Abstract
PURPOSE
The optimal neoadjuvant treatment for resectable carcinoma of the thoracic esophagus (TE) or gastroesophageal junction (GEJ) remains a matter of debate. We performed an individual participant data (IPD) network meta-analysis (NMA) of randomized controlled trials (RCTs) to study the effect of chemotherapy or chemoradiotherapy, with a focus on tumor location and histology subgroups.
PATIENTS AND METHODS
All, published or unpublished, RCTs closed to accrual before December 31, 2015 and having compared at least two of the following strategies were eligible: upfront surgery (S), chemotherapy followed by surgery (CS), and chemoradiotherapy followed by surgery (CRS). All analyses were conducted on IPD obtained from investigators. The primary end point was overall survival (OS). The IPD-NMA was analyzed by a one-step mixed-effect Cox model adjusted for age, sex, tumor location, and histology. The NMA was registered in PROSPERO (CRD42018107158).
RESULTS
IPD were obtained for 26 of 35 RCTs (4,985 of 5,807 patients) corresponding to 12 comparisons for CS-S, 12 for CRS-S, and four for CRS-CS. CS and CRS led to increased OS when compared with S with hazard ratio (HR) = 0.86 (0.75 to 0.99), P = .03 and HR = 0.77 (0.68 to 0.87), P < .001 respectively. The NMA comparison of CRS versus CS for OS gave a HR of 0.90 (0.74 to 1.09), P = .27 (consistency P = .26, heterogeneity P = .0038). For CS versus S, a larger effect on OS was observed for GEJ versus TE tumors (P = .036). For the CRS versus S and CRS versus CS, a larger effect on OS was observed for women (P = .003, .012, respectively).
CONCLUSION
Neoadjuvant chemotherapy and chemoradiotherapy were consistently better than S alone across histology, but with some variation in the magnitude of treatment effect by sex for CRS and tumor location for CS. A strong OS difference between CS and CRS was not identified.
INTRODUCTION
Esophageal carcinoma is one of the most aggressive neoplasias of the digestive tract with 544,076 deaths for 604,100 new cases each year.1 In locally advanced stage, defined as T2 or more (any N) or N1 or more (any T) both with M0, upfront surgery (S) is not recommended anymore.2,3 Multimodal treatment combining chemotherapy followed by surgery (CS) or chemoradiotherapy followed by surgery (CRS) has been separately shown to be superior to S alone.4,5 Yet, few randomized controlled trials (RCTs) comparing these two strategies are readily available and the optimal neoadjuvant treatments therefore remain a matter of debate. Moreover, in the era of precision medicine, esophageal carcinoma is considered as a heterogeneous entity encompassing two histologic subtypes: adenocarcinoma (AC) and squamous cell carcinoma (SCC), and two anatomic locations: thoracic esophagus (TE) and gastroesophageal junction (GEJ). Available RCTs were not powered to study the interaction between these characteristics and the treatment effect. Available meta-analyses were based on aggregated data, and/or did not include all available trial and/or did not performed network meta-analysis (NMA) and thus were not able to answer all questions5-7 (Data Supplement, online only).
CONTEXT
Key Objective
Do neoadjuvant chemotherapy and chemoradiotherapy have a differential effect on survival for esophageal or gastroesophageal junction (GEJ) carcinoma and, in particular, across histology, location, and sex?
Knowledge Generated
Neoadjuvant chemotherapy and chemoradiotherapy were equally effective in adenocarcinoma and squamous cell carcinoma. Variations in treatment efficacy were observed with the tumor location for neoadjuvant chemotherapy and with sex for chemoradiotherapy.
Relevance (A.H. Ko)
-
This network meta-analysis confirms a benefit for either chemotherapy or chemoradiotherapy in the neoadjuvant setting for esophageal and GEJ carcinomas. The incremental benefit conferred by the addition of radiation to chemotherapy in this context appears to be quite modest, a decision that can potentially be informed by specific clinical features.*
*Relevance section written by JCO Associate Editor Andrew H. Ko, MD, FASCO.
NMA is an extension to standard (pairwise) meta-analysis (MA) of use where more than two therapeutic options are available.8 It allows information arising from direct (head to head) and indirect (via a common comparator) comparisons to be combined. Furthermore, individual participant data (IPD) meta-analysis9 is considered to be one of the most effective ways to study interactions between treatment effects and potential modifiers, and IPD-NMA has been proposed to study these interactions across several treatments in the network.10
The MANATEC-02 Collaborative Group recently reported the update of the IPD-MA on CS versus S comparion.4 Similarly, since our previous IPD-MA on the CRS versus S comparison,11 new RCTs and updated information on previous RCTs became available. Moreover, additional trials comparing CS with CRS have been reported. The purpose of this study was to update the information on the CRS versus S comparison and also gather IPD from RCT on the CRS versus CS comparison to perform an IPD-NMA of the neoadjuvant treatments of carcinoma of the TE and GEJ, with two goals: better specify the CRS versus CS comparison through a combination of direct and indirect evidences and perform a subgroup analysis for the other comparison.
PATIENTS AND METHODS
The Protocol (online only) was made a priori, and the meta-analysis was registered in Prospero with number CRD42018107158. The study was approved by the French National Commission of Informatics and Liberty in January 2019.
Eligibility Criteria
There were no language restrictions, and both published and unpublished trials were eligible. Trials had to have been closed to accrual before December 31, 2015, and use a method of random assignment preventing the knowledge of the future treatment arm. The RCT had to have randomly assigned patients with either TE or GEJ tumors. Trials, which have included esophageal and gastric carcinoma, were also eligible, but only the patients with TE or GEJ tumor location were included. Trials had to have randomly assigned patients with surgically resectable SCC or AC without distant metastasis.
Trials had to have compared at least two of the following sequences: upfront S, CS, or CRS. While the smaller trials included in the previous meta-analyses were not excluded, newly identified trials having randomly assigned <60 patients were not eligible for the main analysis.
Study Identification Strategy
The same strategy used in our recent IPD-MA of CS versus S was used.4 Briefly, the searches covered three electronic databases (PubMed, Web of Science, and Scopus), two trial registries (ClinicalTrials.gov and Cochrane Central Register of Controlled Trials), and five conference proceedings (ASCO, American Society of Therapeutic Radiation Oncology, European Society of Medical Oncology, European Society for Radiotherapy and Oncology, and European Cancer Conference Organization; Data Supplement).
Data Collection Process and Checking
The investigators of trials included in the previous MA were asked for updated survival information, whereas investigators of newly identified trials were asked to participate in the IPD-MA. Available data were checked and reanalyzed to identify potential errors or discrepancies between the received data and the associated publication. A standardized protocol, which follows the recommendations of the Cochrane Individual Participant Data Meta-analysis Methods Group12 and PRISMA IPD,13 was used to reduce risk of bias (Data Supplement).
Studied Items and End Points
The primary end point was overall survival (OS), defined as the time from random assignment to the date of death due to any cause. Patients lost to follow-up or alive on the date of last follow-up were censored.
Disease-free survival (DFS) was defined as the time until death because of any cause or any recurrence. The pattern of failure was categorized as (1) local recurrence, (2) distant or synchronous local and distant recurrence, and (3) death without recurrence. For all these secondary end points, a landmark set at 6 months after random assignment was used to allow for differences in the time to S in each arm (Data Supplement).
Other end points were the quality of the surgical resection (defined as an R0 pathologic resection), postoperative mortality (defined as any death in the first 30 postoperative days), and postoperative complications defined as severe if requiring a reintervention or medically treated but life-threatening.
Statistical Analysis
All analyses were performed according to the intention-to-treat principle. The IPD-NMA was based on a frequentist, one-step model. Contrary to the two-step approach, all parameters (treatment effects and interactions) are estimated together by a single model.14 Models for each outcome had an random intercept to account for the clustering of patients within trials (Data Supplement). P values were not adjusted for multiple comparisons.
Heterogeneity was tested as proposed by Rücker15 with a generalization of Cochran's test called the Q test. The within-design heterogeneity corresponds to the variation in treatment effects for a single comparison. The between-design heterogeneity corresponds to the discrepancies between the treatment effect estimated by direct and indirect comparison. Inconsistency (ie, the discrepancy between direct and indirect estimations of the treatment effect) was evaluated by three methods: node-splitting, Q statistics, and an IPD based approach (Data Supplement).
For the time-to-event end points a mixed-effect Cox model was used. For the binomial end point, a generalized linear mixed-effects model with a logarithmic link function and binomial distribution of the residual was used to obtain risk ratio (RR). In the case of nonconvergence, a logistic link function was used instead giving odds ratio. The variable was coded so that a RR > 1 indicates a greater probability of having a noncurative resection and having a morbidity and death.
A competing risk approach was used to study the effects on patterns of relapse, as well as an analysis according to cancer and noncancer death following the method of Peto (Data Supplement).
The survival benefit was also estimated by the difference in Restricted Mean Survival Time (dRMST) at 5 years (Data Supplement).
Preplanned subgroup analyses investigated how treatment effects varied with age, sex, histology, tumor location, and T and N from the TNM. All these analyses were performed by introducing in the model a treatment × covariate interaction term (Data Supplement). Available data were insufficient to estimate the treatment effect according to the T and N from the TNM.
NMA can be biased or exhibit inconsistency if prognostic covariates are not balanced between arms.16 Adjustment for the covariates and the introduction of interaction can lower this bias. Therefore, models were adjusted for age, sex, histology, and anatomic location. Missing data for these covariates were handled by multiple imputation (Data Supplement).
Several sensitivity analyses were performed. The first used an IPD two-step model as proposed by Rücker (Data Supplement). The second used a model without covariate adjustment and missing data imputation. The protocol also prespecified sensitivity analysis excluding clearly outlying trials and small trials from the initial meta-analysis (<60 patients). Finally, an unplanned sensitivity analysis was added to investigate the impact of the exclusion of RCTs either too small (<60 patients) or without IPD. A two-step model, combining IPD and aggregate date from the publications, was used (Data Supplement).
Role of the Funding Source
The funding source had no role in the study design, collection, analysis, and interpretation of the data, in the writing the report, and in the decision to submit for publication.
RESULTS
Characteristics of Trials and Patients
We identified 35 eligible trials, including 10 new trials. Among the 25 previously identified trials, 17 provided IPD and five provided updated survival data. We were unable to contact the investigators of nine trials including one new trial (822 patients, 14.2% of the eligible population). In total, 26 trials (4,985 patients) were available for the primary end point analysis of OS (Figs 1 and 2).
FIG 1.
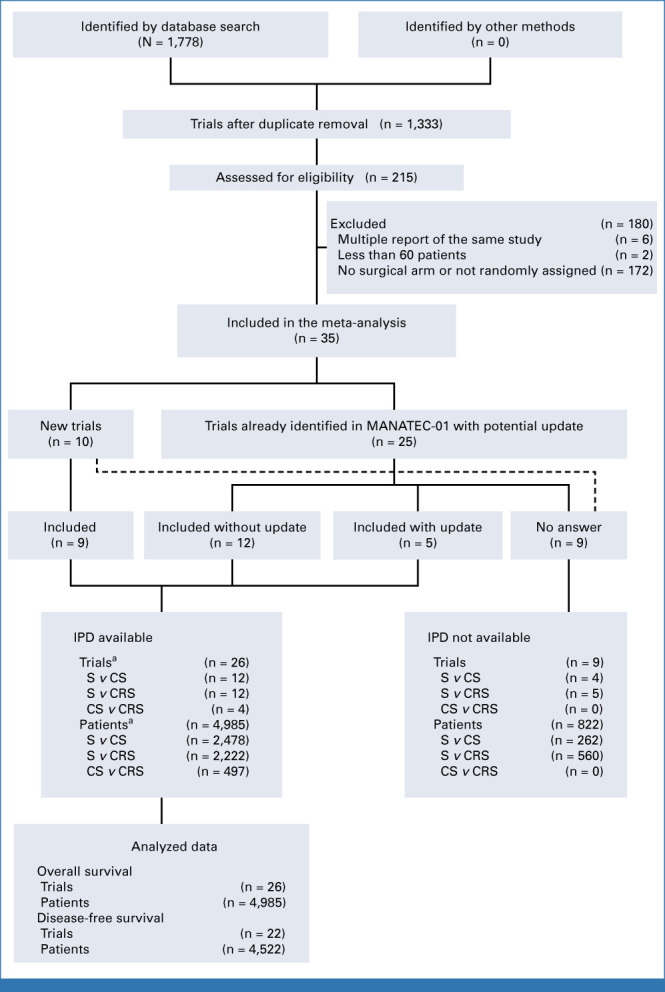
PRISMA flowchart. aOne trial with 2 × 2 factorial design contributing to the three comparisons. CRS, chemoradiotherapy followed by surgery; CS, chemotherapy followed by surgery; IPD, individual participant data; S, surgery.
FIG 2.
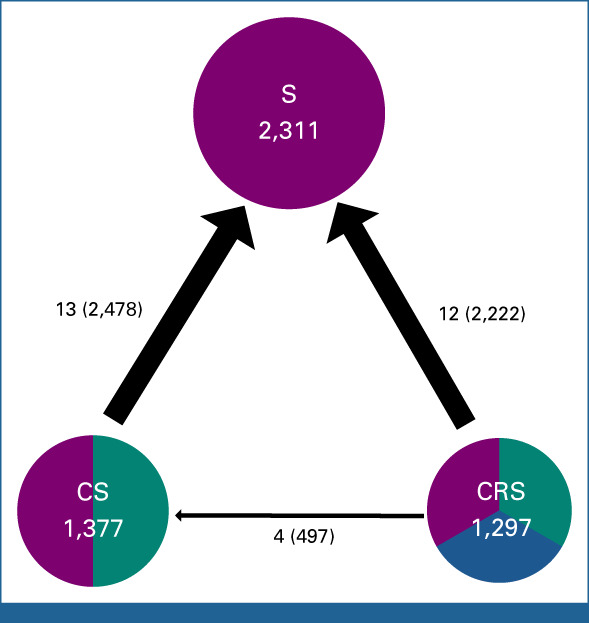
MANATEC-02 network. The numbers above the arrows describe the comparison. The first numbers correspond to the number of trials (or contrasts for multiarm trials) for each comparison, and the second between parentheses to the number of patients. The direction of the arrow indicates the arbitrary-chosen direction of the hazard ratio. One multiarm trial (Scandinavia) provided two estimates (contrasts) for CS-S, one from CRS-RS and one from CS-S, and therefore, the numbers above the arrows can sum up to more than the number within the circles that gives the number of patients randomly assigned to receive the corresponding treatment. CRS, chemoradiotherapy followed by surgery; CS, chemotherapy followed by surgery; RS, radiotherapy followed by surgery; S, surgery.
Sixteen trials comparing CS with S were identified.17-33 IPD were available for 12 trials (90% of eligible patients). All used a platinum-based chemotherapy associated with bleomycin, fluorouracil (5-FU) or epirubicin (Data Supplement). Seventeen trials comparing CRS with S were identified.27,34-53 IPD were available for 12 trials (80% of eligible patients). Radiotherapy delivered 35-50.4 Gy in 10-23 fractions. Concomitant chemotherapies were mostly platinum based with 5-FU (Data Supplement). Four trials comparing CRS with CS were identified.27,54-56 IPD were available for all of them (100%). Radiotherapy was delivered at a dose of 30-40 Gy in 15-20 fractions. Concomitant chemotherapies were mostly cisplatin-5-FU (Data Supplement).
Included patients were mostly men, 4,039 (81%) with a mean age of 61 years (IQR, 54-67). There were 2,743 (55%) SCCs and 2,179 ACs (44%). Tumors were mainly located in the TE, 3,788 (85%). T from the TNM was not available for 2,574 patients (52% of the total), and N from the TNM was missing for 2,908 patients (58%). There were <15% missing data for sex, histologic subtype, age, and anatomic location, which were included as adjustment factors in the models (Data Supplement).
Patients' characteristics were broadly similar across the three comparisons (Data Supplement). The mean age was 62 years (IQR, 54-68) for S versus CS, 59 years (IQR, 53-65) for S versus CRS, and 63 years (IQR, 56-68). There were 80%, 82%, and 86% men in the CS versus S, CRS versus S, and CRS versus CS comparisons, respectively. However, the S versus CRS trials had a higher proportion of SCC (66%) followed by S versus CS (53%) and CS versus CRS (16%). S versus CRS trials also had a higher proportion of tumors located in the TE (93%) followed by CRS versus CS (86%) and CS versus S (78%).
Treatment Effect on Overall Survival
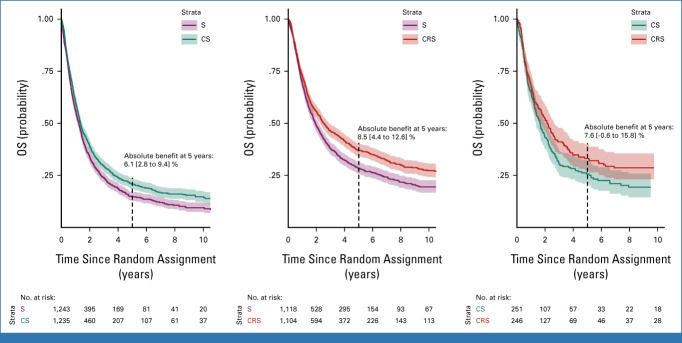
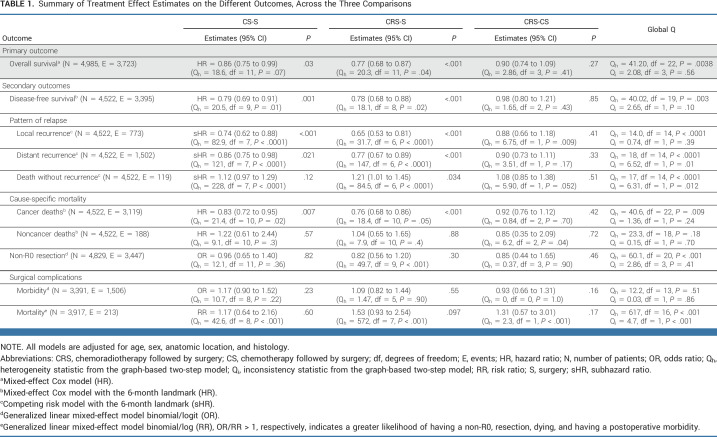
After a median follow-up of 6.2 years (95% CI, 6.0 to 6.4), 3,723 deaths were observed (Fig 3). In the adjusted model, the hazard ratios (HRs) were 0.86 (95% CI, 0.75 to 0.99; P = .03) for CS-S, 0.77 (0.68 to 0.87; P < .001) for CRS-S, and 0.90 (0.74 to 1.09; P = .27) for CRS-CS (Table 1). There was heterogeneity but no inconsistency (Data Supplement). On an absolute scale, the dRMST at 5 years was 3.4 (0.8 to 5.9) months for CS versus S, 4.3 (1.7 to 6.8) months for CRS versus S, and 0.9 (−2.3 to 4.2) months for CRS versus CS.
FIG 3.
Kaplan-Meier estimated overall survival in the trials comparing S with CS (left), CRS with S (middle), and CRS with CS (right). The shaded area corresponds to the 95% CI. Absolute benefits are differences in overall survival rates at 5 years. CRS, chemoradiotherapy followed by surgery; CS, chemotherapy followed by surgery; OS, overall survival; S, surgery.
TABLE 1.
Summary of Treatment Effect Estimates on the Different Outcomes, Across the Three Comparisons
The main subgroup analyses are reported in Figure 4. When age was modeled as a continuous variable, it was not a significant treatment modifier for any comparison (Pinteraction = .98, .71, and .76 for CS-S, CRS-S, and CRS-CS, respectively, Data Supplement). A statistically greater treatment effect in women than men was observed for the comparison of CRS-S (HR = 0.56 [0.44 to 0.71] v HR = 0.82 [0.73 to 0.93], Pinteraction = .003) and CRS-CS (HR = 0.63 [0.44 to 0.89] v HR = 0.99 [0.81 to 1.20], Pinteraction = .012) but not CS-S (Pinteraction = .51; Fig 4). There was no strong evidence that histologic subtype was a treatment effect modifier for either comparison (Fig 4 and Data Supplement). A statistically greater treatment effect was seen for tumors located at the GEJ for the comparison of CS-S (HR = 0.68 [0.54 to 0.86] v HR = 0.88 [0.77 to 1.02], Pinteraction = .036), but not for CRS-S (Pinteraction = .13), although point estimates are quite similar, nor CRS-CS (Pinteraction = .78; Fig 4).
FIG 4.
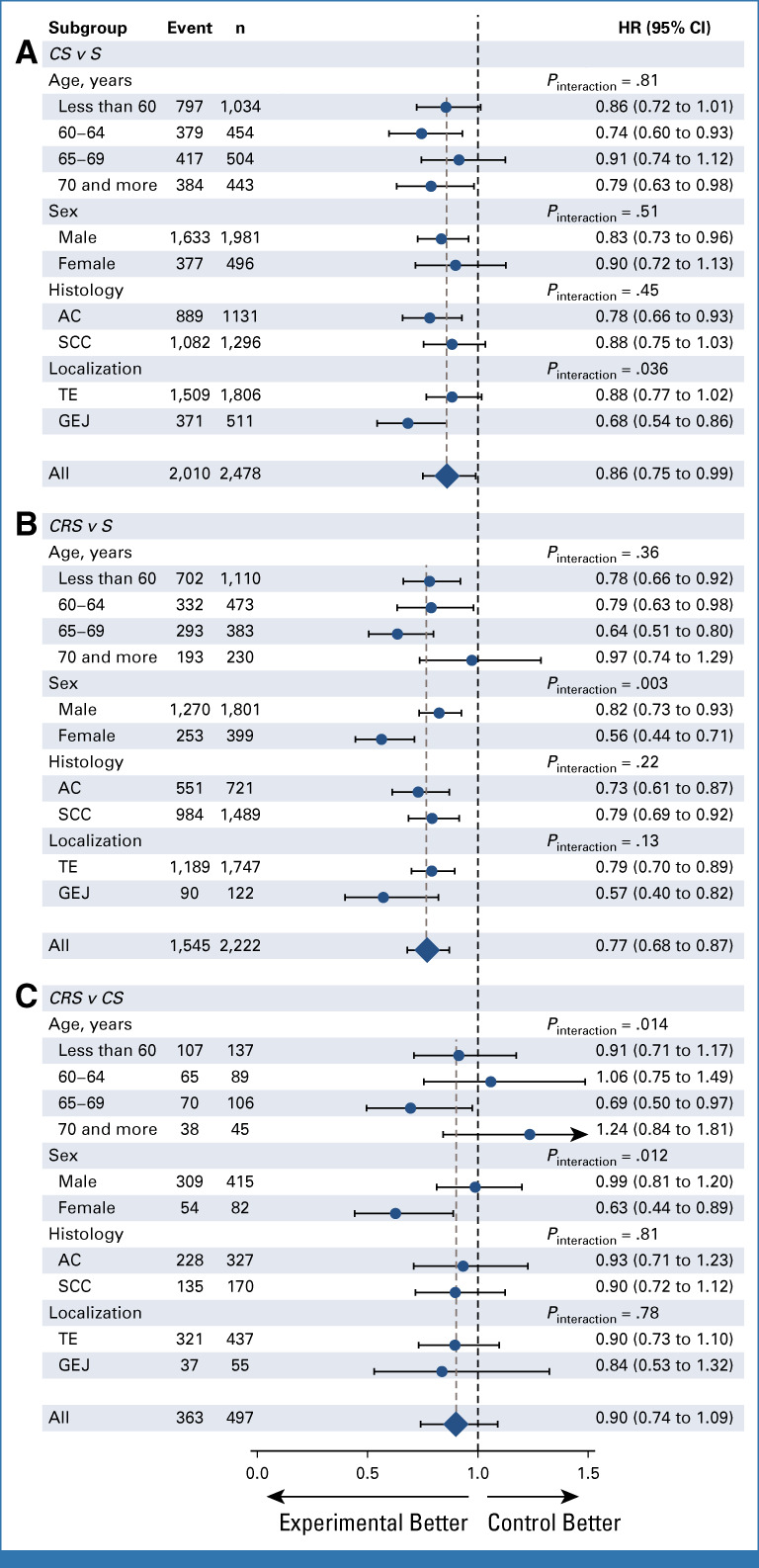
Forest plot of the treatment effect on overall survival according to prespecified subgroups for (A) CS-S, (B) CRS-S, and (C) CRS-CS comparison. Number of patients (n) and number of events (event) are those from the complete case population of the direct comparisons, whereas HR and P values are from the full adjusted models. AC, adenocarcinoma; CRS, chemoradiotherapy followed by surgery; CS, chemotherapy followed by surgery; GEJ, gastroesophageal junction; HR, hazard ratio; S, surgery; SCC, squamous cell carcinoma; TE, thoracic esophagus.
Treatment Effect on Secondary End Points
The treatment effects on secondary end points are summarized in Table 1. During follow-up, 3,395 DFS events were observed, with 1,145 occurring before the 6-month landmark. In the adjusted models, the overall HRs were 0.79 (0.69 to 0.91) for the CS-S comparison, 0.78 (0.68 to 0.88) for CRS-S comparison, and 0.98 (0.80 to 1.21) for CRS-CS comparison. In the full NMA model, there was some evidence for heterogeneity, but less for inconsistency (Data Supplement). Subgroup analyses provided similar results to those for OS (Fig 5).
FIG 5.
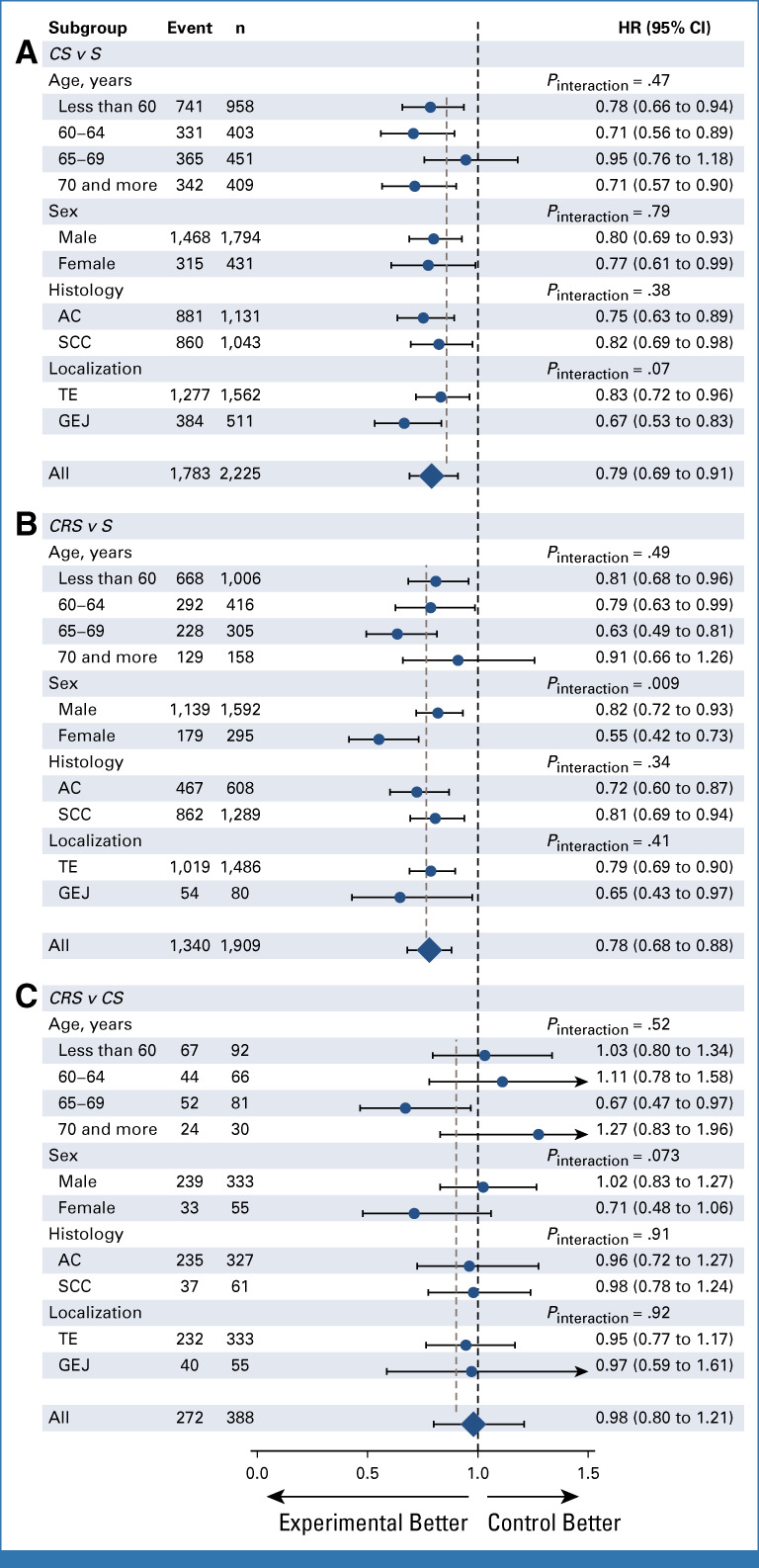
Forest plot of the treatment effect on disease-free survival according to prespecified subgroups for (A) CS-S, (B) CRS-S, and (C) CRS-CS comparison. Number of patients (n) and number of events (event) are those from the complete case population of the direct comparisons, whereas HR and P values are from the full adjusted models. AC, adenocarcinoma; CRS, chemoradiotherapy followed by surgery; CS, chemotherapy followed by surgery; GEJ, gastroesophageal junction; HR, hazard ratio; S, surgery; SCC, squamous cell carcinoma; TE, thoracic esophagus.
Of the 4,522 patients, 773 (17%) had a local recurrence, 1,054 (23%) a distant recurrence, 448 (10%) a combined distant and local recurrence, and 1,119 (25%) a death without recurrence and 1,128 (25%) were alive without recurrence (Data Supplement). Both CS and CRS lead to lower rates of local and distant recurrences when compared with S. When compared with CS, CRS did not significantly lower the risk of local or distant recurrence. CRS led to a significant increase in deaths without recurrence as compared with S (sub HR = 1.21 [1.01 to 1.45]). There was some evidence of heterogeneity for local recurrence, distant recurrence, and death without recurrence and of inconsistency for the latter two.
Information was available for postoperative morbidity in 3,391 patients (missing for 13 trials) and for mortality in 3,917 (missing for nine trials). Morbidity was not significantly influenced by neoadjuvant treatment (P = .23, .55, and .16 for CS-CS, CRS-S, and CRS-CS respectively). Two hundred and thirteen patients (5.4%) died in the postoperative period. No strong differences were observed for the postoperative mortality rates across the comparisons, with some sign of heterogeneity and inconsistency.
Sensitivity analyses are fully detailed in the Data Supplement; they did not substantially alter the main results.
DISCUSSION
To our knowledge, in this first IPD-NMA on preoperative treatment for locally advanced carcinoma of the esophagus and GEJ, we provide clear evidence that the multimodal strategies are superior to upfront S in terms of OS, DFS, local and distant relapse, and cancer death, but not the rate of complete R0 resection. There was no clear difference in the effect on OS for the two options most used today (CRS and CS), but there were a limited number of patients in the direct comparison (n = 497). Surprisingly, no strong benefits were seen on either DFS or the risk of local recurrence for CRS versus CS.
The true novelty of our work was the ability, given by the IPD, to investigate treatment effect modifiers. Beforehand, the histological subtype was thought to be the most likely effect modifier as AC and SCC are thought to be two very different tumors, but no strong difference in treatment effect by histology interaction was seen in any comparison, suggesting that CS and CRS are more or less equally effective in AC and SCC. By contrast, the anatomic location effect of CS over S was greater for tumors located at the GEJ even after adjustment for histologic subtype. There was less evidence for such an interaction in the CRS versus S comparison, but this analysis relied on few patients with a GEJ tumor and point estimates are rather similar. There was no strong evidence in favor of age being a treatment effect modifier. Surprisingly, sex was shown to be the modifier of the chemoradiotherapy effect with a greater efficacy for women than men. The same interaction was not seen in the CS versus S comparison. Yet, women represented only 23% of the population, which can have an influence on these results. The latter unexpected subgroup generates a hypothesis that needs confirmation in other studies. In a 2019 ESMO Workshop,57 gender medicine was acknowledged as an underevaluated topic in oncology. A given explanation, among others, is that trials are rarely powered to detect differences in treatment effect across sex. This workshop identified IPD-MA/NMA as the methodology most adapted to detect such differences. For instance, in a previous IPD-MA from our group for head and neck cancer,58 sex was also identified as a treatment effect modifier, thanks to the MA. In other malignancies, similar findings have even led to trial design in which men and women were given different dosages.59
Several trials are currently ongoing to compare the CS and CRS strategy, TOPGEAR60 (ACTRN12609000035224), CMISG170161 (ClinicalTrials.gov identifier: NCT03001596), ESOPEC62 (ClinicalTrials identifier: NCT02509286), Neo-AEGIS63 (ClinicalTrials identifier: NCT01726452), and NExT (JCOG 1109, UMIN000009482). Primary results of Neo-AEGIS were presented at ASCO annual meeting 202164 and ASCO GI 2023 (Rapid abstract 295). This European RCT randomly assigned 377 patients with AC of the esophagus to receive either a CS approach on the basis of the MAGIC and then FLOT regimen65 or a CRS approach as used in the CROSS trial.45 Preliminary results did not show superiority of the CRS approach in OS (HR = 1.03 [0.77 to 1.38]). Initial results of the NExT trial were presented at ASCO GI 2022.66 This international RCT randomly assigned 601 patients with SCC of the esophagus in three arms: (1) doublet CS: cisplatin-5-FU and then S, (2) triplet CS: docetaxel-cisaplatin-5-FU and then S, and (3) CRS: cisplatin-5-FU plus 41.4 Gy/23 fraction and then S. When compared with the doublet regimen, CRS led to an estimated HR of 0.84 ([0.63 to 1.12], P = .12) in OS. The triplet regimen was better than the doublet (HR = 0.68 [0.50 to 0.92], P = .006), and the triplet versus CRS comparison was not reported. The preliminary results of these two trials are in accordance with ours, suggesting that if there is a survival benefit of the CRS approach compared with CS, it is likely to be small. Yet, these trials have been powered to detect a HR of 0.7 (from 0.645 for CMISG1701 to 0.76 for NExT), which seems quite large given that the lower bound of our 95% CI for this comparison was 0.74 and the trialist could therefore consider a sample size increase. Other trialists have chosen to combine chemotherapy and chemoradiotherapy before S of gastric and GEJ carcinoma.67 Of note, all the ongoing trials restricted their population to either AC or SCC, and therefore, no more data for the treatment interaction with histologic subtype will be available by analyzing them separately. Our data can provide some hints as to why CRS does not provide an important OS treatment effect compared with CS. As distant recurrences were more frequent than local ones, priority should be given to the control of the systemic disease in patient with locally advanced tumors. Death without relapse was also more frequent than local recurrence, illustrating the importance of treating care of comorbidities sharing the same risk factors (obesity, alcohol, tobacco, etc).
A lot of effort is now spent to integrating new therapies in the strategy. Targeted therapies for estimated glomerular filtration rate or vascular endothelial growth factor pathways have been tested in advanced disease68-73 but failed to demonstrate a significant survival benefit. More recently, a phase 3 trial74 evaluated the addition of pembrolizumab to a chemotherapy backbone of 5-FU and cisplatin for the first-line treatment of advanced disease. A significant gain in OS (HR, 0.73; 95% CI, 0.62 to 0.86) was observed. In another phase 3 study75 comparing nivolumab with placebo in the adjuvant setting after CRS, a gain in DFS was observed HR = 0.69 (96.4% CI, 0.56 to 0.86; P < .001). These two studies suggest that checkpoint inhibitor might have a key role to play in the near future.
In this work, we choose to use a frequentist, single-step multilevel model to evaluate treatment effects and treatment by covariate interactions in the NMA. A recent study from our group16 demonstrated that this approach is appropriate when the treatment effect modifier is not evenly distributed across comparisons, which was the case here.
Several limitations of our work should be acknowledged. First, our network was not well balanced for the CRS versus CS comparison. There were 12 times more patients in the indirect comparisons than the direct ones, and the indirect comparisons came from mainly older trials. Since it is considered that four patients in an indirect comparison give as much information as the one in a direct one, we had three times more information coming from the indirect comparison. Yet, even if direct and indirect comparison did not provide strictly similar results, we found no strong evidence of inconsistency in the network. Moreover, the inconsistency approach by IPD revealed that a part of the visible inconsistency may be related to a different distribution of confounders. Another limitation is that not all end points should be considered equal. Our primary end point of OS is a robust end point, unlikely to be affected by differences in centers and across time. The same may not be true for DFS, which can be affected by how postoperative surveillance is performed and what methods were available. Morbidities and the rate of R0 procedures should be interpreted with the greatest caution, as contemporary classification and standardization were not usual during the time frame of the included trials, and large variability in absolute rates across trials was observed. Yet, even if the absolute risk is subject to caution, the relative risk may be of interest given that patients in both arms were evaluated in the same way inside a trial. Another limitation is that our study expands over a long time period. Yet, the statistical model we used allows for a variation of the baseline risk for each trial (random intercept) and a variation in treatment effect (random slope) taking this phenomenon into account. Although more recent treatments are thought to be more effective, we did not observe a clear trend in the treatment effects over time (Data Supplement). Moreover, although the most recent regimen (FLOT)65 has been shown to be superior for gastric cancers, little is known for esophageal cancers, and in the metastatic setting, there is no clear evidence that the addition of taxanes to FOLFOX should become the standard of care for these tumors. Another limitation was that T staging and N staging are prognostic factors and potential treatment effect modifiers, but we had too many missing data to examine this. Toxicities were also seldom available, and when they were, often, they were not graded. Thus, formal statistical comparisons between CS and CRS were not possible for that end point.
The ongoing RCTs may be added when available to the NMA following the principle of the living network meta-analysis.76-78 This would have the advantage to strengthen the direct CRS versus CS comparison. New treatment modalities like the promising immunotherapy may be added too if they are compared with a node already existing in the network.
In conclusion, this IPD-NMA shows clearly that both CS and CRS improve OS and DFS compared with S, without raising the risk of postoperative mortality or morbidities. CRS does not appear to be superior to CS, and any potential benefit is most likely small. Treatment effects were consistent across histology subtypes, but the CRS seemed to be more beneficial for women and CS seemed to be more beneficial for GEJ tumors, which, however, needs confirmation in further trials.
ACKNOWLEDGMENT
The project was initiated by Gustave Roussy, France; see Appendix 1 (online only) for the full list of members.
APPENDIX 1. MANATEC-02 Collaborative Group
MANATEC-02: Individual patient data Meta-Analysis of chemotherapy or chemoradiotherapy as NeoAdjuvant Treatment of Esophageal or gastro esophageal junction Carcinoma
Project Management Group
Matthieu Faron, Armel Maurice Cheugoua-Zanetsie, Jean Pierre Pignon, Pierre Blanchard, Michel Ducreux, Stefan Michiels
Advisory Board
Pierre Thirion, Jayne Tierney, Val Gebski, Bryan H. Burmeister, Xavier Paoletti, Johanna Van Sandick, Jianhua Fu
Investigators
Chanvitan Apinop (Prince of Songkla University, Songkla, Thailand), Anne Arezina (Alliance for Clinical Trial in Oncology, Chicago, IL), Emilie Barbier (Fédération Francophone de Cancérologie Digestive, Dijon, France), Gary Bass (Connolly Hospital Blanchardstown, Dublin, Ireland), Pierre Blanchard (Gustave Roussy, Villejuif, France), Jurjen Boonstra (Erasmus University Medical Center, Rotterdam, the Netherlands), Jean-François Bosset (Chru Jean Minjoz, Besançon, France), Bryan H. Burmeister (Princess Alexandra, Brisbane, Australia), Armel Maurice Cheugoua-Zanetsie (Gustave Roussy, Villejuif, France), David Cunningham (Royal Marsden Hospital, London, United Kingdom), Michel Ducreux (Gustave Roussy, Villejuif, France), Matthieu Faron (Gustave Roussy, Villejuif, France), Jianhua Fu (Sun Yat-Sen University Cancer Center, Guangzhou, China), Val Gebski (Nhmrc, Sydney, Australia), D. J. Girling (MRC, London, United Kingdom), David Kelsen (Memorial Sloan Kettering Cancer Center, New York, NY), Fredrik Klevebro (Karolinska University Hospital, Stockholm, Sweden), Simon Law (Queen Mary Hospital, Hong-Kong SAR, China), Elisabeth Le Prise (Centre Eugene Marquis, Rennes, France), Florian Lordick (Technische University, München, Germany), Tanaphon Maipang (Prince of Songkla University, Songkla, Thailand), Murielle Mauer (EORTC, Brussel, Belgium), Stefan Michiels (Gustave Roussy, Villejuif, France), Jennifer Moughan (NRG Oncology Statistics Data Management Center, Philadelphia, PA), Matthew Nankivell (MRC, London, United Kingdom), Knut Nygaard (Aker University Hospital, Oslo, Norway), Patrick Owens (Saint Luke's Hospital, Dublin, Ireland), Xavier Paoletti (Institut Curie, Paris, France), Guillaume Piessen (CHU de Lille, Lille, France), Jean Pierre Pignon (Gustave Roussy, Villejuif, France), Jean-Luc Raoul (Centre Eugene Marquis, Rennes, France), Jack Roth (MD Anderson, Houston, TX), Alberto Ruol (Universita Degli Studi di Padova, Padova, Italia), Peter Schlag (Association Surgical Oncology der Deutschen, Germany), Christoph Schumacher (Technische University München, Munich, Germany), Joel Shapiro (Erasmus University Medical Center, Rotterdam, the Netherlands), B. Mark Smithers (University of Queensland, Brisbane, Australia), Michael Stahl (Evang. Kliniken Essen-Mitte, Essen, Germany), Joel Tepper (University of North Carolina School of Medicine, Chapel Hill, NC), Pierre Thirion (St Luke, Dublin, Ireland), Janine M. Thomas (Princess Alexandra Hospital, Woolloongabba, Australia), Jayne Tierney (MRC, London, United Kingdom), Susan Urba (University of Michigan Medical Center, Ann Arbor, MI), Michele Valmasoni (Padova University Hospital, Padova, Italy), Ate van der Gaast (Erasmus, Rotterdam, the Netherlands), Johanna van Sandick (The Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, the Netherlands), Danièle Williaume (Centre Eugène Marquis, Rennes, France), Kathryn Winter (NRG Oncology Statistics and Data Management Center, Philadelphia, PA), John Wong (Queen Mary Hospital, University of Hong Kong, Hong Kong, China), Hong Yang (Sun Yat-Sen University Cancer Center, Guangzhou, China), Marc Ychou (Val d’Aurelles, Montpellier, France)
Matthieu Faron
Travel, Accommodations, Expenses: Vifor Pharma, PharmaMar, Ipsen
Pierre Thirion
Honoraria: Janssen
Dewi Vernerey
Consulting or Advisory Role: OSE Immunotherapeutics, Janssen-Cilag, HalioDx, Pfizer, CellProthera, GERCOR, INCYTE, Fondazione Smith Kline, INVECTYS, AC BioScience, Veracyte
Travel, Accommodations, Expenses: MSD
Guillaume Piessen
Consulting or Advisory Role: Bristol Myers Squibb, Nestle Health Science, MSD Oncology, Astellas Pharma
Travel, Accommodations, Expenses: Medtronic
Uncompensated Relationships: Medtronic
Magnus Nilsson
Travel, Accommodations, Expenses: Medtronic
Marc Ychou
Honoraria: Bayer
David Cunningham
Stock and Other Ownership Interests: OVIBIO
Consulting or Advisory Role: OVIBIO
Research Funding: MedImmune (Inst), Bayer (Inst), 4SC (Inst), Clovis Oncology (Inst), Lilly (Inst), Roche (Inst), Leap Oncology (Inst)
Michael Stahl
Honoraria: Bristol Myers Squibb/Medarex, MSD Oncology, Novartis/Pfizer, Roche/Genentech
Consulting or Advisory Role: BMS GmbH & Co KG, MSD Oncology, Lilly/ImClone
Florian Lordick
Honoraria: Lilly, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Elsevier, BioNTech, SERVIER, Merck KGaA, Roche, Medscape, Incyte, Art Tempi, Medupdate, Streamed Up!, Daiichi Sankyo Europe GmbH, Novartis, Falk Foundation
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, Astellas Pharma, Zymeworks, Amgen, Daichi Sankyo, Novartis
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Lilly
Joel Tepper
Consulting or Advisory Role: EMD Serono
Other Relationship: Elsevier
Jack Roth
Stock and Other Ownership Interests: Genprex
Consulting or Advisory Role: Genprex
Research Funding: Genprex, Varian Medical Systems
Patents, Royalties, Other Intellectual Property: Patents licensed by Genprex
Bryan Burmeister
Employment: GenesisCare Oncology
Research Funding: Regeneron (Inst)
Travel, Accommodations, Expenses: GenesisCare Oncology
Xavier Paoletti
Honoraria: MSD Oncology
Consulting or Advisory Role: MSD Oncology, Daiichi Sankyo
Speakers' Bureau: Ipsen
Michel Ducreux
Employment: Sandoz
Honoraria: Bayer, TERUMO, Pierre Fabre, Roche/Genentech
Consulting or Advisory Role: Roche, Merck Serono, Servier, Amgen, Novartis, Ipsen, Lilly, Pierre Fabre, HalioDx, Daiichi Sankyo/Astra Zeneca, AstraZeneca, Basilea, Bayer, GlaxoSmithKline, MSD, Rafael Pharmaceuticals, Sotio, Zymeworks
Speakers' Bureau: Roche, Merck KGaA, Bayer, SERVIER, Amgen, Pierre Fabre, AstraZeneca, HalioDx, GlaxoSmithKline, Lilly, MSD
Research Funding: Roche (Inst), Keocyt (Inst)
Travel, Accommodations, Expenses: Roche, Merck Serono, Bayer, Pierre Fabre, SERVIER
Stefan Michiels
Consulting or Advisory Role: Sensorion, Biophytis, SERVIER, Yuhan, Roche, Kedrion Biopharma, IQVIA
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the European Society for Medical Oncology GI World Congress, Barcelona, Spain, June 29-July 2, 2022.
SUPPORT
Supported by the French government Programme Hospitalier de Recherche Clinique en Cancérologie (PHRC-K18-187).
AUTHOR CONTRIBUTIONS
Conception and design: Matthieu Faron, Maurice Cheugoua-Zanetsie, Jayne Tierney, Marc Ychou, Val Gebski, Johanna van Sandick, Jean-Pierre Pignon, Michel Ducreux, Stefan Michiels
Financial support: Jean-Pierre Pignon
Administrative support: Jean-Pierre Pignon
Provision of study materials or patients: Matthew Nankivell, Hong Yang, Joel Shapiro, B. Mark Smithers, Thomas Walsh, Guillaume Piessen, Jurjen Boonstra, Marc Ychou, Simon Law, David Cunningham, Florent de Vathaire, Susan Urba, Michele Valmasoni, Danièle Williaume, Florian Lordick, Joel Tepper, Jack Roth, Val Gebski, Bryan Burmeister, Jianhua Fu
Collection and assembly of data: Matthieu Faron, Maurice Cheugoua-Zanetsie, Matthew Nankivell, Kathryn Winter, Hong Yang, Joel Shapiro, Dewi Vernerey, B. Mark Smithers, Thomas Walsh, Guillaume Piessen, Magnus Nilsson, Marc Ychou, Simon Law, Florent de Vathaire, Michael Stahl, Michele Valmasoni, Danièle Williaume, Janine Thomas, Joel Tepper, Jack Roth, Bryan Burmeister, Jianhua Fu, Jean-Pierre Pignon, Stefan Michiels
Data analysis and interpretation: Matthieu Faron, Maurice Cheugoua-Zanetsie, Pierre Thirion, Hong Yang, Magnus Nilsson, Jurjen Boonstra, David Cunningham, Michael Stahl, Susan Urba, Florian Lordick, Joel Tepper, Xavier Paoletti, Jean-Pierre Pignon, Michel Ducreux, Stefan Michiels
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Individual Participant Data Network Meta-Analysis of Neoadjuvant Chemotherapy or Chemoradiotherapy in Esophageal or Gastroesophageal Junction Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Matthieu Faron
Travel, Accommodations, Expenses: Vifor Pharma, PharmaMar, Ipsen
Pierre Thirion
Honoraria: Janssen
Dewi Vernerey
Consulting or Advisory Role: OSE Immunotherapeutics, Janssen-Cilag, HalioDx, Pfizer, CellProthera, GERCOR, INCYTE, Fondazione Smith Kline, INVECTYS, AC BioScience, Veracyte
Travel, Accommodations, Expenses: MSD
Guillaume Piessen
Consulting or Advisory Role: Bristol Myers Squibb, Nestle Health Science, MSD Oncology, Astellas Pharma
Travel, Accommodations, Expenses: Medtronic
Uncompensated Relationships: Medtronic
Magnus Nilsson
Travel, Accommodations, Expenses: Medtronic
Marc Ychou
Honoraria: Bayer
David Cunningham
Stock and Other Ownership Interests: OVIBIO
Consulting or Advisory Role: OVIBIO
Research Funding: MedImmune (Inst), Bayer (Inst), 4SC (Inst), Clovis Oncology (Inst), Lilly (Inst), Roche (Inst), Leap Oncology (Inst)
Michael Stahl
Honoraria: Bristol Myers Squibb/Medarex, MSD Oncology, Novartis/Pfizer, Roche/Genentech
Consulting or Advisory Role: BMS GmbH & Co KG, MSD Oncology, Lilly/ImClone
Florian Lordick
Honoraria: Lilly, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Elsevier, BioNTech, SERVIER, Merck KGaA, Roche, Medscape, Incyte, Art Tempi, Medupdate, Streamed Up!, Daiichi Sankyo Europe GmbH, Novartis, Falk Foundation
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, Astellas Pharma, Zymeworks, Amgen, Daichi Sankyo, Novartis
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Lilly
Joel Tepper
Consulting or Advisory Role: EMD Serono
Other Relationship: Elsevier
Jack Roth
Stock and Other Ownership Interests: Genprex
Consulting or Advisory Role: Genprex
Research Funding: Genprex, Varian Medical Systems
Patents, Royalties, Other Intellectual Property: Patents licensed by Genprex
Bryan Burmeister
Employment: GenesisCare Oncology
Research Funding: Regeneron (Inst)
Travel, Accommodations, Expenses: GenesisCare Oncology
Xavier Paoletti
Honoraria: MSD Oncology
Consulting or Advisory Role: MSD Oncology, Daiichi Sankyo
Speakers' Bureau: Ipsen
Michel Ducreux
Employment: Sandoz
Honoraria: Bayer, TERUMO, Pierre Fabre, Roche/Genentech
Consulting or Advisory Role: Roche, Merck Serono, Servier, Amgen, Novartis, Ipsen, Lilly, Pierre Fabre, HalioDx, Daiichi Sankyo/Astra Zeneca, AstraZeneca, Basilea, Bayer, GlaxoSmithKline, MSD, Rafael Pharmaceuticals, Sotio, Zymeworks
Speakers' Bureau: Roche, Merck KGaA, Bayer, SERVIER, Amgen, Pierre Fabre, AstraZeneca, HalioDx, GlaxoSmithKline, Lilly, MSD
Research Funding: Roche (Inst), Keocyt (Inst)
Travel, Accommodations, Expenses: Roche, Merck Serono, Bayer, Pierre Fabre, SERVIER
Stefan Michiels
Consulting or Advisory Role: Sensorion, Biophytis, SERVIER, Yuhan, Roche, Kedrion Biopharma, IQVIA
No other potential conflicts of interest were reported.
REFERENCES
- 1.World Health Organization . GLOBOCAN—Cancer Today. 2020. http://gco.iarc.fr/today/home [Google Scholar]
- 2.Obermannová R, Alsina M, Cervantes A, et al. : Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 33:992-1004, 2022 [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network : Esophageal and Esophagogastric Junction Cancers. NCCN. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1433 [DOI] [PubMed] [Google Scholar]
- 4.Faron M, Cheugoua-Zanetsie AM, Thirion P, et al. : Individual patient data meta-analysis of neoadjuvant chemotherapy followed by surgery versus upfront surgery for carcinoma of the oesophagus or the gastro-oesophageal junction. Eur J Cancer 157:278-290, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. : Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: Systematic review with meta-analysis combining individual patient and aggregate data. Eur J Cancer 49:3149-3158, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, Wang H, Luo G, et al. : A systematic review and network meta-analysis of neoadjuvant therapy combined with surgery for patients with resectable esophageal squamous cell carcinoma. Int J Surg 38:41-47, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Sjoquist KM, Burmeister BH, Smithers BM, et al. : Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol 12:681-692, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Lumley T: Network meta-analysis for indirect treatment comparisons. Stat Med 21:2313-2324, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Pignon JP, Hill C: Meta-analyses of randomised clinical trials in oncology. Lancet Oncol 2:475-482, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Riley RD, Dias S, Donegan S, et al. : Using individual participant data to improve network meta-analysis projects. BMJ Evid Based Med 28:197-203, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thirion P, Maillard E, Pignon J: Individual patient data-based meta-analysis assessing the effect of preoperative chemo-radiotherapy in resectable oesophageal carcinoma. Int J Radiat Oncol Biol Phys 72:S71-S72, 2008 [Google Scholar]
- 12.Tierney JF, Stewart LA, Clarke M, et al. : Individual participant data, in Cochrane Handbook for Systematic Reviews of Interventions. Oxford, UK, John Wiley & Sons, 2019, pp 643-658. https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119536604.ch26 [Google Scholar]
- 13.Stewart LA, Clarke M, Rovers M, et al. : Preferred reporting items for a systematic review and meta-analysis of individual participant data: The PRISMA-IPD statement. JAMA 313:1657, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Salanti G, Higgins JPT, Ades AE, et al. : Evaluation of networks of randomized trials. Stat Methods Med Res 17:279-301, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Rücker G: Network meta-analysis, electrical networks and graph theory. Res Synth Methods 3:312-324, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Faron M, Blanchard P, Ribassin-Majed L, et al. : A frequentist one-step model for a simple network meta-analysis of time-to-event data in presence of an effect modifier. PLoS One 16:e0259121, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allum WH, Stenning SP, Bancewicz J, et al. : Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27:5062-5067, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Ancona E, Ruol A, Santi S, et al. : Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: Final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer 91:2165-2174, 2001 [PubMed] [Google Scholar]
- 19.Baba M, Natsugoe S, Shimada M, et al. : Prospective evaluation of preoperative chemotherapy in resectable squamous cell carcinoma of the thoracic esophagus. Dis Esophagus 13:136-141, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Boonstra JJ, Kok TC, Wijnhoven BP, et al. : Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: Long-term results of a randomized controlled trial. BMC Cancer 11:181, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham D, Allum WH, Stenning SP, et al. : Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11-20, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Kelsen DP, Ginsberg R, Pajak TF, et al. : Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 339:1979-1984, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Kelsen DP, Winter KA, Gunderson LL, et al. : Long-term results of RTOG trial 8911 (USA Intergroup 113): A random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 25:3719-3725, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Law S, Fok M, Chow S, et al. : Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: A prospective randomized trial. J Thorac Cardiovasc Surg 114:210-217, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Maipang T, Vasinanukorn P, Petpichetchian C, et al. : Induction chemotherapy in the treatment of patients with carcinoma of the esophagus. J Surg Oncol 56:191-197, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Medical Research Council Oesophageal Cancer Working Group : Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet 359:1727-1733, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Nygaard K, Hagen S, Hansen HS, et al. : Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: A randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second scandinavian trial in esophageal cancer. World J Surg 16:1104-1109, 1992; discussion 1110 [DOI] [PubMed] [Google Scholar]
- 28.Roth JA, Pass HI, Flanagan MM, et al. : Randomized clinical trial of preoperative and postoperative adjuvant chemotherapy with cisplatin, vindesine, and bleomycin for carcinoma of the esophagus. J Thorac Cardiovasc Surg 96:242-248, 1988 [PubMed] [Google Scholar]
- 29.Schlag PM: Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. Arch Surg 127:1446-1450, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Schuhmacher C, Gretschel S, Lordick F, et al. : Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 28:5210-5218, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Ding T, Chang L: A randomized clinical study of preoperative chemotherapy for esophageal carcinoma. Zhonghua Zhong Liu Za Zhi 23:254-255, 2001 [PubMed] [Google Scholar]
- 32.Wang XL, Wu GX, Zhang MD, et al. : A favorable impact of preoperative FPLC chemotherapy on patients with gastric cardia cancer. Oncol Rep 7:241-244, 2000 [PubMed] [Google Scholar]
- 33.Ychou M, Boige V, Pignon J-P, et al. : Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29:1715-1721, 2011 [DOI] [PubMed] [Google Scholar]
- 34.An F, Huang J, Xie Y, et al. : A prospective study of combined chemoradiotherapy followed by surgery in the treatment of esophageal carcinoma. Zhonghua Zhong Liu Za Zhi 25:376-379, 2003 [PubMed] [Google Scholar]
- 35.Apinop C, Puttisak P, Preecha N: A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology 41:391-393, 1994 [PubMed] [Google Scholar]
- 36.Bass GA, Furlong H, O’Sullivan KE, et al. : Chemoradiotherapy, with adjuvant surgery for local control, confers a durable survival advantage in adenocarcinoma and squamous cell carcinoma of the oesophagus. Eur J Cancer 50:1065-1075, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Bosset JF, Gignoux M, Triboulet JP, et al. : Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 337:161-167, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Burmeister BH, Smithers BM, Gebski V, et al. : Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncol 6:659-668, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. : Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: The randomized controlled CROSS trial. J Clin Oncol 39:1995-2004, 2021 [DOI] [PubMed] [Google Scholar]
- 40.Prise EL, Etienne PL, Meunier B, et al. : A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 73:1779-1784, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Lee J-L, Park SI, Kim S-B, et al. : A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol 15:947-954, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Lv J, Cao XF, Zhu B, et al. : Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol 16:1649-1654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mariette C, Dahan L, Mornex F, et al. : Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: Final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 32:2416-2422, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Peng L, Xie T-P, Han Y-T, et al. : Randomized controlled study on preoperative concurrent chemoradiotherapy versus surgery alone for esophageal squamous cell carcinoma. Tumor 28:620-622, 2008 [Google Scholar]
- 45.Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. : Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol 16:1090-1098, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Tepper J, Krasna MJ, Niedzwiecki D, et al. : Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 26:1086-1092, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urba SG, Orringer MB, Turrisi A, et al. : Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 19:305-313, 2001 [DOI] [PubMed] [Google Scholar]
- 48.van Hagen P, Hulshof MCCM, van Lanschot J, et al. : Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074, 2084, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Walsh TN, Grennell M, Mansoor S, et al. : Neoadjuvant treatment of advanced stage esophageal adenocarcinoma increases survival. Dis Esophagus 15:121-124, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Walsh TN, Noonan N, Hollywood D, et al. : A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 335:462-467, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Yang H, Fu J, Liu M, et al. : A multi-centered randomized controlled study of neo-adjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of esophagus: An interim analysis. Zhonghua Yi Xue Za Zhi 92:1028-1032, 2012 [PubMed] [Google Scholar]
- 52.Yang H, Liu H, Chen Y, et al. : Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol 36:2796-2803, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiao X, Zhang J, Wang J, et al. : Concurrent neoadjuvant chemoradiotherapy for Siewert II and III adenocarcinoma at gastroesophageal junction. Am J Med Sci 349:472-476, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burmeister BH, Thomas JM, Burmeister EA, et al. : Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer 47:354-360, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Stahl M, Walz MK, Riera-Knorrenschild J, et al. : Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur J Cancer 81:183-190, 2017 [DOI] [PubMed] [Google Scholar]
- 56.Klevebro F, Alexandersson von Döbeln G, Wang N, et al. : A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 27:660-667, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Wagner AD, Oertelt-Prigione S, Adjei A, et al. : Gender medicine and oncology: Report and consensus of an ESMO workshop. Ann Oncol 30:1914-1924, 2019 [DOI] [PubMed] [Google Scholar]
- 58.Lacas B, Carmel A, Landais C, et al. : Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother Oncol 156:281-293, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfreundschuh M, Murawski N, Zeynalova S, et al. : Optimization of rituximab for the treatment of DLBCL: Increasing the dose for elderly male patients. Br J Haematol 179:410-420, 2017 [DOI] [PubMed] [Google Scholar]
- 60.Leong T, Smithers BM, Haustermans K, et al. : TOPGEAR: A randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: Interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol 24:2252-2258, 2017 [DOI] [PubMed] [Google Scholar]
- 61.Tang H, Tan L, Shen Y, et al. : CMISG1701: A multicenter prospective randomized phase III clinical trial comparing neoadjuvant chemoradiotherapy to neoadjuvant chemotherapy followed by minimally invasive esophagectomy in patients with locally advanced resectable esophageal squamous cell carcinoma (cT3-4aN0-1M0) (NCT03001596). BMC Cancer 17:450, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoeppner J, Lordick F, Brunner T, et al. : ESOPEC: Prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 16:503, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reynolds JV, Preston SR, O’Neill B, et al. : ICORG 10-14: NEOadjuvant trial in adenocarcinoma of the oEsophagus and oesophagoGastric junction International Study (Neo-AEGIS). BMC Cancer 17:401, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reynolds JV, Preston SR, O’Neill B, et al. : Neo-AEGIS (Neoadjuvant trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): Preliminary results of phase III RCT of CROSS versus perioperative chemotherapy (Modified MAGIC or FLOT protocol). (NCT01726452). J Clin Oncol 39:4004, 2021. 34672688 [Google Scholar]
- 65.Al-Batran S-E, Homann N, Pauligk C, et al. : Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 393:1948-1957, 2019 [DOI] [PubMed] [Google Scholar]
- 66.Kato K, Ito Y, Daiko H, et al. : A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol 40:238, 2022 [Google Scholar]
- 67.Slagter AE, Jansen EPM, van Laarhoven HWM, et al. : CRITICS-II: A multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. BMC Cancer 18:877, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chu L, Chen Y, Liu Q, et al. : A phase II study of apatinib in patients with chemotherapy-refractory esophageal squamous cell carcinoma (ESO-Shanghai 11). Oncologist 26:e925-e935, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dutton SJ, Ferry DR, Blazeby JM, et al. : Gefitinib for oesophageal cancer progressing after chemotherapy (COG): A phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol 15:894-904, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Moehler M, Maderer A, Thuss-Patience PC, et al. : Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: A prospective, open-label, randomised phase III AIO/EORTC trial (POWER). Ann Oncol 31:228-235, 2020 [DOI] [PubMed] [Google Scholar]
- 71.Waddell T, Chau I, Cunningham D, et al. : Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open-label phase 3 trial. Lancet Oncol 14:481-489, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yanwei L, Feng H, Ren P, et al. : Safety and efficacy of apatinib monotherapy for unresectable, metastatic esophageal cancer: A single-arm, open-label, phase II study. Oncologist 25:e1464-e1472, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoon HH, Bendell JC, Braiteh FS, et al. : Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: A randomized, double-blind, multicenter phase II trial. Ann Oncol 27:2196-2203, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun J-M, Shen L, Shah MA, et al. : Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 398:759-771, 2021 [DOI] [PubMed] [Google Scholar]
- 75.Kelly RJ, Ajani JA, Kuzdzal J, et al. : Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 384:1191-1203, 2021 [DOI] [PubMed] [Google Scholar]
- 76.Créquit P, Trinquart L, Yavchitz A, et al. : Wasted research when systematic reviews fail to provide a complete and up-to-date evidence synthesis: The example of lung cancer. BMC Med 14:8, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vandvik PO, Brignardello-Petersen R, Guyatt GH: Living cumulative network meta-analysis to reduce waste in research: A paradigmatic shift for systematic reviews? BMC Med 14:59, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Créquit P, Martin-Montoya T, Attiche N, et al. : Living network meta-analysis was feasible when considering the pace of evidence generation. J Clin Epidemiol 108:10-16, 2019 [DOI] [PubMed] [Google Scholar]