Abstract
PURPOSE
The Atezo-Brain study evaluated atezolizumab combined with chemotherapy in patients with advanced non–small-cell lung cancer (NSCLC) with untreated brain metastases, a population traditionally excluded from trials.
METHODS
This single-arm phase II clinical trial enrolled patients with advanced nonsquamous NSCLC with untreated brain metastases without neurologic symptoms or asymptomatic with medical treatment. Dexamethasone was allowed up to 4 mg once daily. Atezolizumab plus carboplatin and pemetrexed was given for four to six cycles followed by atezolizumab plus pemetrexed until progression for a maximum of 2 years. The primary end points were to determine the progression-free survival (PFS) rate at 12 weeks and the incidence of grade ≥3 adverse events during the first 9 weeks. Intracranial outcomes were assessed using response assessment in neuro-oncology brain metastases criteria.
RESULTS
Forty patients were enrolled and 22 (55%) were receiving corticosteroids at baseline. The overall 12-week PFS rate was 62.2% (95% credibility interval [CrI], 47.1 to 76.2). The rate of grade 3/4 adverse events during the first 9 weeks was 27.5%. Most neurologic events were grade 1 and 2 but five patients (12.5%) experienced grade 3-4 neurologic events. With a median follow-up of 31 months, intracranial median PFS was 6.9 months and response rate was 42.7% (95% CrI, 28.1 to 57.9). Systemic median PFS was 8.9 months and response rate was 45% (95% CrI, 28.1 to 57.9). The median overall survival (OS) was 11.8 months (95% CI, 7.6 to 16.9) and the 2-year OS rate was 27.5% (95% CI, 16.6 to 45.5).
CONCLUSION
Atezolizumab plus carboplatin and pemetrexed demonstrates activity in patients with advanced nonsquamous NSCLC with untreated brain metastases with an acceptable safety profile.
INTRODUCTION
Lung cancer is the most common solid tumor to metastasize to the CNS and about one quarter of patients diagnosed with stage IV non–small-cell lung cancer (NSCLC) have brain metastases at diagnosis.1 Historically, whole-brain radiotherapy (WBRT) had been the cornerstone of treatment for brain metastases in patients with advanced NSCLC, especially when stereotactic radiosurgery (SRS) and surgery were not feasible or indicated. However, the use of WBRT has diminished because of modest survival benefit and its negative impact on cognitive function and quality of life (QOL), and because of emerging evidence that SRS may benefit patients with higher disease burden in the brain metastases.2-5 In addition, the incorporation of highly CNS-penetrant targeted therapies in oncogene-addicted NSCLC modified the clinical management of patients with asymptomatic brain metastases harboring EGFR and ALK positive tumors such that local therapy could be deferred.6
CONTEXT
Key Objective
There is a high need for effective and safe treatment options for patients with lung cancer with brain metastases. In this Bayesian clinical trial, we evaluated the combination of atezolizumab plus carboplatin and pemetrexed in patients with advanced nonsquamous non–small-cell lung cancer, who had brain metastases that were neurologically asymptomatic or controlled with corticosteroids and had not previously received any local brain therapies.
Knowledge Generated
Initiating systemic therapy with chemoimmunotherapy in patients with untreated brain metastases was safe and yielded relevant intracranial and systemic responses that were concordant in most patients. When disease progressed in the brain, most patients were rescued with brain radiotherapy. We observed that chemoimmunotherapy made it possible to delay whole-brain radiotherapy without a decline in quality of life and neurologic status.
Relevance (T.E. Stinchcombe)
-
This study provides prospective data about the activity and safety of chemotherapy and immunotherapy in patients with untreated brain metastases. Chemotherapy and immunotherapy as the initial therapy with imaging surveillance of the brain metastases is an option in select patients.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
Although most clinical trials exclude or under-represent patients with NSCLC who have previously untreated brain metastases,7 immunotherapy has shown encouraging intracranial efficacy in patients with oncogene driver–negative PD-L1–positive NSCLC who have brain metastases that are untreated or progressing after previous radiotherapy.8 Several post hoc exploratory analyses of phase III clinical trials showed that chemotherapy combined with immunotherapy improved overall survival (OS) compared with chemotherapy alone regardless of the presence of brain metastases.9,10 However, these trials were not specifically designed to evaluate the intracranial efficacy of chemoimmunotherapy and excluded patients receiving corticosteroids at doses higher than 10 mg daily of prednisone or equivalent.
We designed this phase II clinical trial to evaluate the safety and the efficacy of atezolizumab combined with carboplatin and pemetrexed in patients with advanced nonsquamous NSCLC with untreated brain metastases. We present the final analysis in this report.
METHODS
Study Design and Eligibility
This was an open-label, single-arm, phase II clinical trial conducted at 15 hospitals in Spain. Patients were age 18 years and older, had Eastern Cooperative Oncology Group performance status (ECOG PS) 0-1, and had stage IV nonsquamous NSCLC with untreated brain metastases that did not exhibit neurologic symptoms or that were controlled with anticonvulsants or dexamethasone at a maximum dose of 4 mg daily, with measurable disease in the body by computed tomography (CT) per RECIST version 1.1 criteria in the body and in the brain by magnetic resonance imaging (MRI) per response assessment in neuro-oncology brain metastases (RANO-BM), with at least one measurable lesion with a minimum size of 10 mm.11 There was no limitation on the maximum number of brain metastases or the maximum size per lesion. Patients had adequate bone marrow, liver, and renal function. Exclusion criteria included known EGFR mutations or ALK rearrangements; presence of leptomeningeal carcinomatosis or metastases in the brainstem, medulla, or lesions causing obstructive hydrocephalus; contraindication for immunotherapy (history of active autoimmune, infectious disease, or interstitial lung disease); previous malignancies within 3 years of study entry; previous treatment with immune checkpoint inhibitors; and hepatitis B or C infection or HIV positivity.
The study complied with the Declaration of Helsinki, International Council on Harmonisation Good Clinical Practice, local laws, and regulatory requirements. Independent ethics committees or institutional review boards approved the Protocol (online only). Patients provided written informed consent.
Study Procedures and Treatment
Patients were treated with atezolizumab 1,200 mg intravenously once every 3 weeks in combination with carboplatin (AUC 5) plus pemetrexed (500 mg per square meter). All the patients received premedication with folic acid, vitamin B12, and dexamethasone according to guidelines for pemetrexed use. After completing four to six cycles, patients continued with pemetrexed plus atezolizumab maintenance until unacceptable toxicity, disease progression, patient decision, completion of 2 years of therapy, or patient withdrawal of consent (Data Supplement [Fig S1], online only).
A body CT scan and a brain MRI were performed at baseline, every 6 weeks until the 12th week, and thereafter every 9 weeks until disease progression. Patients with progression exclusively in the brain could receive brain radiotherapy while staying within the study if they continued to derive clinical benefit and had ECOG PS ≤2. In case of systemic progression without brain progression, a novel line of systemic treatment was considered.
Adverse events and abnormal laboratory findings were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. Investigators determined whether adverse events were treatment-related according to the study protocol and standard regulatory requirements. Dose reductions were not permitted for atezolizumab, which could be interrupted, delayed, or discontinued depending on tolerability. Reductions were permitted for pemetrexed and carboplatin in accordance with two levels of dosage specified in the trial protocol. PD-L1 tumor proportion score expression was conducted at each center by immunohistochemical analysis after clinical practice using the Dako 22C3 and Ventana SP263 antibodies. PD-L1 expression was considered positive when ≥1% of tumor cells had PD-L1 membranous staining.
Study Outcomes
The two primary end points were safety assessed by NCI-CTCAE version 4.0 defined as the incidence of grade 3 or higher adverse events during the first 9 weeks and progression-free survival (PFS) according to RANO-BM and RECIST v1.1 criteria. Overall PFS was defined as the time from time from inclusion until death from any cause or objective tumor progression evaluated by the investigators using RANO-BM in the brain and RECIST v1.1 in the body, whichever occurred first.11
Secondary end points were the intracranial and systemic response rate (overall response rate [ORR]), the duration of response ([DOR] time from first documented complete or partial response [PR] to disease progression or death), and OS. OS was defined as the time from inclusion to death from any cause. The exploratory end points consisted of determining the time to brain radiotherapy and the event-free survival (EFS) defined as time from inclusion to brain radiotherapy or death, and to evaluate the correlation between PD-L1 expression in tumor tissue and efficacy end points. Additional exploratory end points were the assessment of QOL using the European Organisation for Research and Treatment of Cancer QLQ-C30 questionnaire that was administered at baseline and every 3 months. Global health status and overall QOL were analyzed in patients who had a baseline measurement.
Statistics
Safety and efficacy were assessed in the intention-to-treat population cohort of 40 patients using the Bayesian Multc Lean design (Data Supplement [Methods]).12,13 Details about previous and posterior distributions are detailed in the protocol. A sample size of 40 patients ensured that, if the trial is not terminated early, a posterior 90% credibility interval (CrI) would have width of 0.257 at most, under the assumption of a 50% PFS rate at 12 weeks. Futility and excess of toxicity stopping rules on the basis of posterior probabilities were evaluated sequentially every five patients (Data Supplement [Fig S2]). Posterior distributions with CrI at 95% were calculated for response or toxicity rates. Kaplan-Meier method was used to estimate PFS and OS. Data for patients who were alive or lost to follow-up were censored for OS at the time they were last known to be alive. Data for patients who were alive and did not have disease progression or who were lost to follow-up were censored for the analysis of PFS at the time of the last imaging assessment. When patients had tumor progression in one compartment (brain or systemic), they were censored for the PFS analysis in the other compartment. The stratified log-rank test was used to assess differences in PFS and OS among different subgroups. Hazard ratios and associated 95% CI were calculated with the use of a stratified Cox proportional-hazards model.
Data collection and data quality was ensured by the Spanish Lung Cancer Group. All analyses were based on the study database lock on March 31, 2022. All statistical analysis was performed using R Statistical Software (v 4.1.3; San Francisco, CA).
RESULTS
Patients and Disease Characteristics
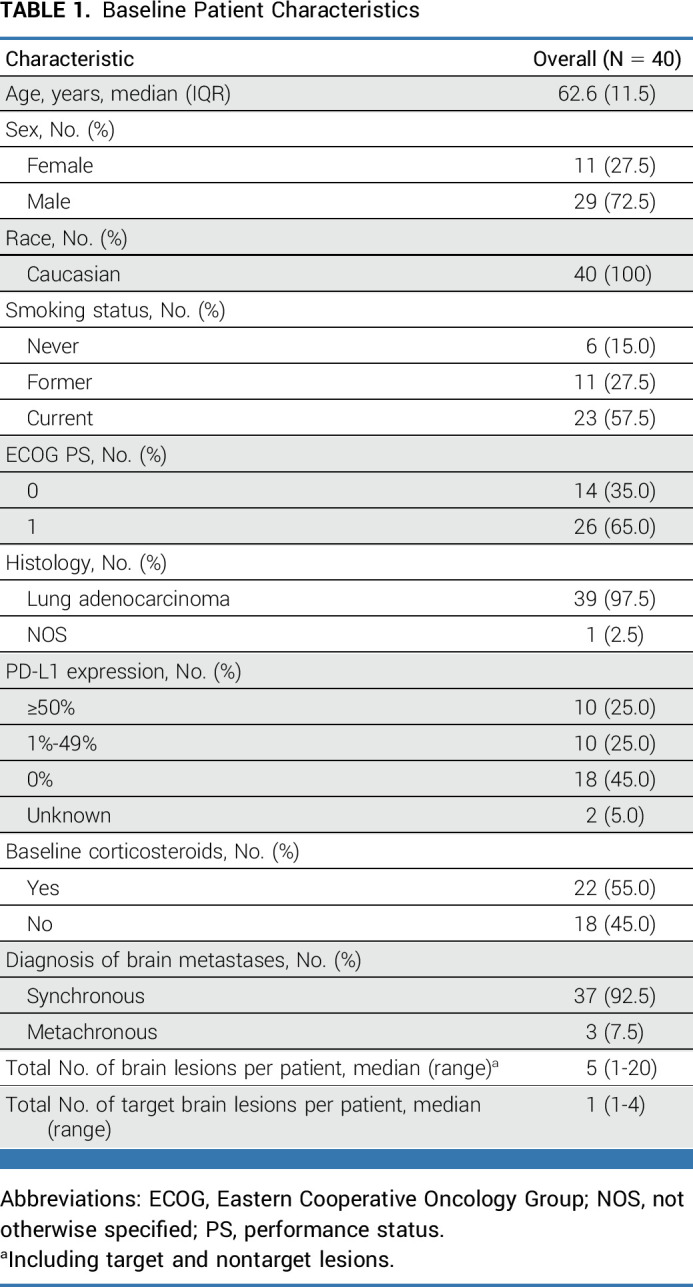
Study enrollment was completed between November 2018 and December 2019. Forty-three patients were registered, but 40 patients met eligibility criteria and were enrolled at 13 sites (Fig 1; Data Supplement [Table S1]). Baseline patient characteristics are shown in Table 1. The median age was 62.5 years. Twenty-nine (72.5%) patients were male and most had a smoking history (85.0%). Brain metastases were diagnosed concurrently with lung cancer in most patients (92.5%). PD-L1 expression was ≥1% in 20 patients (50.0%), negative in 18 patients (45.0%), and unknown in two patients (5.0%). The median total number of brain metastases per patient was 5, ranging from 1 to 20. The median size of the sum of all target lesions per patient was 13 mm, ranging from 10 to 42 mm. Twenty-two (55.0%) patients were receiving dexamethasone during the week before inclusion in the study to control neurologic symptoms with continued administration throughout the study, regardless of pemetrexed premedication. Sixteen patients were receiving 4 mg of dexamethasone once daily while six patients received <4 mg once daily.
FIG 1.
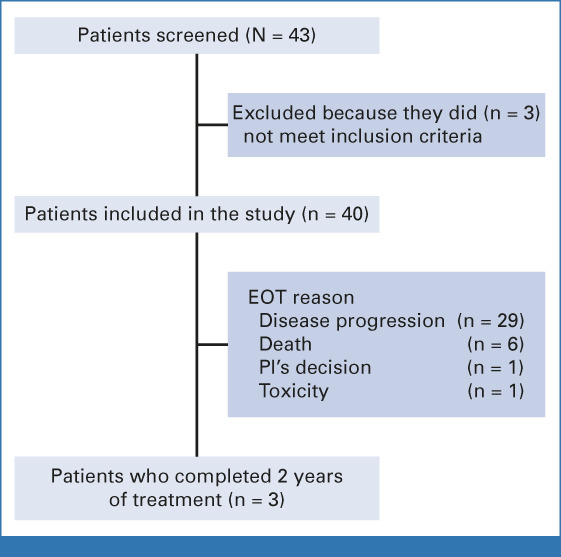
Study flowchart. EOT, end of treatment; PI, principal investigator.
TABLE 1.
Baseline Patient Characteristics
Efficacy
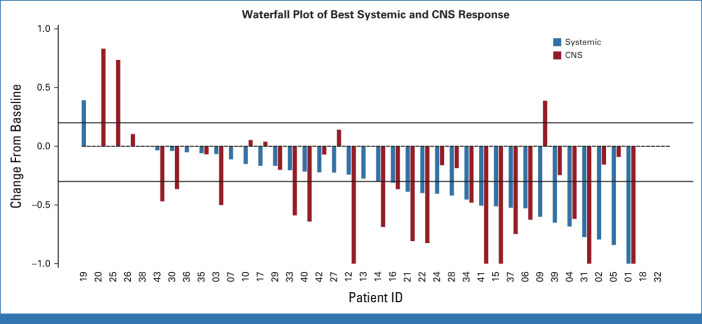
The study was completed since the boundaries for futility or unacceptable toxicity were not reached (NR) at any moment. The overall PFS rate at 12 weeks was 62.2% (95% CrI, 47.1 to 76.2; Data Supplement [Fig S3]), above the expected 50%. Seventeen patients (42.7%; 95% CrI, 28.1 to 57.9; Table 2) had a confirmed intracranial response (12 [70.6%] PRs and 5 [29.4%] complete responses [CRs]), while 17 patients had stable disease (SD) in the brain and five had progressive disease (PD). The median time to intracranial response was 82 days and the median DOR in the brain among patients with CNS response was 14 months (95% CI, 10 to NR; Data Supplement [Fig S4]). Eighteen patients (45%; 95% CrI, 28.1 to 57.9; Table 2) achieved a systemic response (17 [94%] PRs and 1 [6%] CR), while 16 patients achieved SD and four patients had PD. Most responses were concordant in the brain and in the body, except for 6 (15%) patients (Fig 2). Three patients had systemic progression with SD in the brain and three patients had intracranial progression with PR or SD in the body. In an exploratory analysis, the intracranial ORR was similar among patients receiving corticosteroids (50%) compared with those who did not receive them (38.9%). The systemic ORR was also consistent between patients who were being treated (52.6%) or not with corticosteroids (44.4%). No differences were observed in the intracranial or systemic ORR according to PD-L1 expression (Data Supplement [Fig S5]).
TABLE 2.
Efficacy Results
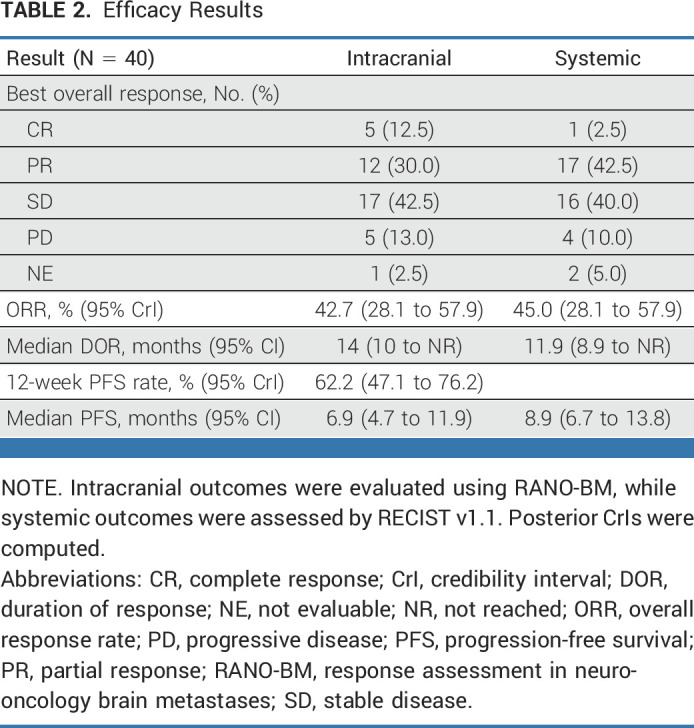
FIG 2.
Waterfall plot of best ORR according to RECIST version 1.1 (depicted by the red bars) and RANO-BM (depicted by blue bars). Two patients are not shown because of on-treatment assessments not being available. ORR, overall response rate; RANO-BM, response assessment in neuro-oncology brain metastases.
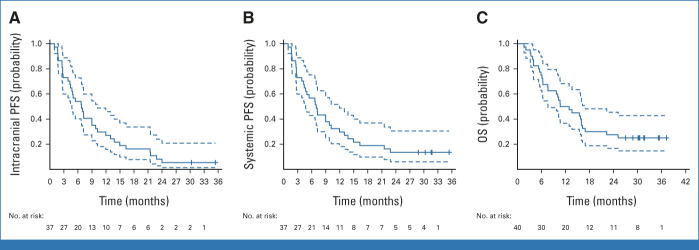
With a minimum follow-up of 27 months, the median intracranial PFS was 6.9 months (95% CI, 4.7 to 11.9; Fig 3A) and the median systemic PFS was 8.9 months (95% CI, 6.7 to 13.8; Fig 3B). The median OS was 11.8 months (95% CI, 7.6 to 16.9; Fig 3C). Estimated 1- and 2-year OS rates were 50% (95% CI, 36.7 to 68.2) and 27.5% (95% CI, 16.6 to 45.5), respectively. No significant differences were observed in OS according to PD-L1 expression and baseline corticosteroids (Data Supplement [Fig S6]). Nevertheless, the 2-year OS rate for patients with PD-L1 ≥1% was 40.0% (95% CI, 23.4 to 68.4), while in patients with PD-L1 <1%, it was 16.7% (95% CI, 5.9 to 46.8).
FIG 3.
Kaplan-Meier plots of (A) intracranial PFS according to RANO-BM and (B) systemic PFS according to RECIST v1.1. (C) Kaplan-Meier plot of OS. Dotted lines indicate 95% CI. OS, overall survival; PFS, progression-free survival; RANO-BM, response assessment in neuro-oncology brain metastases.
Treatment at Intracranial Progression
During the study, 24 patients (60%) received brain radiotherapy because of PD. Sixteen patients received WBRT and eight patients received SRS. Median time to brain radiotherapy was 10.9 months (95% CI, 7.8 to 15.9; Data Supplement [Fig S7]). Thirteen patients died before receiving brain radiotherapy mainly because of complications related with extracranial progression. Median EFS, defined as the time from inclusion to brain radiotherapy or death, was 7.6 months (95% CI, 5.5 to 10.9).
Toxicity
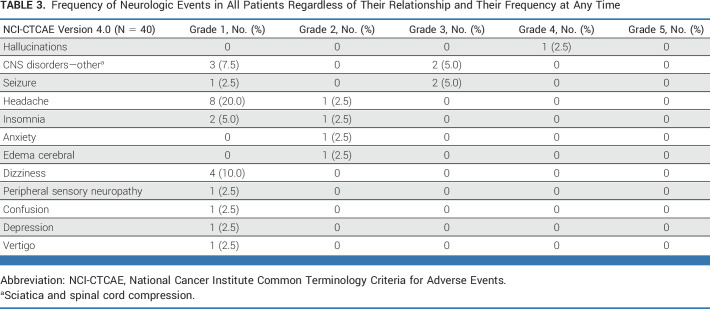
The median number of cycles of carboplatin was four and of atezolizumab and pemetrexed was 8; three patients completed the planned 2 years of treatment. The rate of grade 3-4 toxicity during the first 9 weeks was 27.5%, below the predefined boundary of 35%. No grade 5 adverse events were reported during the first 9 weeks. All neurologic adverse events, irrespective of causality to systemic therapy and time of onset, are shown in Table 3. Most neurologic adverse events were grade 1 and 2, and only five patients (12.5%) experienced grade 3-4 neurologic toxicity consisting of grade 4 hallucinations in one patient, grade 3 seizure in two patients, and grade 3 sciatica and spinal cord compression in one patient each. The median time to the appearance of treatment-related neurologic toxicity was NR and the median time to the appearance of any neurologic toxicity was 15.7 months (Data Supplement [Figs S8A and S8B]). Global health status and overall QOL scores were maintained throughout the study (Data Supplement [Figs S9A and S9B]).
TABLE 3.
Frequency of Neurologic Events in All Patients Regardless of Their Relationship and Their Frequency at Any Time
Twenty-eight patients (70%) had grade 3-4 treatment-related adverse events, but only 7 (17.5%) were deemed serious: acute kidney injury and pneumonitis in two patients each, while nephritis, pulmonary embolism, and febrile neutropenia were seen in one patient each (Data Supplement [Table S2]). One patient experienced grade 5 toxicity, consisting of febrile neutropenia and sepsis, that was considered related to chemotherapy.
DISCUSSION
The results of this phase II clinical trial show that patients with advanced NSCLC with untreated brain metastases can benefit from initiating systemic treatment with atezolizumab combined with chemotherapy. Previous exploratory analysis from pivotal phase III clinical trials have demonstrated that patients with brain metastases benefited from the addition of immunotherapy to chemotherapy versus chemotherapy alone. However, these reports were based on subgroup analyses with significant attrition since most studies excluded patients with untreated brain lesions or receiving corticosteroids or were not designed to evaluate the efficacy outcomes in the CNS.
To our knowledge, this is the first study centered on this special population that evaluates the activity and safety of a PD-L1 inhibitor combined with platinum-based chemotherapy in patients with NSCLC with untreated brain metastases. This phase II trial has a Bayesian sequential design to minimize the risk of harm or futility on this vulnerable population. Forty patients were included, and the study was successfully completed. Some patients had significant disease burden on the basis of the total number of brain metastases and half of the patients were receiving corticosteroids at baseline. The patients received atezolizumab combined with carboplatin and pemetrexed, which was previously evaluated in the IMpower132 trial in advanced nonsquamous NSCLC; however, this study excluded patients with untreated brain metastases and those requiring corticosteroids.14
We assessed efficacy outcomes in the brain using RANO-BM to allow future cross-trial comparisons.11 In our study, chemoimmunotherapy yielded similar ORR in the brain and in the body, and responses were highly concordant in both compartments. Chemoimmunotherapy led to a complete intracranial response in five patients. Brain pseudoprogression is uncommon but has been reported with single immunotherapy in a large NSCLC cohort.15 In our study, we did not observe any case of pseudoprogression. An exploratory analysis did not show significant correlation between intracranial response and PD-L1 expression or corticosteroids treatment at baseline.
In our study, the median intracranial PFS by RANO-BM was 6.9 months, consistent with previous PFS reported in the pooled analysis of Keynote-021G, -189, and -407 trials evaluating pembrolizumab plus chemotherapy.9 This exploratory analysis included a subset of patients with untreated brain metastases but did not report the intracranial efficacy, and PFS was exclusively measured by RECIST v1.1. In an exploratory analysis of the CheckMate-9LA trial, the combination of two cycles of chemotherapy plus nivolumab and ipilimumab yielded a promising median intracranial PFS of 13.5 months in patients with previously treated and stable brain metastases, without corticosteroids and asymptomatic.10 However, this study population was highly selected and cannot be directly compared with our study, which is enriched with more vulnerable patients. Nevertheless, in our study, the 2-year OS was 27.5% in the overall population and 40% in patients with PD-L1 expression ≥1%, similar to that reported in the seminal study of pembrolizumab enrolling patients with PD-L1–positive NSCLC with untreated brain metastases.8 Because of the limited statistical power of our study, it is not possible to determine whether the baseline corticosteroids have an effect on ORR or OS.
Most patients who progressed in the brain were considered candidates for salvage brain radiotherapy, predominantly consisting of WBRT, although some were treated with SRS. The greater use of WBRT was attributed to regional practice trends and to a high number of brain metastases in certain patients. Median time to brain radiotherapy was 10.9 months. In our study, we observed that systemic therapy allowed for the deferral of WBRT without a decline in QOL.16 However, when considering the initiation of initiating systemic therapy and the deferral of brain-directed local therapy in patients with lung cancer, it is important to discuss within a multidisciplinary tumor board that includes radiation oncologists and neurosurgeons.6
Most neurologic adverse events were mild or moderate, except four patients who had grade 3 and 1 with grade 4 neurologic events that fully recovered. Treatment-related serious adverse events were observed only in 17.5% and there was only one patient with grade 5 toxicity related to chemotherapy. This safety profile is consistent with the previously published data using this treatment combination.14
The major limitation of the study is the single-arm design, which precludes us from establishing the optimal treatment strategy for patients with NSCLC and synchronous brain metastases. Randomized clinical trials would help to understand how to integrate brain local therapy with immunotherapy or chemoimmunotherapy in this patient population. Another limitation is the relatively small sample size, which reduces the statistical power to conduct subgroup analyses.
In summary, atezolizumab combined with carboplatin and pemetrexed has activity in patients with treatment-naïve advanced nonsquamous NSCLC with untreated brain metastases. Systemic and intracranial efficacy was similar, thus highlighting that atezolizumab plus chemotherapy could be a therapeutic option in this highly vulnerable population of patients. Future studies targeting additional immune checkpoints or integrating systemic therapies with stereotactic radiotherapy to improve the control rate and minimize the risk of brain toxicity are warranted.
ACKNOWLEDGMENT
Copyediting editorial support was provided by Aurora O'Brate. The authors are grateful to Cristina Muñoz-Pinedo and Joaquim Moreno from the Bellvitge Biomedical Research Institute (IDIBELL) for storing the patients' samples collected during the study. The authors thank the CERCA Program/Generalitat de Catalunya for institutional support and La Marató de TV3, project number 201929-30, for the funding support.
Ernest Nadal
Consulting or Advisory Role: MSD, Bristol Myers Squibb, Roche, Boehringer Ingelheim, Pfizer, Takeda, AstraZeneca, Lilly, Amgen, Bayer, Sanofi, Merck Serono, Janssen Oncology, Qiagen, Pierre Fabre
Research Funding: Roche (Inst), Pfizer (Inst), Roche (Inst), Bristol Myers Squibb (Inst), Merck Serono (Inst)
Travel, Accommodations, Expenses: MSD, Bristol Myers Squibb, Pfizer, Roche, Lilly
Delvys Rodríguez-Abreu
Consulting or Advisory Role: Roche, Bristol Myers Squibb, MSD, AstraZeneca Spain, Novartis
Speakers' Bureau: Roche, Bristol Myers Squibb, MSD
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb, MSD, Novartis
Marta Simó
Speakers' Bureau: Aventik Medial, Solti
Bartomeu Massutí
Consulting or Advisory Role: Roche, Boehringer Ingelheim, AstraZeneca, Merck Serono, Janssen
Speakers' Bureau: Roche, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Sanofi/Regeneron, Janssen Oncology, Pfizer
Travel, Accommodations, Expenses: Roche, MSD Oncology, AstraZeneca
Oscar Juan
Honoraria: Bristol Myers Squibb, Roche/Genentech, MSD Oncology, AstraZeneca/MedImmune, Takeda
Consulting or Advisory Role: Bristol Myers Squibb, Lilly, Takeda, AstraZeneca Spain, Janssen Oncology
Research Funding: AstraZeneca Spain (Inst)
Travel, Accommodations, Expenses: Takeda, AstraZeneca/MedImmune
Rafael López
Honoraria: Roche, Takeda, AstraZeneca Spain, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Roche, Novartis, Boehringer Ingelheim, AstraZeneca Spain, MSD Oncology
Research Funding: Roche (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: Roche, Novartis, Boehringer Ingelheim, MSD, Takeda
Javier De Castro
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp and Dohme, Roche, Pfizer, Takeda, GlaxoSmithKline, Janssen Oncology, Sanofi, Bayer, Lilly, Amgen
Travel, Accommodations, Expenses: AstraZeneca Spain, Merck Sharp & Dohme, Roche
Anna Estival
Consulting or Advisory Role: GlaxoSmithKline, Roche, MSD Oncology
Speakers' Bureau: PharmaMar, Roche, MSD Oncology, AstraZeneca Spain, Pfizer, Bristol Myers Squibb Foundation/Janssen, Takeda, GlaxoSmithKline
Travel, Accommodations, Expenses: Roche, Pfizer, Lilly, MSD Oncology, BMSi
Ivana Sullivan
Consulting or Advisory Role: Takeda, Roche, Sanofi, Novartis, Bristol Myers Squibb Spain
Speakers' Bureau: Roche, Merck Sharp & Dohme, AstraZeneca Spain, Bristol Myers Squibb Spain, Pfizer
Travel, Accommodations, Expenses: Roche, Takeda, Pfizer, AstraZeneca Spain, Bristol Myers Squibb Spain, Lilly
Enriqueta Felip
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Roche, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Turning Point Therapeutics, Daiichi Sankyo
Speakers' Bureau: Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, Merck Sharp & Dohme, Peervoice, Pfizer, Sanofi, Takeda, Touch Oncology
Travel, Accommodations, Expenses: AstraZeneca, Janssen, Roche
Other Relationship: Grifols
Uncompensated Relationships: Member of the Scientific Advisory Committee -Hospital Universitari Parc Taulí, SEOM (Sociedad Española de Oncología Médica), President from 2021-2023, “ETOP IBCSG Partners” Member of the Scientific Committee
Ana Blasco
Honoraria: Sanofi/Aventis, Janssen Oncology, Takeda, AstraZeneca Spain, Bristol Myers Squibb Foundation, Pfizer
Consulting or Advisory Role: Amgen, Mylan, AstraZeneca Spain
Travel, Accommodations, Expenses: Janssen Oncology
Maria Guirado
Speakers' Bureau: Roche/Genentech, MSD Oncology, Takeda, Bristol Myers Squibb/Medarex
Travel, Accommodations, Expenses: Roche/Genentech, MSD Oncology
Noelia Vilariño
Consulting or Advisory Role: Boehringer Ingelheim
Expert Testimony: Roche/Genentech, MSD Oncology, Boehringer Ingelheim
Travel, Accommodations, Expenses: Roche/Genentech, Boehringer Ingelheim
Valentín Navarro
Employment: Institut Català d'Oncologia
Jordi Bruna
Honoraria: Takeda
Travel, Accommodations, Expenses: Novocure, Eisai Europe, CSL Behring
No other potential conflicts of interest were reported.
See accompanying Editorial, p. 4462
DISCLAIMER
The content is solely the responsibility of the authors. Roche had no role in data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and provided approval to submit the manuscript for publication. The corresponding author had final responsibility for the decision to submit for publication.
PRIOR PRESENTATION
Presented in part at the World Congress of Lung Cancer, virtual, September 8-14, 2021, and the ASCO annual meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by Roche, sponsored and conducted through the Spanish Lung Cancer Group (GECP).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.02561.
AUTHOR CONTRIBUTIONS
Conception and design: Ernest Nadal, Marta Simó, Valentín Navarro, Jordi Bruna
Provision of study materials or patients: Ernest Nadal, Delvys Rodríguez-Abreu, Bartomeu Massutí, Oscar Juan, Gerardo Huidobro, Javier De Castro, Anna Estival, Ivana Sullivan, Enriqueta Felip, Ana Blasco, Maria Guirado
Collection and assembly of data: Ernest Nadal, Marta Simó, Bartomeu Massutí, Oscar Juan, Gerardo Huidobro, Javier De Castro, Anna Estival, Joaquín Mosquera, Ivana Sullivan, Enriqueta Felip, Ana Blasco, Maria Guirado, Eva Pereira, Noelia Vilariño, Valentín Navarro, Jordi Bruna
Data analysis and interpretation: Ernest Nadal, Delvys Rodríguez-Abreu, Marta Simó, Rafael López, Noelia Vilariño, Valentín Navarro, Jordi Bruna
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Trial of Atezolizumab Combined With Carboplatin and Pemetrexed for Patients With Advanced Nonsquamous Non–Small-Cell Lung Cancer With Untreated Brain Metastases (Atezo-Brain, GECP17/05)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ernest Nadal
Consulting or Advisory Role: MSD, Bristol Myers Squibb, Roche, Boehringer Ingelheim, Pfizer, Takeda, AstraZeneca, Lilly, Amgen, Bayer, Sanofi, Merck Serono, Janssen Oncology, Qiagen, Pierre Fabre
Research Funding: Roche (Inst), Pfizer (Inst), Roche (Inst), Bristol Myers Squibb (Inst), Merck Serono (Inst)
Travel, Accommodations, Expenses: MSD, Bristol Myers Squibb, Pfizer, Roche, Lilly
Delvys Rodríguez-Abreu
Consulting or Advisory Role: Roche, Bristol Myers Squibb, MSD, AstraZeneca Spain, Novartis
Speakers' Bureau: Roche, Bristol Myers Squibb, MSD
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb, MSD, Novartis
Marta Simó
Speakers' Bureau: Aventik Medial, Solti
Bartomeu Massutí
Consulting or Advisory Role: Roche, Boehringer Ingelheim, AstraZeneca, Merck Serono, Janssen
Speakers' Bureau: Roche, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Sanofi/Regeneron, Janssen Oncology, Pfizer
Travel, Accommodations, Expenses: Roche, MSD Oncology, AstraZeneca
Oscar Juan
Honoraria: Bristol Myers Squibb, Roche/Genentech, MSD Oncology, AstraZeneca/MedImmune, Takeda
Consulting or Advisory Role: Bristol Myers Squibb, Lilly, Takeda, AstraZeneca Spain, Janssen Oncology
Research Funding: AstraZeneca Spain (Inst)
Travel, Accommodations, Expenses: Takeda, AstraZeneca/MedImmune
Rafael López
Honoraria: Roche, Takeda, AstraZeneca Spain, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Roche, Novartis, Boehringer Ingelheim, AstraZeneca Spain, MSD Oncology
Research Funding: Roche (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: Roche, Novartis, Boehringer Ingelheim, MSD, Takeda
Javier De Castro
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp and Dohme, Roche, Pfizer, Takeda, GlaxoSmithKline, Janssen Oncology, Sanofi, Bayer, Lilly, Amgen
Travel, Accommodations, Expenses: AstraZeneca Spain, Merck Sharp & Dohme, Roche
Anna Estival
Consulting or Advisory Role: GlaxoSmithKline, Roche, MSD Oncology
Speakers' Bureau: PharmaMar, Roche, MSD Oncology, AstraZeneca Spain, Pfizer, Bristol Myers Squibb Foundation/Janssen, Takeda, GlaxoSmithKline
Travel, Accommodations, Expenses: Roche, Pfizer, Lilly, MSD Oncology, BMSi
Ivana Sullivan
Consulting or Advisory Role: Takeda, Roche, Sanofi, Novartis, Bristol Myers Squibb Spain
Speakers' Bureau: Roche, Merck Sharp & Dohme, AstraZeneca Spain, Bristol Myers Squibb Spain, Pfizer
Travel, Accommodations, Expenses: Roche, Takeda, Pfizer, AstraZeneca Spain, Bristol Myers Squibb Spain, Lilly
Enriqueta Felip
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Roche, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Turning Point Therapeutics, Daiichi Sankyo
Speakers' Bureau: Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, Merck Sharp & Dohme, Peervoice, Pfizer, Sanofi, Takeda, Touch Oncology
Travel, Accommodations, Expenses: AstraZeneca, Janssen, Roche
Other Relationship: Grifols
Uncompensated Relationships: Member of the Scientific Advisory Committee -Hospital Universitari Parc Taulí, SEOM (Sociedad Española de Oncología Médica), President from 2021-2023, “ETOP IBCSG Partners” Member of the Scientific Committee
Ana Blasco
Honoraria: Sanofi/Aventis, Janssen Oncology, Takeda, AstraZeneca Spain, Bristol Myers Squibb Foundation, Pfizer
Consulting or Advisory Role: Amgen, Mylan, AstraZeneca Spain
Travel, Accommodations, Expenses: Janssen Oncology
Maria Guirado
Speakers' Bureau: Roche/Genentech, MSD Oncology, Takeda, Bristol Myers Squibb/Medarex
Travel, Accommodations, Expenses: Roche/Genentech, MSD Oncology
Noelia Vilariño
Consulting or Advisory Role: Boehringer Ingelheim
Expert Testimony: Roche/Genentech, MSD Oncology, Boehringer Ingelheim
Travel, Accommodations, Expenses: Roche/Genentech, Boehringer Ingelheim
Valentín Navarro
Employment: Institut Català d'Oncologia
Jordi Bruna
Honoraria: Takeda
Travel, Accommodations, Expenses: Novocure, Eisai Europe, CSL Behring
No other potential conflicts of interest were reported.
REFERENCES
- 1.Waqar SN, Samson PP, Robinson CG, et al. : Non-small-cell lung cancer with brain metastasis at presentation. Clin Lung Cancer 19:e373-e379, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Brown PD: The diminishing role of whole-brain radiation therapy in the treatment of brain metastases. JAMA Oncol 3:1023-1024, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Mulvenna P, Nankivell M, Barton R, et al. : Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet 388:2004-2014, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown PD, Jaeckle K, Ballman KV, et al. : Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA 316:401-409, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang EL, Wefel JS, Hess KR, et al. : Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol 10:1037-1044, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Vogelbaum MA, Brown PD, Messersmith H, et al. : Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol 40:492-516, 2022 [DOI] [PubMed] [Google Scholar]
- 7.Vilarino N, Bruna J, Bosch-Barrera J, et al. : Immunotherapy in NSCLC patients with brain metastases. Understanding brain tumor microenvironment and dissecting outcomes from immune checkpoint blockade in the clinic. Cancer Treat Rev 89:102067, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Goldberg SB, Schalper KA, Gettinger SN, et al. : Pembrolizumab for management of patients with NSCLC and brain metastases: Long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol 21:655-663, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell SF, Rodriguez-Abreu D, Langer CJ, et al. : Outcomes with pembrolizumab plus platinum-based chemotherapy for patients with NSCLC and stable brain metastases: Pooled analysis of KEYNOTE-021, -189, and -407. J Thorac Oncol 16:1883-1892, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Carbone D, Ciuleanu T, Cobo M, et al. : OA09.01 first-line nivolumab + ipilimumab + chemo in patients with advanced NSCLC and brain metastases: Results from CheckMate 9LA. J Thorac Oncol 16:S862, 2021 [Google Scholar]
- 11.Lin NU, Lee EQ, Aoyama H, et al. : Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol 16:e270-e278, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Thall PF, Simon RM, Estey EH: New statistical strategy for monitoring safety and efficacy in single-arm clinical trials. J Clin Oncol 14:296-303, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Thall PF, Sung HG: Some extensions and applications of a Bayesian strategy for monitoring multiple outcomes in clinical trials. Stat Med 17:1563-1580, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Nishio M, Barlesi F, West H, et al. : Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: Results from the randomized Phase 3 IMpower132 trial. J Thorac Oncol 16:653-664, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Hendriks LEL, Henon C, Auclin E, et al. : Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thorac Oncol 14:1244-1254, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Parsons MW, Dietrich J: Assessment and management of cognitive symptoms in patients with brain tumors. Am Soc Clin Oncol Educ Book 41:e90-e99, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.02561.