Abstract
BACKGROUND:
The recovery of severe traumatic brain injury (TBI) survivors with long-term favorable outlook is understudied. Time to follow commands varies widely in this patient population but has important clinical implications.
OBJECTIVE:
To (1) evaluate time to follow commands in severe patients with TBI with favorable outcomes, (2) characterize their trajectory of recovery, and (3) identify predictors associated with delayed cognitive improvement.
METHODS:
Participants were recruited prospectively at a Level I trauma center through the Brain Trauma Research Center from 2003 to 2018. Inclusion criteria were age 16 to 80 years, Glasgow Coma Scale score ≤8 and motor score <6, and Glasgow Outcome Scale-Extended measure ≥4 at 2 years postinjury.
RESULTS:
In 580 patients, there were 229 (39.5%) deaths and 140 (24.1%) patients had favorable outcomes at 2 years. The mean age was 33.7 ± 14.5 years, median Glasgow Coma Scale was 7 (IQR 6-7), and median Injury Severity Score was 30 (IQR 26-38). The mean time to follow commands was 12.7 ± 11.8 days. On multivariable linear regression, the presence of diffuse axonal injury (B = 9.2 days [4.8, 13.7], P < .0001) or intraventricular hemorrhage (B = 6.4 days [0.5, 12.3], P < .035) was associated with longer time before following commands and patients who developed nosocomial infections (B = 6.5 days [1.6-11.4], P < .01).
CONCLUSION:
In severe TBI survivors with favorable outcomes, time to follow commands varied widely. Most patients began to follow commands within 2 weeks. Evidence of diffuse axonal injury, intraventricular hemorrhage, and infections can delay cognitive improvement in the acute period. Patients make considerable recovery up to 2 years after their injury.
KEY WORDS: Diffuse axonal injury, Intraventricular hemorrhage, Polytrauma, Glasgow Outcome Scale-Extended, Traumatic brain injury, Favorable recovery, Prognosis
ABBREVIATIONS:
- ATV
all-terrain vehicle
- BTRC
Brain Trauma Research Center
- DAI
diffuse axonal injury
- GOS
Glasgow Outcome Scale
- GOS-E
Glasgow Outcome Scale-Extended
- EDH
epidural hemorrhage
- IPH
intraparenchymal hemorrhage
- ISS
Injury Severity Score
- IVH
intraventricular hemorrhage
- MCC
motorcycle collision
- mGCS
motor GCS
- MVC
motor vehicle collision
- SAH
subarachnoid hemorrhage
- SDH
subdural hemorrhage
- TBI
traumatic brain injury.
Traumatic brain injury (TBI) is a complex disorder encompassing a spectrum of intracranial pathologies, many of which are prognostic and management challenges. TBI is associated with 56 000 deaths annually in the United States, and over 5 million Americans currently live with TBI-related disabilities.1-4 Accurate prognostication after severe head injury is crucial to discussions on goals of care and difficult medical decisions.
To the neurointensivist, the capacity to follow commands indicates clinical evidence of consciousness and time to following commands is a potential predictor of better outcomes after severe head injury.5 However, the timing of arousal from unconsciousness, along with long-term outcomes, can widely vary after severe TBI.6 The Corticosteroid Randomisation After Significant Head Injury (CRASH) and International Mission for Prognosis and Analysis of Clinical Trials (IMPACT) trials have identified patient age, Glasgow Coma Scale (GCS) score on admission, and pupillary reactivity as predictors of functional outcomes at 6 months on the Glasgow Outcome Scale-Extended (GOS-E),7,8 but prognostication models remain nonspecific. Severe TBI survivors can improve up to 24 months after their initial injury,9,10 even up to 5 years in certain patients.11 Ongoing collaborative efforts are evaluating the utility of biomarkers and genetics to further risk stratify patients.12,13
In this study, we investigate the trajectory of recovery in a cohort of prospectively enrolled severe patients with TBI with favorable outcomes at 2 years, to assist with decision-making and prognostication throughout the course of care for the patient. The goal is to identify predictors of time to follow commands. We hypothesize that time to follow commands can widely vary after injury based on specific patterns of intracranial hemorrhage.
METHODS
Study Design
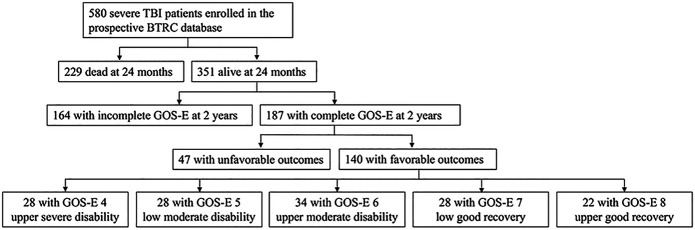
All study patients were enrolled at Brain Trauma Research Center through a prospective cohort study at a single level I trauma center. As part of an Institutional Review Board-approved longitudinal cohort study that enrolled consecutive patients from October 2003 to February 2018, the legal-authorized representative was approached for proxy consent. Inclusion criteria for this study were age 16 to 80 years, severe TBI with GCS score ≤8 and motor GCS (mGCS) ≤5, and GOS-E ≥4 at the 2-year follow up. Patients were excluded if they had GCS of 3 and bilateral fixed pupils, or imminent brain death, and those who did not survive the acute hospital setting (Figure 1). The results from this study were reported in adherence to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
FIGURE 1.
Study profile of enrolled patients with severe TBI. Flow diagram depicting longitudinal inclusion criteria of severe TBI survivors in the BTRC database with favorable outcomes on GOS-E ≥4 at 2-year follow-up after the initial injury from 2003 to 2018. BTRC, Brain Trauma Research Center; GOS-E, Glasgow Outcome Scale-Extended; TBI, traumatic brain injury.
Demographic and Outcome Measures
Demographic and clinical information was collected by research nurse coordinators. Admission GCS scores were categorized into 3 to 4, 5 to 6, and 7 to 8. Trauma registry ISS scores were categorized into minor/moderate (1-15), serious (16-24), severe (25-49), and critical/maximum (50-75). Occurrences of inpatient infections were recorded. Imaging findings were documented from head computed tomography and/or MRI. After stabilization from their acute injuries, patients underwent an MRI of the brain to evaluate the extent of their TBI burden within the first week post-trauma. Traumatic pathologies included intraparenchymal hemorrhage (IPH), subarachnoid hemorrhage (SAH), subdural hemorrhage (SDH), epidural hemorrhage (EDH), intraventricular hemorrhage (IVH), and diffuse axonal injury (DAI). Treatment was provided in accordance with the Brain Trauma Foundation guidelines.14
Daily physician progress notes were reviewed for documentation of GCS motor score (mGCS) of command following. Time to follow commands was defined as the duration of time between injury and demonstration of ability to follow verbal commands mGCS = 6. Long-term functional outcome was measured on the GOS-E at 3, 6, 12, and 24 months after injury. The GOS-E is a structured 8-point system: death (1), vegetative state (2), lower (3) and upper (4) severe disability, lower (5) and upper (6) moderate disability, and lower (7) and upper (8) good recovery.15 Similar to the Randomized Evaluation of Surgery with Craniectomy for Uncontrollable Elevation of Intracranial Pressure (RESCUE-ICP) trial, outcomes were dichotomized as favorable (upper severe disability or better GOS-E 4-8) or unfavorable (GOS-E 3).16 Severe TBI survivors with favorable outcomes at 2 years ranged from patients dependent for daily support but could be left alone for more than 8 hours to patients who were able to resume normal life with minor deficits.17
Statistical Analysis
Descriptive statistics are presented as means, SDs, median, and IQR for continuous variables and proportions for categorical variables. The Pearson chi-squared test (χ2) for categorical variables and analysis of variance for continuous variables were performed. We conducted multivariable linear regression analysis on the association between type of intracranial bleed with time to following commands, while controlling for sex, age, GCS, ISS, and occurrence of in-hospital infections. Binary logistic regression was conducted to evaluate for predictors of good recovery outcomes at 24 months (GOS-E score 7 or 8). Pearson correlation analysis was performed for time to following commands and functional outcome at 2 years on the GOS-E in these patients. The correlation coefficient and significance are reported. Significance was assessed at P = .05. All analyses were performed using the Statistical Package for Social Sciences (SPSS; version 27; IBM Corporation). The data sets generated during and/or analyzed during this study are not publicly available but could be acquired from the corresponding author on reasonable request.
RESULTS
Demographics and Injury Characteristics
In total, 580 patients with severe TBI were enrolled between 2003 and 2018 and 229 (39.5%) patients died from their acute injuries. One hundred forty (24.1%) patients showed favorable outcomes (GOS-E ≥4) at 2 years post-trauma and were included in this study (Figure 1). The average age (SD) was 33.7 ± 14.5 years, 106 (75.7%) patients were male, and 130 (92.9%) patients were Caucasian. The median GCS score was 7 (IQR 6-7; Table 1). The median Injury Severity Score (ISS) was 30 (IQR 26-38). Mechanisms of injury included motor vehicle collision (47.1%), fall (17.9%), motorcycle collision (15.0%), all-terrain vehicle (7.1%), pedestrian vs vehicle (4.3%), bicyclist (2.9%), and other (5.7%; Figure 2).
TABLE 1.
Demographic and Clinical Characteristics of the Patients in This Study
| Parameters | Overall N = 140 |
Days to follow commands (SD) | P value |
|---|---|---|---|
| Age (y) | .669 | ||
| 16-29 | 72 (51.4%) | 12.4 (12.7) | |
| 30-44 | 33 (23.6%) | 14.9 (12.4) | |
| 45-59 | 26 (18.6%) | 11.5 (9.3) | |
| 60-80 | 9 (6.4%) | 11.2 (8.4) | |
| Sex | .003 | ||
| Female | 34 (24.3%) | 17.8 (16.3) | |
| Male | 106 (75.7%) | 11.1 (9.4) | |
| Admission GCS | .219 | ||
| 3-4 | 26 (18.6%) | 15.2 (13.0) | |
| 5-6 | 38 (27.1%) | 14.1 (13.8) | |
| 7-8 | 76 (54.3%) | 11.2 (10.0) | |
| ISS | .739 | ||
| Minor/moderate | 7 (5.0%) | 11.3 (11.5) | |
| Serious | 22 (15.7%) | 12.6 (11.5) | |
| Severe | 94 (67.1%) | 11.7 (11.4) | |
| Critical/maximum | 12 (8.6%) | 15.5 (10.3) | |
| Mechanism of injury | .909 | ||
| MVC | 66 (47.1%) | 13.2 (13.0) | |
| Fall | 25 (17.9%) | 14.2 (11.4) | |
| MCC | 21 (15.0%) | 12.8 (12.6) | |
| ATV | 10 (7.1%) | 11.4 (7.4) | |
| Pedestrian struck | 6 (4.3%) | 11.5 (10.4) | |
| Bicyclist | 4 (2.9%) | 5.5 (4.0) | |
| Other | 8 (5.7%) | 13.0 (10.1) | |
| Intracranial bleed | .010 | ||
| IPH | 74 (52.9%) | 12.1 (10.0) | |
| SAH | 79 (56.4%) | 14.8 (13.3) | |
| SDH | 62 (44.3%) | 14.3 (14.8) | |
| EDH | 22 (15.7%) | 10.9 (10.9) | |
| IVH | 15 (10.7%) | 20.1 (20.6) | |
| DAI | 38 (27.1%) | 20.2 (15.5) | |
| Craniectomy/craniotomy | .914 | ||
| No | 86 (61.4%) | 12.8 (12.3) | |
| Yes | 54 (38.6%) | 12.6 (11.0) | |
| Other surgery | .844 | ||
| No | 57 (40.7%) | 12.5 (11.1) | |
| Yes | 83 (59.3%) | 12.9 (12.3) | |
| In-hospital infection | .010 | ||
| No | 23 (16.4%) | 7.0 (6.6) | |
| Yes | 117 (83.6%) | 13.9 (12.2) |
ATV, all-terrain vehicle; DAI, diffuse axonal injury; EDH, epidural hematoma; GCS, Glasgow Coma Scale; IPH, intraparenchymal hematoma; ISS, Injury Severity Score; IVH, intraventricular hematoma; MCC, motorcycle crash; MVC, motor vehicle crash; SAH, subarachnoid hematoma; SDH, subdural hematoma.
FIGURE 2.
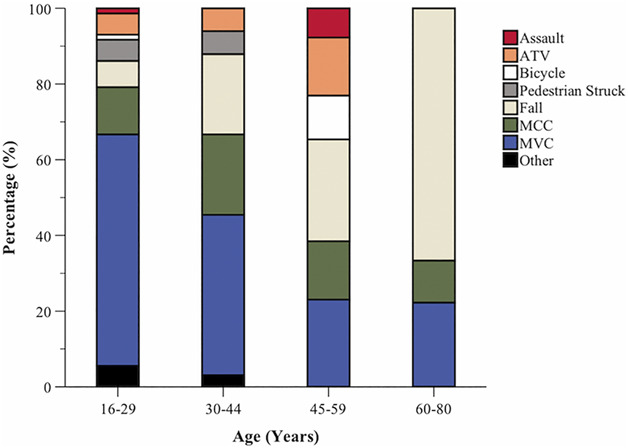
Bar graphs depicting the types of injury mechanisms of injury by age groups in severe traumatic brain injury survivors with favorable outcomes at 2 years. ATV, all-terrain vehicle; MCC, motorcycle crash; MVC, motor vehicle crash.
The most common traumatic intracranial pathology was SAH (56.4%), followed by IPH (52.9%), SDH (44.3%), DAI (27.1%), EDH (15.7%), and IVH (10.7%; Table 1). Fifty-four patients (38.6%) underwent craniotomy/craniectomy, and 83 patients also had nonneurosurgical procedures (59.3%). There were 117 (83.5%) patients who experienced in-hospital infections and 44 (31.4%) patients with more than 1 infection during the hospitalization.
Time to Follow Commands
The mean time to follow commands was 12.7 ± 11.8 days, and the median time was 8.5 days. Sixty-three (45.0%) patients followed commands ≤7 days after the initial injury, 29 (20.7%) patients between 8 and 14 days, 23 (16.4%) patients between 15 and 21 days, 12 (8.6%) patients between 22 and 28 days, and 13 (9.3%) patients between 28 and 78 days (Figure 3A). There was 1 patient who followed commands on day 78. The mean length of mechanical ventilation was 12.0 ± 6.5 days, mean length of stay in the intensive care unit was 18.5 ± 11.0 days, and mean length of hospital stay was 24.2 ± 13.4 days.
FIGURE 3.
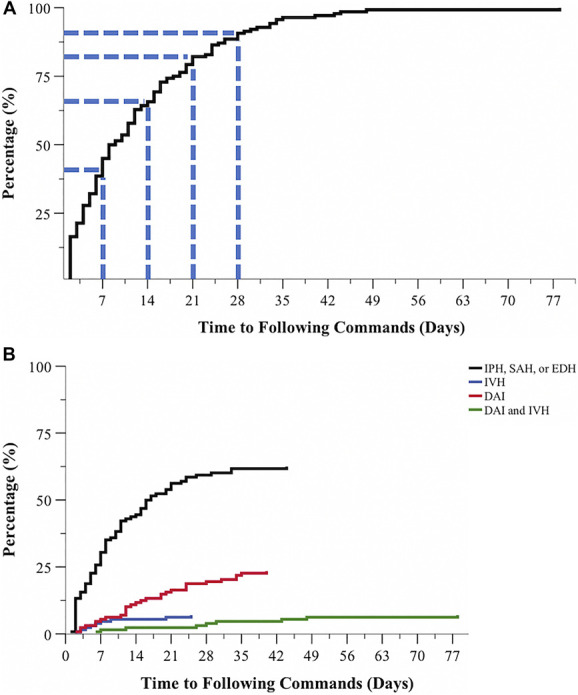
Distribution curves of time to following commands in severe traumatic brain injury with favorable outcomes. A, Overall, 45.0% of patients started to follow commands ≤7 days after injury and 65.7% of patients by 2 weeks. There was 1 patient who followed commands on day 78. B, Patients with evidence of DAI and IVH experienced delayed clinical arousal from unconsciousness compared with patients with other types of intracranial trauma. DAI, diffuse axonal injury; EDH, epidural hemorrhage; IVH, intraventricular hemorrhage; IPH, intraparenchymal hemorrhage, SAH, subarachnoid hemorrhage.
Multivariable linear regression showed that patients with DAI took significantly longer time to follow commands than patients without DAI (B = +9.2 days, [4.8, 13.7], P < .0001), as did patients with IVH (B = +6.4 days, [0.5, 12.3], P = .035; Figure 3B). Patients with in-hospital infections also took longer time to follow commands (B = +6.5 days, [1.6, 11.4], P < .01) compared with patients without infections.
Functional Outcomes
At 24 months postinjury, there were 90 (64.3%) patients of GOS-E 4 to 6; 50 (35.7%) patients had GOS-E 7 to 8 (Table 2). Figure 4 shows the functional recovery of patients at 24 months on the GOS-E relative to their GOS-E at 3 months. The median 3-month GOS-E was 4 IQR (3-5), and the median 6-month GOS-E was 5 IQR (3-6). The median 12-month GOS-E was 5 IQR (4-7), compared with 6 IQR (5-7) on 24-month GOS-E. Multivariable binary logistic regression demonstrated that relative to female patients at 2 years after injury, male patients were over 4 times more likely to have good recovery with GOS-E of 7 to 8 (odd ration [OR] 4.8 [1.5, 16.1], P = .010; Table 3). Pearson correlation analysis showed a weak negative correlation (r = 0.040, P = .017) between time to following commands in these patients and functional recovery at 2 years on the GOS-E. Patients presenting with critical/maximum polytrauma on ISS were less likely to have good recovery (OR 0.04 [0.002, 0.8], P = .036) compared with patients with minor/moderate polytrauma on ISS.
TABLE 2.
Functional Outcomes of Severe TBI Survivors at 2 Years With Favorable Recovery
| Parameters | GOS-E 4-6 N = 90 |
GOS-E 7-8 N = 50 |
P value |
|---|---|---|---|
| Age (y) | .082 | ||
| 16-29 | 39 (54.2%) | 33 (45.8%) | |
| 30-44 | 25 (75.8%) | 8 (24.2%) | |
| 45-59 | 19 (73.1%) | 7 (26.9%) | |
| 60-80 | 7 (77.8%) | 2 (22.2%) | |
| Sex | .004 | ||
| Female | 29 (85.3%) | 5 (14.7%) | |
| Male | 61 (57.5%) | 45 (42.5%) | |
| Admission GCS | .054 | ||
| 3-4 | 22 (84.6%) | 4 (15.4%) | |
| 5-6 | 22 (57.9%) | 16 (42.1%) | |
| 7-8 | 46 (60.5%) | 30 (39.5%) | |
| ISS | .084 | ||
| Minor/moderate | 3 (42.9%) | 4 (57.1%) | |
| Serious | 13 (59.1%) | 9 (40.9%) | |
| Severe | 58 (61.7%) | 36 (38.3%) | |
| Critical/maximum | 11 (91.7%) | 1 (8.3%) | |
| Mechanism of injury | .749 | ||
| MVC | 44 (66.7%) | 22 (33.3%) | |
| Fall | 15 (60.0%) | 10 (40.0%) | |
| MCC | 12 (57.1%) | 9 (42.9%) | |
| ATV | 7 (70.0%) | 3 (30.0%) | |
| Pedestrian struck | 5 (83.3%) | 1 (16.7%) | |
| Bicyclist | 3 (75.0%) | 1 (25.0%) | |
| Other | 2 (40.0%) | 3 (60.0%) | |
| Intracranial bleed | .054 | ||
| IPH | 48 (64.9%) | 26 (35.1%) | |
| SAH | 61 (77.2%) | 18 (22.8%) | |
| SDH | 41 (66.1%) | 21 (33.9%) | |
| EDH | 10 (45.5%) | 12 (54.5%) | |
| IVH | 9 (60.0%) | 6 (40.0%) | |
| DAI | 25 (65.8%) | 13 (34.2%) | |
| In-hospital infection | .185 | ||
| No | 12 (52.2%) | 11 (47.8%) | |
| Yes | 78 (66.7%) | 39 (33.3%) |
ATV, all-terrain vehicle; DAI, diffuse axonal injury; EDH, epidural hematoma; GCS, Glasgow Coma Scale; GOS-E, Glasgow Outcome Scale-Extended; IPH, intraparenchymal hematoma; ISS, Injury Severity Score; IVH, intraventricular hematoma; MCC, motorcycle crash; MVC, motor vehicle crash; SAH, subarachnoid hematoma; SDH, subdural hematoma.
FIGURE 4.
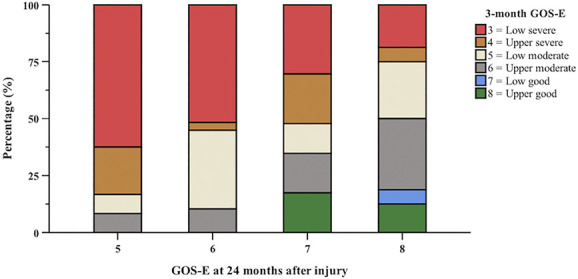
Bar graphs showing the progressive functional recovery of patients in this study 24 months after injury relative to their 3-month GOS-E scores. At 3 months, 58 (41.4%) patients had GOS-E <4. The median 3-month GOS-E was 4 IQR (3-5) compared with 6 IQR (5-7) on a 24-month GOS-E. GOS-E, Glasgow Outcome Scale-Extended
TABLE 3.
Multivariable Regression of Time to Follow Commands and Functional Outcomes at 2 Years
| Parameters | Days to follow commands | Good recovery on GOS-E 7-8 | ||
|---|---|---|---|---|
| B (95% CI) | P value | OR (95% CI) | P value | |
| Age (y) | ||||
| 16-29 | Reference | Reference | ||
| 30-44 | 4.3 (−0.4-9.1) | .075 | 0.7 (0.2-2.1) | .494 |
| 45-59 | 1.6 (−3.4-6.6) | .526 | 0.4 (0.1-1.3) | .113 |
| 60-80 | −2.7 (−10.4-5.1) | .502 | 0.6 (0.1-4.4) | .646 |
| Sex | ||||
| Female | Reference | |||
| Male | −3.6 (−7.9-0.7) | .102 | 4.8 (1.5-16.1) | .010 |
| Admission GCS | ||||
| 3-4 | Reference | Reference | ||
| 5-6 | 1.0 (−4.5-6.4) | .728 | 4.1 (0.9-18.8) | .066 |
| 7-8 | −0.4 (−5.3-4.6) | .886 | 2.3 (0.6-9.1) | .242 |
| ISS | ||||
| Minor/moderate | Reference | Reference | ||
| Serious | −4.7 (−12.3-2.9) | .221 | 0.5 (0.05-3.9) | .479 |
| Severe | −5.6 (−12.2-1.0) | .096 | 0.3 (0.04-2.0) | .198 |
| Critical/maximum | −1.6 (−10.5-7.3) | .727 | 0.04 (0.002-0.8) | .036 |
| IPH | ||||
| No | Reference | Reference | ||
| Yes | −1.0 (−4.7-2.6) | .577 | 0.9 (0.4-2.3) | .861 |
| SAH | ||||
| No | Reference | Reference | ||
| Yes | 3.1 (−0.8-7.0) | .116 | 0.4 (0.2-1.0) | .062 |
| SDH | ||||
| No | Reference | Reference | ||
| Yes | 2.9 (−0.9-6.6) | .139 | 1.0 (0.4-2.5) | .966 |
| EDH | ||||
| No | Reference | Reference | ||
| Yes | 2.6 (−2.4-7.6) | .304 | 2.5 (0.8-8.0) | .128 |
| IVH | ||||
| No | Reference | Reference | ||
| Yes | 6.4 (0.5-12.3) | .035 | 1.0 (0.3-3.8) | .991 |
| DAI | ||||
| No | Reference | Reference | ||
| Yes | 9.2 (4.8-13.7) | <.0001 | 1.4 (0.5-4.2) | .534 |
| Infection | ||||
| No | Reference | Reference | ||
| Yes | 6.5 (1.6-11.4) | <.01 | 0.4 (0.1-1.3) | .117 |
B, mean increase or decrease; DAI, diffuse axonal injury; EDH, epidural hematoma; GCS, Glasgow Coma Scale; GOS-E, Glasgow Outcome Scale-Extended; IPH, intraparenchymal hematoma; ISS, Injury Severity Score; IVH, intraventricular hematoma; MCC, motorcycle crash; MVC, motor vehicle crash; OR, odds ratio; SAH, subarachnoid hematoma; SDH, subdural hematoma.
DISCUSSION
Recovery from severe TBI relies on collaborative care across a broad range of clinical and social disciplines. Recent studies have provided valuable insights into the potential for functional improvement beyond 6 months of the initial injury.9,11,18 This study reveals distinctive patterns of cognitive recovery, which can aid with prognosis and initiate therapeutic treatment as early as possible.
Command Following After Severe TBI
Evaluation of command following is an integral aspect of neurological assessments of patients with TBI.19 For clinical and prognostic purposes, following commands assumes early importance in post-traumatic care and throughout the course of rehabilitation. In our patients, the average time to follow commands was 12.7 days, with over 60% of patients doing so by 2 weeks. This behavior reflects a higher level of cognitive function and motor processing, thereby differentiating patients who are waking from unconsciousness from those remaining in a vegetative state.20,21 Prognosis after severe head trauma is often uncertain and medical decisions can be difficult for the family.5,22 For this reason, clinical evidence of consciousness, or lack thereof, could assist with ongoing discussions on goals of care and continuing life-sustaining therapies.
Notably, patients with radiographic evidence of DAI and IVH—an indirect marker of DAI—experienced prolonged periods of time before demonstrating behavioral consciousness at the bedside. TBI patients with DAI and IVH took approximately 9 and 6 days longer, respectively, to begin to follow commands. First described by Strich and colleagues in 1956,23 DAI is characterized by microhemorrhages to neural tracts, corpus callosum, and brainstem, occurring frequently after high-velocity trauma that involves rotational acceleration-deceleration forces.24,25 In this study, patients with DAI experienced delays in cognitive improvement, possibly because of additional time needed for injured neural connections to remodel after stabilization from the initial injury.26
Patients with severe TBI are at high risk for developing nosocomial infections, most being pulmonary infections, bacteremia, and surgical site infections.27 Hospital infections are associated with greater inpatient mortality, longer duration of mechanical ventilation, and increased multiorgan dysfunction.28 Even in TBI survivors who achieved favorable recovery, we found that the occurrence of nosocomial infections delayed the time to follow commands by over 6 days. Systemic infection can further drive inflammation in patients with severe traumatic injuries. TBI predisposes patients to developing nosocomial infections, which in turn could worsen secondary brain injury after the initial trauma. As seen in our severe TBI cohort, immunological dysfunction, even when treated appropriately with antibiotics, can delay overall neurological recovery. Acute brain injury can precipitate significant immunosuppression through T-cell dysfunction and sympathetic activation, thereby increasing the likelihood of infections.29,30 Recognition and timely treatment of infections are crucial to reducing mortality and improving the cognitive status of patients with neurotrauma. Prognosis of these patients should be approached with caution.
Functional Recovery After Acute Inpatient Stay
Similar to earlier reports, we find that the mortality rate after severe TBI is high at 39.1%1,2,9; however, the long-term prognosis for the survivors is better than broadly appreciated. The improvement from vegetative state, severe disability (conscious but dependent for daily support due to disabilities), or moderate disability (disabled but independent at home) is meaningful from a personal, social, and economic perspective. Patients with severe TBI require significantly longer time to improve than patients with moderate TBI.10,11 Early cognitive delay can occur with certain bleed patterns and with nosocomial infections, but there was no strong correlation with long-term functional recovery. In this patient population, we find that time to following commands may more so reflect various patterns of neurotrauma and medical comorbidities during the acute period, rather than it being a clear predictor of long-term functional recovery. These patients continue to make considerable progress toward regaining independence.
Once the medical condition of the patient allows for evaluation, the physical therapist, occupational therapist, speech therapist, and physiatry team assess for individualized needs and goals. Rehabilitation is achieved through an interdisciplinary approach. The duration of inpatient rehabilitation can span 4 to 8 weeks, during which a minimum of 3 hours daily of therapeutic interventions to target cognitive, speech, and physical conditioning are provided to optimize home and community integration.31
We found that male TBI survivors had better functional trajectories reported at 2 years than female patients. This is in line with investigations in the mild and moderate TBI populations, but sex differences remain poorly understood.18,32,33 Proposed theories include differences in post-traumatic symptom perception and reporting between sexes as well as differences in morphological variation and cortical thickness.34,35 Differential access to postinjury rehabilitation and support could also affect outcomes after TBI. There are disparities between human and animal studies, with human investigations in general reporting worse outcomes in women, whereas animal models found that females tend to do better after injury.36,37 The neuroprotective advantage of estrogen and progesterone has been characterized in controlled experimental models; however, its translation into clinical findings has been equivocal.38
Finally, patients who initially presented with concomitant polytrauma, frequently after high velocity traffic accidents, were less likely to gain functional independence. The presence of polytrauma is common in the context of TBI, with reported incidences of up to 70% in large population-based investigations.39 These patients are associated with additional physiological and socioeconomic challenges.40 In our cohort, patients with ISS 50 to 75 continued to experience moderate disabilities at 2 years postinjury. These findings have been reported in mild-moderate TBI literature, but less so in patients with severe TBI.41,42 Thus, multisystem trauma is a risk factor for functional dependence, and multidisciplinary communication and collaboration are necessary for long-term rehabilitation of these at-risk patients.
Limitations
Although this study adds to the body of knowledge on outcomes after severe TBI, it is not without limitations. The retrospective data analysis of this cohort, which was necessaryto understand time to follow commands as an outcome parameter, is a potential limitation of this study. Second, we use the GOS-E as the widely adopted functional outcome assessment in the severe TBI population37,38; however, the GOS-E lacks sensitivity in detecting more specific impairments to correlate with injury patterns.43,44 Third, our data on radiographic findings of intracranial pathology were based on computed tomography scans technology and did not specify the locations of injury, which will be a topic for future investigation. Patients without complete 2-year follow-up were not included in this study. As illustrated in Figure 1, there were 164 enrolled patients who did not have documented GOS-E measures as evaluation of their functional status 2 years from their initial injury. The rate of loss to follow-up was 28.3%. Nonetheless, these results can assist with understanding the spectrum of recovery and prognostication after severe TBI in compliance with the current Guidelines for the Management of Severe TBI.31 Time to follow commands in patients with poor outcomes was not studied. Further analysis of these patients is part of the scope of a separate future study.
CONCLUSION
In patients with severe TBI who make a favorable recovery, most patients show clinical evidence of following commands by 2 weeks but evidence of DAI, IVH, or nosocomial infections can delay cognitive improvement in the acute phase. Patients can make considerable functional recovery after acute hospitalization, emphasizing better clinical prognostication than expected, and the need for continued support in these patients.
Footnotes
The abstract of this manuscript was an oral presentation at the Congress of Neurological Surgeons Annual Meeting, October 19, 2021, Austin, TX.
Contributor Information
Enyinna L. Nwachuku, Email: nwachukuel@upmc.edu.
Tiffany E. Wilkins, Email: tiffelizabethwilkins@gmail.com.
John K. Yue, Email: john.yue@ucsf.edu.
Anita Fetzick, Email: fetzal@upmc.edu.
Yue-Fang Chang, Email: yuc2@pitt.edu.
Sue R. Beers, Email: beersSR@upmc.edu.
David O. Okonkwo, Email: okonkwodo@upmc.edu.
Ava M. Puccio, Email: puccioam@upmc.edu.
Funding
This study was supported by the federally funded Grants R00-NR013176, National Institutes of Health (NS30318; Brain Trauma Research Center, University of Pittsburgh).
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Bryan-Hancock C, Harrison J. The global burden of traumatic brain injury: preliminary results from the Global Burden of Disease Project. Inj Prev. 2010;16(supplement 1):A17. [Google Scholar]
- 2.Flaada JT, Leibson CL, Mandrekar JN, et al. Relative risk of mortality after traumatic brain injury: a population-based study of the role of age and injury severity. J Neurotrauma. 2007;24(3):435-445. [DOI] [PubMed] [Google Scholar]
- 3.Galvagno SM, Fox EE, Appana SN, et al. Outcomes after concomitant traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg. 2017;83(4):668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whyte J, Cifu D, Dikmen S, Temkin N. Prediction of functional outcomes after traumatic brain injury: a comparison of 2 measures of duration of unconsciousness. Arch Phys Med Rehabil. 2001;82(10):1355-1359. [DOI] [PubMed] [Google Scholar]
- 6.Vedantam A, Robertson CS, Gopinath SP. Clinical characteristics and temporal profile of recovery in patients with favorable outcomes at 6 months after severe traumatic brain injury. J Neurosurg. 2018;129(1):234-240. [DOI] [PubMed] [Google Scholar]
- 7.Roozenbeek B, Lingsma HF, Lecky FE, et al. Prediction of outcome after moderate and severe traumatic brain injury: external validation of the International Mission on Prognosis and Analysis of Clinical Trials (IMPACT) and corticoid randomisation after significant head injury (CRASH) prognostic models. Crit Care Med. 2012;40(5):1609-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lingsma H, Andriessen TM, Haitsema I, et al. Prognosis in moderate and severe traumatic brain injury: external validation of the IMPACT models and the role of extracranial injuries. J Trauma Acute Care Surg. 2013;74(2):639-646. [DOI] [PubMed] [Google Scholar]
- 9.Wilkins TE, Beers SR, Borrasso AJ, et al. Favorable Functional recovery in severe traumatic brain injury survivors beyond six months. J Neurotrauma. 2019;36(22):3158-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corral L, Ventura JL, Herrero JI, et al. Improvement in GOS and GOSE scores 6 and 12 months after severe traumatic brain injury. Brain Inj. 2007;21(12):1225-1231. [DOI] [PubMed] [Google Scholar]
- 11.Whyte J, Nakase-Richardson R, Hammond FM, et al. Functional outcomes in traumatic disorders of consciousness: 5-year outcomes from the national institute on disability and rehabilitation research traumatic brain injury model systems. Arch Phys Med Rehabil. 2013;94(10):1855-1860. [DOI] [PubMed] [Google Scholar]
- 12.Yue JK, Yuh EL, Korley FK, et al. Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: a prospective multicentre study. Lancet Neurol. 2019;18(10):953-961. [DOI] [PubMed] [Google Scholar]
- 13.Deng H, Zusman BE, Nwachuku EL, et al. B-cell lymphoma 2 (Bcl-2) gene is associated with intracranial hypertension after severe traumatic brain injury. J Neurotrauma. 2021;38(2):291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(suppl 1):S1-S106. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JT, Pettigrew LE, Teasdale GM, Teasdale GM. Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: guidelines for their use. J Neurotrauma. 1998;15(8):573-585. [DOI] [PubMed] [Google Scholar]
- 16.Hutchinson PJ, Kolias AG, Timofeev IS, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375(12):1119-1130. [DOI] [PubMed] [Google Scholar]
- 17.Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow outcome scale. J Neurotrauma. 1998;15(8):587-597. [DOI] [PubMed] [Google Scholar]
- 18.Forslund MV, Perrin PB, Røe C, et al. Global outcome trajectories up to 10 years after moderate to severe traumatic brain injury. Front Neurol. 2019;10(14):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. Lancet. 1974;304(7872):81-84. [DOI] [PubMed] [Google Scholar]
- 20.Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol. 2014;10(2):99-114. [DOI] [PubMed] [Google Scholar]
- 21.Whyte J, DiPasquale MC, Vaccaro M. Assessment of command-following in minimally conscious brain injured patients. Arch Phys Med Rehabil. 1999;80(6):653-660. [DOI] [PubMed] [Google Scholar]
- 22.Turgeon AF, Lauzier F, Simard JF, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183(14):1581-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurol Neurosurg Psychiatry. 1956;19(3):163-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mata-Mbemba D, Mugikura S, Nakagawa A, et al. Intraventricular hemorrhage on initial computed tomography as marker of diffuse axonal injury after traumatic brain injury. J Neurotrauma. 2015;32(5):359-365. [DOI] [PubMed] [Google Scholar]
- 25.Abu Hamdeh S, Marklund N, Lannsjö M, et al. Extended anatomical grading in diffuse axonal injury using MRI: hemorrhagic lesions in the substantia nigra and mesencephalic tegmentum indicate poor long-term outcome. J Neurotrauma. 2017;34(2):341-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieira RdCA, Paiva WS, de Oliveira DV, Teixeira MJ, de Andrade AF, de Sousa RMC. Diffuse axonal injury: epidemiology, outcome and associated risk factors. Front Neurol. 2016;7(10):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kourbeti IS, Papadakis JA, Neophytou C, et al. Infections in patients with traumatic brain injury who undergo neurosurgery. Br J Neurosurg. 2011;25(1):9-15. [DOI] [PubMed] [Google Scholar]
- 28.Zygun DA, Zuege DJ, Boiteau PJ, et al. Ventilator-associated pneumonia in severe traumatic brain injury. Neurocrit Care. 2006;5(2):108-114. [DOI] [PubMed] [Google Scholar]
- 29.Dziedzic T, Slowik A, Szczudlik A. Nosocomial infections and immunity: lesson from brain-injured patients. Crit Care. 2004;8(4):266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoyt DB, Ozkan AN, Hansbrough JF, Marshall L, vanBerkum-Clark M. Head injury: an immunologic deficit in T-cell activation. J Trauma. 1990;30(7):759-766; discussion 766-767. [PubMed] [Google Scholar]
- 31.Carney N, Totten AM, O'Reilly C, et al. Guidelines for the management of severe traumatic brain injury, Fourth Edition. Neurosurgery. 2017;80(1):6-15. [DOI] [PubMed] [Google Scholar]
- 32.Willemse-van Son AHP, Ribbers GM, Verhagen AP, Stam HJ. Prognostic factors of long-term functioning and productivity after traumatic brain injury: a systematic review of prospective cohort studies. Clin Rehabil. 2007;21(11):1024-1037. [DOI] [PubMed] [Google Scholar]
- 33.Yue JK, Levin HS, Suen CG, et al. Age and sex-mediated differences in six-month outcomes after mild traumatic brain injury in young adults: a TRACK-TBI study. Neurol Res. 2019;41(7):609-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17(7):1550-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farace E, Alves WM. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg. 2000;93(4):539-545. [DOI] [PubMed] [Google Scholar]
- 36.Gupte R, Brooks W, Vukas R, Pierce J, Harris J. Sex differences in traumatic brain injury: what we know and what we should know. J Neurotrauma. 2019;36(22):3063-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renner C, Hummelsheim H, Kopczak A, et al. The influence of gender on the injury severity, course and outcome of traumatic brain injury. Brain Inj. 2012;26(11):1360-1371. [DOI] [PubMed] [Google Scholar]
- 38.Späni CB, Braun DJ, Van Eldik LJ. Sex-related responses after traumatic brain injury: considerations for preclinical modeling. Front Neuroendocrinol. 2018;50(7):52-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Q, Liu H, Wu X, et al. Characteristics of acute treatment costs of traumatic brain injury in Eastern China—a multi-centre prospective observational study. Injury. 2012;43(12):2094-2099. [DOI] [PubMed] [Google Scholar]
- 40.Yue JK, Satris GG, Dalle Ore CL, et al. Polytrauma is associated with increased three- and six-month disability after traumatic brain injury: a TRACK-TBI Pilot study. Neurotrauma Rep. 2020;1(1):32-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stulemeijer M, van der Werf SP, Jacobs B, et al. Impact of additional extracranial injuries on outcome after mild traumatic brain injury. J Neurotrauma. 2006;23(10):1561-1569. [DOI] [PubMed] [Google Scholar]
- 42.Lingsma H, Teuntje M J, Haitsema I, et al. Prognosis in moderate and severe traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):639-646. [DOI] [PubMed] [Google Scholar]
- 43.Levin HS, Boake C, Song J, et al. Validity and sensitivity to change of the extended Glasgow outcome scale in mild to moderate traumatic brain injury. J Neurotrauma. 2001;18(6):575-584. [DOI] [PubMed] [Google Scholar]
- 44.Weir J, Steyerberg EW, Butcher I, et al. Does the extended Glasgow Outcome Scale add value to the conventional Glasgow Outcome Scale? J Neurotrauma. 2012;29(1):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]