 | 0.68 | 28.9±18.2 |
| Ghimire et al.21 | Cross-sectional | Nobel Medical College, Morang | 47 | NA | 47 | 0.47 | NA |
| Chapagain et al.22 | Prospective, cross-sectional | Kanti Hospital, Kathmandu | 1155 | 1155 | 100 | 0.96 | 7.2±4.0 |
| Blacksell et al.23 | Retrospective, cross-sectional | Dhulikhel Hospital, Kavre | 103 | 103 | 23 | 1.22 | 20a |
| Bajracharya, 24 | Retrospective, cross-sectional | TUTH, Kathmandu | 84 | NA | 84 | 0.87 | 10.5 |
| Agrawal et al.25 | Retrospective, cross-sectional | Sukraraj Tropical and Infectious Disease Hospital, Kathmandu | 20 | NA | 20 | 0.43 | 11.45±3.1 |
| Upadhyay et al.26 | Cross-sectional study | NPHL, Kathmandu | 434 | 434 | 175 | 0.58 | NA |
| Pokhrel et al.27 | Prospective, cross-sectional | Sukraraj Tropical and Infectious Disease Hospital, Kathmandu | 2070 | 2070 | 253 | 0.87 | 36.42±15.6 |
Median age.
AUFI, acute undifferentiated febrile illness; CMCTH, Chitwan Medical College & Teaching Hospital; COMSTH, College of Medical Sciences and Teaching Hospital; NMCTH, National Medical College & Teaching Hospital; NPHL, National Public Health Laboratory; PHC, Primary Health Care; ST, scrub typhus; TUTH, Tribhuvan University Teaching Hospital; NA, Not available.
Diagnostic methods used for the confirmation of ST cases
Immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA) was used in nine out of 15 studies, and a majority of cases (n=1948, 72.28%) were confirmed with this diagnostic method. Other confirmatory diagnostic tests used were the Rapid Diagnostic Kit (RDT) (n=677, 25.12%), which was used in four studies, the Weil Felix Test (WFT) (n=397, 14.73%), and Immunofluorescent Assay (IFA) (n=70, 2.6%). More than one diagnostic method (IgM ELISA and WFT) for case confirmation was used in one study16.
Seroprevalence of ST in AUFI
The seroprevalence of ST among the AUFI cases in Nepal was 19.31% (95% confidence interval (CI): 18.55–20.08%). The seroprevalence of ST among AUFI ranged from 5.37 to 40.32% in Nepal. The details of the pooled seroprevalence of ST, along with the seroprevalence of individual studies, are provided in Figure 1 of the Supplementary File.
Demographics of ST cases
The mean age of ST patients, as provided by nine studies, was 19.9 years (95% CI: 10.03–29.77). The data on the age-wise distribution of ST cases was provided in seven studies. According to these studies, young people at or below 20 years of age were most commonly affected and accounted for 36.73% (n=630) of total cases. Likewise, 33.18% (n=569) of the ST cases were between 20 and 40 years of age, and 23.79% (n=408) of the cases were between 40 and 60 years of age. Only 6.30% (n=108) of the total cases were above 60 years of age.
The data on the gender-wise distribution of ST was available in all studies. The proportion of males affected by ST was less than that of females. The total number of ST infections among males and females was 1165 (42.3%) and 1585 (57.7%), respectively. The occupations of the cases were reported in only three (n=929) studies. The common occupations of the patients were students (n=222, 42.26%), farmers (n=182, 30.89%), and homemaker women (n=96, 18.73%).
Geographical and temporal distribution
A total of eight studies (n=1,406) reported on the provincial distribution of ST cases. The maximum number of cases was reported from Bagmati province (n=836, 59.46%). It was followed by Lumbini province (n=217, 15.43%), Sudurpaschim province (n=166, 11.80%), Gandaki province (n=81, 5.76%), Madhesh province (n=79, 5.61%), Koshi province (n=24, 70%), and Karnali province (n=3, 0.21%). Chitwan, Nawalparasi, Kailai, Dhading, Kavre, and Gorkha were some of the districts with the highest number of ST cases, according to the included studies. The details on the geographical distribution of cases in Nepal are shown in Figure 3.
Figure 3.
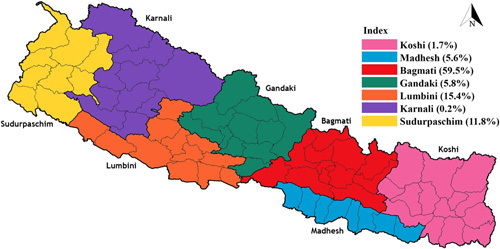
Provincial distribution of scrub typhus cases in Nepal.
A total of nine studies described the month-wise distribution of ST cases in Nepal. Our analysis showed that most of the ST cases were reported in the month of August (n=469, 26.33%). It was followed by October (n=417, 23.41%), September (n=326, 18.30%), November (n=260, 14.60%), and July (n=205, 11.51%). The monthly distribution of total ST cases is illustrated in Figure 4.
Figure 4.
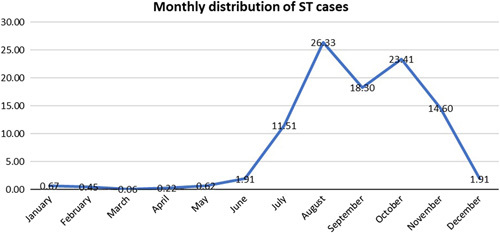
Monthly distribution of scrub typhus cases. ST, scrub typhus.
Clinical characteristics of laboratory-confirmed ST cases
A total of 11 studies reported various clinical characteristics of patients with ST. All of the patients included in our analysis (n=2018, 100%) had fever. The second most common clinical symptom reported was anorexia (58.36%), followed by headache (56.7%), cough (39.58%), shortness of breath (34.61%), nausea (32.11%), and abdominal pain (22.55%). The clinical signs present were lymphadenopathy (17.45%), jaundice (10.86%), rashes (8.01%), and eschar (7.84%). Further details on these clinical characteristics are provided in Table 2.
Table 2.
Clinical features of scrub typhus.
| Clinical features | Number of studies | Frequency (%) | Range% |
|---|---|---|---|
| Anorexia | 5 | 687 (58.4) | 8–97.9 |
| Headache | 10 | 1,087(56.7) | 38–83 |
| Vomiting/nausea | 9 | 406 (32.11) | 4.3–63.5 |
| Cough | 7 | 150 (39.58) | 26–74.4 |
| Breathlessness | 8 | 335 (34.61) | 5–57.7 |
| Abdominal pain | 7 | 258 (22.55) | 15–54 |
| Hepatomegaly | 5 | 140 (45.19) | 5–65.5 |
| Splenomegaly | 6 | 99 (17.6) | 1–46.4 |
| Lymphadenopathy | 6 | 274 (17.45) | 1.9–84.6 |
| Eschar | 10 | 157 (7.84) | 3.1–29.27 |
| Rash | 6 | 45 (8.01) | 1–20 |
| Jaundice | 5 | 176 (11.81) | 3.6–30.7 |
Organ involvement and CFR
ST cases with various organ involvements were reported in 11 out of 15 studies. The most common complication in Nepalese patients was acute kidney injury (AKI) (33.37%), followed by respiratory problems (25.52%), cardiac issues (12.61%), and neurological manifestations (12.10%). The details of organ involvement among ST cases are provided in Table 3. The overall CFR of ST, as identified from seven studies, was found to be 2.56%.
Table 3.
Organ involvement among scrub typhus cases.
| Organ involvement | Number of studies | Frequency (%) | Range% |
|---|---|---|---|
| Renal (with AKI) | 6 | 262 (33.37) | 1–65.4 |
| Respiratory (with ARDS, pneumonia) | 4 | 187 (25.5) | 8–78.72 |
| Cardiovascular (with myocarditis) | 5 | 99 (12.6) | 1–67.3 |
| Neurological (with meningitis, meningoencephalitis) | 6 | 95 (12.10) | 6.37–34.5 |
AKI, acute kidney injury; ARDS, acute respiratory distress syndrome.
Laboratory parameters
Thrombocytopenia was one of the most common derangements in hematological parameters, affecting up to 66.60% of the total cases. Leucocytosis (313, 16.53%) was twice as commonly observed as leucopenia (105, 8.11%) among the patients. Similarly, raised aspartate aminotransferase (AST) and raised alanine aminotransferase (ALT) were seen in 70.26% and 63.63% of the patients, respectively. The details of other common lab parameter derangements are provided in Table 1 in the Supplementary File.
Treatment
Among seven studies with a total of 692 cases, 309 (44.7%) were treated with doxycycline alone, 108 (15.6%) with azithromycin alone, 75 (10.8%) with doxycycline and azithromycin, 64 (9.2%) with doxycycline and ciprofloxacin, and 42 (6%) with chloramphenicol. Other drugs administered in ST cases in Nepal were ceftriaxone, piperacillin/tazobactam, amikacin, levofloxacin, ceftriaxone, cefepime, etc.
Discussion
This systematic review included 15 observational studies involving a total of 10,977 participants, to investigate the prevalence and characteristics of ST in Nepal. The seroprevalence of ST among AUFI cases, as per our review, was 19.31%. This is similar to the burden of ST observed among febrile patients seeking medical attention in hospitals in India (25.3%)29. The existing pool of research on sero-epidemiological data indicates that O. tsutsugamushi infection is widespread throughout Asia, with an average seroprevalence of 22.2%30. Moreover, a nationwide database study conducted in Taiwan showed that 17.8% of suspected cases were diagnosed as ST31. Similarly, a study from South Korea reported that 20.35% of suspected cases were diagnosed as ST32. Notably, a higher seroprevalence of 31.8% was observed in Thai men33.
The studies included in the review have shown a wide range of seroprevalence, ranging from 5 to 40%, which could be due to the lack of standardization among study designs and diagnostic modalities used. One particular study reported a significantly higher seroprevalence of up to 40%26. The higher seroprevalence observed in this study could potentially be attributed to the timing of sample collection. Specifically, the samples were collected between July and November, which coincides with the rainy monsoon and post-monsoon seasons and the peak growth of vegetation and mite populations. Notably, the majority of studies included in our review consistently reported higher seroprevalence (over 90% of total cases) during the period of July–November14,16,17,19,20,24.
In all the studies included in this systematic review, indirect methods of diagnosis were employed for the diagnosis of ST. The most commonly used confirmatory test was the IgM ELISA, which detected 72.28% of all cases. It is worth noting that although the recommended gold standard test for ST is IFA, IgM ELISA is commonly used in Nepal34,35. This can be attributed to the higher reported sensitivity and specificity of the IgM ELISA compared to the IFA as well as the lower cost of the ELISA compared to the IFA34–36. The sensitivity of RDTs was higher than that of IFA, making RDTs suitable for early diagnosis during the acute phase of ST, thereby aiding prompt treatment initiation37. Furthermore, another study reported that RDTs exhibited a sensitivity of 82% and a specificity of 98% when compared to samples defined as positive using IFA38. Similarly, a study highlighted the higher sensitivity and specificity of RDTs, reaching up to 92%36. Because of their low cost and comparable sensitivity and specificity to ELISA and IFA, RDTs could be used in rural areas of Nepal for early detection of ST37. On the other hand, the conventional WFT demonstrated lower sensitivity and specificity compared to other diagnostic tests36. While four studies included in this review employed WFT as a diagnostic test, routine use of WFT is not recommended by the authors. Although polymerase chain reaction (PCR) is known to have high sensitivity (98%) and specificity (100%), its routine use in low–middle income countries like Nepal is hindered by its higher cost36.
The areas with dense scrub vegetation are particularly infested with the infected chiggers of trombiculid mites. The chiggers flourish in different environments, such as grassy plains, forest clearings, and riverbanks. The southern plains of Nepal, known as the ‘Terai’ region, provide a propitious environment with a hot and humid climate essential for the transmission of ST39. Despite a wide distribution of ST in various regions of Nepal, a higher burden has been observed in the Terai and a relatively lower burden in elevated areas in the mid-hills and mountains. The districts of Chitwan and Nawalparasi, which are located in Terai, have reported a substantial incidence of ST cases. These areas, which are densely populated, serve as major population hubs in the country40. Contrary to this, the fewest cases were reported in the Karnali province (0.21%), which is a remote and mountainous region. Our review also showed that the burden of ST within the country exhibited heterogeneity, with the highest proportion of cases reported in the Bagmati province (59.465%), followed by the Lumbini province (15.43%).
According to our review, the period from July to November showed the highest number of ST cases in Nepal. This period corresponds to the monsoon and post-monsoon seasons in Nepal. During the monsoon season, mites find optimal conditions for laying eggs, and the subsequent post-monsoon period leads to the growth of scrub vegetation, which serves as a habitat for mites41. During these months, agricultural workers are primarily working in fields, which puts them at a high risk of contracting an infection from larval mite bites. A similar pattern of distribution has been observed in other countries located in the same geographical area as Nepal. In India, most states experience a high frequency of ST cases during the monsoon and post-monsoon seasons between July and February29. In Japan, the disease shows a bimodal pattern, with a larger peak occurring from October to December and a smaller peak from April to June42. Likewise, South Korea reports a peak in disease incidence from October to December, with lower latitudes associated with a later peak43. In Taiwan, the disease incidence follows a biphasic pattern, with the main peak in June and July and a second smaller peak in September and October, as per the Taiwan National Infectious Disease Statistics System (https://nidss.cdc.gov.tw/en/nndss/disease?id=0812). In Thailand, the peak season for ST is from June to October44, whereas in Bangladesh, the highest number of cases is reported during the months of August and September45.
The most commonly affected age group in Nepal with ST was children and young adults. This finding aligns with studies conducted in Bangladesh and India29,45. In contrast, the prevalence of ST among older age groups was higher in the other five countries, particularly in Japan and South Korea, where there are aging farming communities42,46,47. Females were found to be more affected than males in this review. This could be attributed to the higher exposure of women to mites while working in scrub-prone areas, such as agricultural fields during harvesting or cultivation of crops, or in forests16,17,19,48. Conversely, most men were less exposed to mites as they were predominantly engaged in indoor work, such as office jobs, factory work, or employment in companies16. In a study by Gautam et al.28, female gender, rural residential location, house near grasslands, and working in the fields were identified as significant associated risk factors. Shrubs, precipitation, farmland, and maximum temperature were crucial factors impacting the disease’s spatial distribution49. Rodents play a significant role in the ecology of ST, and it is probable that infected rodents are responsible for the presence of various strains of O. tsutsugamushi within an individual larva. These rodents in rural areas are the main culprits for transmitting the disease to humans50. To prevent the spread of the disease, it is essential to implement precautionary measures aimed at controlling the rodent population in rural areas. According to a study in Vietnam, factors related to ecological and household hygiene were found to have a stronger association with ST infection compared to individual-level exposure activities in the highly endemic area51.
Among the Nepalese patients with ST, common symptoms reported were anorexia (58.4%), headache (56.7%), cough (39.54%), shortness of breath (34.61%), and abdominal pain (22.55%). The presence of an eschar, which is considered a pathognomonic sign of ST, was observed in only 7.84% of Nepalese patients. The occurrence of signs and symptoms varied significantly among the included studies3,29,52. While the presence of eschar is characteristic of ST, its occurrence varies widely from 7 to 80%. Notably, eschar formation is rarely reported in patients from Southeast Asian regions53. In our review, we observed that among cases with various organ involvement, the most frequent complications were AKI (32.73%), respiratory problems (25.52%), cardiac issues (12.61%), and neurological manifestations (12.10%). When comparing our findings to studies conducted globally, varying frequencies of complications were reported. Renal problems, including AKI, ranged from 16 to 51%, pulmonary problems from 16 to 69%, cardiac problems from 2 to 19.3%, neurological issues from 11 to 50%, while multi-organ dysfunction syndrome (MODS) ranged from 6 to 24%; and septic shock from 14 to 46%54–60. In our review, thrombocytopenia was prevalent in 66.60% of cases, and elevated AST/ALT levels were observed in 70.26% of patients. These findings in Nepalese patients align with observations made by studies conducted in India and Thailand55,59,61. Leukocytosis (16.53%) was twice as frequent as leucopenia (8.11%) in the Nepalese population, according to our study. A similar observation was made in a study conducted on Taiwanese patients62. The clinicians working in Nepal should be mindful of these clinical presentations along with complications. This is necessary for early and accurate diagnosis of ST, which can prevent the progression to severe disease.
The literature reveals significant variability in the rates of fatality due to ST. In a study among untreated ST cases, CFR was as high as 70%, with a median of 6.0%3. In a review by Bonell et al.30 on the Asian population, the CFR for treated ST cases was reported to be 1.4%. In our review, the median CFR of Nepalese ST patients was 2.56%, ranging from 0.9 to 7.7%. It is lower than the CFR in India (6.3%) and Northern Thailand (6.2%)29,63. However, in Japan, the CFR is only 0.48% at the time of notification42. Similarly, the CFR of ST in Korea is only 1.32%46. The low CFR in these countries could be attributed to excellent medical care and efficient healthcare systems.
According to our review, doxycycline was prescribed to 44.7% of Nepalese patients, azithromycin to 15.6%, and combinations of doxycycline with azithromycin or ciprofloxacin to even smaller proportions. However, recent evidence from network analysis indicates that chloramphenicol and minocycline have the highest cure rates in pediatric and adult patients, respectively. Second-generation quinolones had lower adverse event rates compared to doxycycline64. The reasons behind the common use of doxycycline and azithromycin could be easy availability, lower costs, and the native prescribing patterns of physicians. There is a need for change in antibiotic prescription patterns to obtain better outcomes in terms of cure rate and patient safety in Nepal. In another analysis, rifampicin showed the highest cure rate and lowest risk of complications, except in areas with tuberculosis endemicity. Moreover, Azithromycin was effective with fewer adverse effects, making it suitable for pregnant women and children65.
Despite being the first systematic review of the epidemiology of ST in Nepal, this study has its limitations. The seroprevalence of ST was highly variable throughout the studies, showing non-uniformity in the sample population, diagnostic methods used, and study site in Nepal. This review included hospital-based studies due to the lack of availability of community-based studies in the current literature. Hence, the findings of the study might have limited applicability and generalizability. There is a need for future studies to explore the seroprevalence of ST through community-based research to precisely estimate the true burden of the disease.
Conclusion
ST is one of the neglected tropical diseases that pose a significant burden in Nepal. Hence, Case finding should be strengthened, especially during monsoon and post-monsoon seasons, post-natural calamities, etc., using RDTs in highly prevalent regions of Nepal. Moreover, the availability and affordability of effective health services for ST cases should be improved in Nepal to decrease the high CFR in Nepal.
Ethical approval
No ethical approval was obtained for this systematic review.
Consent
Informed consent was not obtained for this systematic review.
Sources of funding
The authors received no funding to carry out the research.
Author contribution
P.L., K.M.P., K.K., and A.A.: conceptualization; P.L., K.K., and B.A.: methodology; P.L. and K.K.: software; P.L. and K.M.P.: validation; P.L. and K.M.P.: formal analysis; P.L., K.M.P., B.A., K.K., and A.Y.: investigation; P.L.: resources; P.L., K.K., M.B., and A.Y.: data curation; P.L., K.K., B.A., A.A., K.M.P., M.B., A.R.Z.Z., and M.Z.A.: writing – original draft preparation; P.L., A.R.Z.Z., and M.Z.A.: writing – review and editing; P.L.: visualization. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest disclosure
The authors declare that there are no conflicts to declare.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO.
Unique identifying number or registration ID: CRD42023416353.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=416353.
Guarantor
Dr Pratik Lamichhane.
Data availability statement
The data will be made available upon reasonable request to the readers.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
None.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/annals-of-medicine-and-surgery.
Published online 5 September 2023
Contributor Information
Pratik Lamichhane, Email: pratiklamichhane@iom.edu.np.
Kailash M. Pokhrel, Email: kailashmanipokhrel@iom.edu.np.
Baraa Alghalyini, Email: Balghalyini@alfaisal.edu.
Abdul Rehman Zia Zaidi, Email: Arzaidi@alfaisal.edu.
Maied Z. Alshehery, Email: mzalsherery@kfmc.med.sa.
Kapil Khanal, Email: kapilkhanal@iom.edu.np.
Madhur Bhattarai, Email: madhurbhattarai180@gmail.com.
Alisha Yadav, Email: alishayadavofficial@gmail.com.
References
- 1.Paris DH, Shelite TR, Day NP, et al. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg 2013;89:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerrant RL, Walker DH, Weller PF. Tropical Infectious Diseases: Principles, Pathogens and Practice E-Book. Elsevier Health Sciences; 2011. [Google Scholar]
- 3.Taylor AJ, Paris DH, Newton PN, et al. Review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis 2015;9:e0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh GC, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg 2010;82:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silpapojakul K, Varachit B, Silpapojakul K. Paediatric scrub typhus in Thailand: a study of 73 confirmed cases. Trans R Soc Trop Med Hyg 2004;98:354–359. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Huang L, Zhang W. Scrub typhus with multi-organ dysfunction syndrome and immune thrombocytopenia: a case report and review of the literature. J Med Case Rep 2019;13:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saxena A, Khiangte B, Tiewsoh I. Scrub typhus complicated by acute respiratory distress syndrome and multiorgan failure; an unrecognized alarming entity in central India: a report of two cases. J Family Med Prim Care 2014;3:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu G, Walker DH, Jupiter D, et al. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis 2017;11:e0006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma P, Kakkar R, Kaore SN, et al. Geographical distribution, effect of season & life cycle of scrub typhus. JK Science 2010;12:63. [Google Scholar]
- 10.Karki KB, Acharya BM, Dhimal M, et al. Descriptive Epidemiology of Scrub Typhus in Nepal. Government of Nepal, Nepal Health Research Council; 2017. [Google Scholar]
- 11.Department of Health Services, Annual Report, Ministry of Health and Population, Nepal, 2016: 566.
- 12.Department of Health Services, Annual Health Report, Ministry of Health and Population, 2021: 466.
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedhain A, Bhattarai GR. Clinical presentation of scrub typhus during a major outbreak in central Nepal, Asian. J Med Sci 2017;8:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra A, Thakur R, Thakur R. Clinical and laboratory profile of scrub typhus in paediatric age group. Asian J Med Sci 2020;11:63–67. [Google Scholar]
- 16.Thapa S, Hamal P, Chaudhary NK, et al. Burden of scrub typhus among patients with acute febrile illness attending tertiary care hospital in Chitwan, Nepal. BMJ Open 2020;10:e034727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhup S, Shrestha R, Katuwal N, et al. Seroprevalence of scrub typhus in patients attending Dhulikhel Hospital, Kavre. Kathmandu Univ Med J 2021;19:494–498. [PubMed] [Google Scholar]
- 18.Gurung S, Karki S, Pokharel S, et al. Scrub typhus in a primary health care center of Nepal: a case series. Ann Med Surg 2022;75:103490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhimal M, Dumre SP, Sharma GN, et al. An outbreak investigation of scrub typhus in Nepal: confirmation of local transmission. BMC Infect Dis 2021;21:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhandari I, Malla KK, Ghimire P, et al. Scrub typhus among febrile children in a tertiary care center of central Nepal: a descriptive cross-sectional study. JNMA J Nepal Med Assoc 2021;59:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghimire RH, Ghimire A, Dhungana S, et al. Clinical profile and outcome of adult patients with scrub typhus in a tertiary care centre of eastern Nepal. JNMA J Nepal Med Assoc 2020;58:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapagain RH, Agrawal S, Pokharel S, et al. Clinico-laboratory profile, complications and therapeutic outcome of scrub typhus in children. J Nepal Health Res Counc 2020;18:282–287. [DOI] [PubMed] [Google Scholar]
- 23.Blacksell SD, Sharma NP, Phumratanaprapin W, et al. Serological and blood culture investigations of Nepalese fever patients. Trans R Soc Trop Med Hyg 2007;101:686–690. [DOI] [PubMed] [Google Scholar]
- 24.Bajracharya L. Scrub typhus in children at Tribhuvan University Teaching Hospital in Nepal. Pediatric Health Med Ther 2020;11:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agrawal S, Subedi KH, Shah RK, et al. Clinical and laboratory profile and therapeutic response of scrub typhus in children in a tertiary care centre in Nepal. Birat J Health Sci 2020;5:897–901. [Google Scholar]
- 26.Upadhayay BP, Shakya G, Adhikari S, et al. Scrub typhus: an emerging neglected tropical disease in Nepal. J Nepal Health Res Counc 2016;14:122–127. [PubMed] [Google Scholar]
- 27.Pokhrel A, Rayamajhee B, Khadka S, et al. Seroprevalence and clinical features of scrub typhus among febrile patients attending a referral hospital in Kathmandu, Nepal. Trop Med Infect Dis 2021;6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gautam R, Parajuli K, Sherchand JB. Epidemiology, risk factors and seasonal variation of scrub typhus fever in Central Nepal. Trop Med Infect Dis 2019;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devasagayam E, Dayanand D, Kundu D, et al. The burden of scrub typhus in India: a systematic review. PLoS Negl Trop Dis 2021;15:e0009619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis 2017;11:e0005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang YC, Sun W, Lin JN, et al. Epidemiology and risk factors of scrub typhus in Taiwan: a nationwide database study from 1996 to 2014. Zoonoses Public Health 2021;68:876–883. [DOI] [PubMed] [Google Scholar]
- 32.Won EJ, Kim SH, Byeon KH, et al. Under-diagnosis of vector-borne diseases among individuals suspected of having Scrub Typhus in South Korea. PLoS One 2023;18:e0286631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonwong S, Mason CJ, Chuenchitra T, et al. Nationwide seroprevalence of scrub typhus, typhus, and spotted fever in young Thai men. Am J Trop Med Hyg 2022;106:1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kala D, Gupta S, Nagraik R, et al. Diagnosis of scrub typhus: recent advancements and challenges. 3 Biotech 2020;10:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautam R, Parajuli K, Tshokey T, et al. Diagnostic evaluation of IgM ELISA and IgM immunofluorescence assay for the diagnosis of Acute Scrub Typhus in central Nepal. BMC Infect Dis 2020;20:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kannan K, John R, Kundu D, et al. Performance of molecular and serologic tests for the diagnosis of scrub typhus. PLoS Negl Trop Dis 2020;14:e0008747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, He S, Wang S, et al. Comparison of a rapid diagnostic test and microimmunofluorescence assay for detecting antibody to Orientia tsutsugamushi in scrub typhus patients in China, Asian Pacific. J Trop Med 2011;4:666–668. [DOI] [PubMed] [Google Scholar]
- 38.Kingston HW, Blacksell SD, Tanganuchitcharnchai A, et al. Comparative accuracy of the InBios scrub typhus detect IgM rapid test for the detection of IgM antibodies by using conventional serology. Clin Vaccine Immunol 2015;22:1130–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson CN, Blacksell SD, Paris DH, et al. Undifferentiated febrile illness in Kathmandu, Nepal. Am Soc Trop Med Hyg 2015;92:875–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acharya BK, Chen W, Ruan Z, et al. Mapping environmental suitability of scrub typhus in Nepal using MaxEnt and random forest models. Int J Environ Res Public Health 2019;16:4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kore VB, Mahajan SM. Recent threat of scrub typhus in India: a narrative review. Cureus 2022;14:e30092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinoshita H, Arima Y, Shigematsu M, et al. Descriptive epidemiology of rickettsial infections in Japan: scrub typhus and Japanese spotted fever, 2007–2016. Int J Infect Dis 2021;105:560–566. [DOI] [PubMed] [Google Scholar]
- 43.Jeung YS, Kim CM, Yun NR, et al. Effect of latitude and seasonal variation on scrub typhus, South Korea, 2001–2013. Am J Trop Med Hyg 2016;94:22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wangrangsimakul T, Elliott I, Nedsuwan S, et al. The estimated burden of scrub typhus in Thailand from national surveillance data (2003–2018). PLoS Negl Trop Dis 2020;14:e0008233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nila SS, Paul SK, Kobayashi N, et al. Socio-demographic and clinico-epidemiological study of scrub typhus in a tertiary care hospital of Mymensingh, Bangladesh. Mymensingh Med J 2022;31:66–71. [PubMed] [Google Scholar]
- 46.Lee HW, Cho PY, Moon SU, et al. Current situation of scrub typhus in South Korea from 2001–2013. Parasit Vectors 2015;8:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma CJ, Oh GJ, Kang GU, et al. Differences in agricultural activities related to incidence of scrub typhus between Korea and Japan. Epidemiol Health 2017;39:e2017051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.George T, Rajan SJ, Peter JV, et al. Risk factors for acquiring scrub typhus among the adults. J Glob Infect Dis 2018;10:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Zhang M, Qin Y, et al. Epidemiological analysis and risk prediction of scrub typhus from 2006 to 2021 in Sichuan, China. Front Public Health 2023;11:1177578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frances SP, Watcharapichat P, Phulsuksombati D, et al. Transmission of Orientia tsutsugamushi, the aetiological agent for scrub typhus, to co-feeding mites. Parasitology 2000;120:601–607. [DOI] [PubMed] [Google Scholar]
- 51.Tran HTD, Hattendorf J, Do HM, et al. Ecological and behavioural risk factors of scrub typhus in central Vietnam: a case–control study. Infect Dis Poverty 2021;10:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.M, Pathania, Amisha, Malik P, et al. Scrub typhus: overview of demographic variables, clinical profile, and diagnostic issues in the sub-Himalayan region of India and its comparison to other Indian and Asian studies. J Family Med Prim Care 2019;8:1189–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vivekanandan M, Mani A, Priya YS, et al. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India 2010;58:24–28. [PubMed] [Google Scholar]
- 54.Kim DM, Kim SW, Choi SH, et al. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis 2010;10:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varghese GM, Trowbridge P, Janardhanan J, et al. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis 2014;23:39–43. [DOI] [PubMed] [Google Scholar]
- 56.Takhar RP, Bunkar ML, Arya S, et al. Scrub typhus: a prospective, observational study during an outbreak in Rajasthan, India. Natl Med J India 2017;30:69–72. [PubMed] [Google Scholar]
- 57.Premraj SS, Mayilananthi K, Krishnan D, et al. Clinical profile and risk factors associated with severe scrub typhus infection among non-ICU patients in semi-urban south India. J Vector Borne Dis 2018;55:47–51. [DOI] [PubMed] [Google Scholar]
- 58.Basu S, Chakravarty A. Neurological manifestations of scrub typhus. Curr Neurol Neurosci Rep 2022;22:491–498. [DOI] [PubMed] [Google Scholar]
- 59.Kamath SD, Kumari S, Sunder A. A study of the profile of scrub typhus in a tertiary care hospital in Jharkhand: an underestimated problem. Cureus 2022;14:e26503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Narayanasamy DK, Arunagirinathan AK, Kumar RK, et al. Clinico - laboratory profile of scrub typhus – an emerging rickettsiosis in India. Indian J Pediatr 2016;83:1392–1397. [DOI] [PubMed] [Google Scholar]
- 61.Suputtamongkol Y, Suttinont C, Niwatayakul K, et al. Epidemiology and clinical aspects of rickettsioses in Thailand. Ann N Y Acad Sci 2009;1166:172–179. [DOI] [PubMed] [Google Scholar]
- 62.Lim HK, Wang JM. Scrub typhus: seven-year experience and literature review. J Acute Med 2018;8:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thipmontree W, Tantibhedhyangkul W, Silpasakorn S, et al. Scrub typhus in Northeastern Thailand: eschar distribution, abnormal electrocardiographic findings, and predictors of fatal outcome. Am J Trop Med Hyg 2016;95:769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng BS, Zeng BY, Hung CM, et al. The efficacy and tolerability of antibiotics in scrub typhus: an updated network meta-analysis of randomized controlled trials. Int J Infect Dis 2022;122:461–468. [DOI] [PubMed] [Google Scholar]
- 65.Lu D, Wang T, Luo Z, et al. Evaluation of the therapeutic effect of antibiotics on scrub typhus: a systematic review and network meta-analysis. Front Public Health 2022;10:883945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be made available upon reasonable request to the readers.


