Abstract
Background:
Pelvic floor training with biofeedback has been shown to significantly reduce symptoms of urinary incontinence. The present study aimed to evaluate the effectiveness of pelvic floor training with the ACTICORE1 biofeedback device, which uses a noninsertable pelvic floor sensor with a digital interface.
Materials and methods:
A multicenter randomized controlled clinical pilot study in Germany was conducted between October 2021 and January 2022. The intervention group was instructed to use ACTICORE1 for 6 min daily to train the pelvic floor for 12 weeks. The control group was instructed not to do any pelvic floor training. Over 18-year-old men and women with urinary incontinence and an International Consultation on Incontinence Questionnaire score (ICIQ) of ≥5 were included in the study. The primary endpoint was the ICIQ score 12 weeks after enrollment. The secondary endpoints were the ICIQ score and quality of life using the EG-5D-3L questionnaire 4, 8, and 12 weeks after patients’ enrollment.
Results:
A total of 40 individuals with urinary incontinence were recruited for the present study (35 females, 5 males; 40% lost to follow-up). In terms of biometric data, both groups did not differ. At 4, 8, and 12 weeks, the ICIQ scores of those in the ACTICORE1 group decreased from 12.9 to 7.5. The ICIQ score in the control group decreased from 11.0 to 10.5. The intraindividual improvement of patients in the ACTICORE group was statistically significant.
Conclusion:
Biofeedback training with ACTICORE1 significantly reduces symptoms of urinary incontinence after 12 weeks.
Keywords: ACTICORE1, biofeedback training, stress incontinence, urge incontinence, urinary incontinency
Background
Highlights
ACTICORE1 is a device for pelvic floor biofeedback training.
ACTICORE1 uses a noninsertable pelvic floor sensor with a digital interface.
Biofeedback training with ACTICORE1 significantly reduces symptoms of urinary incontinence.
Urinary incontinence is commonly found in adult women and men. Results of a Germany-wide study show that 13% of individuals across all age groups are affected by urinary incontinence. Women are more frequently affected (15 vs. 10%)1. The prevalence increases with age. In the group of 18-year-olds to 40-year-olds, about 6% of participants reported being incontinent. A total of 23% of individuals over the age of 60 years suffered from urinary incontinence1–3. Therefore, the disease has a significant socioeconomic impact.
Pelvic floor training is an effective form of therapy for urinary incontinence, in particular for stress and mixed incontinence. Training supported by biofeedback has been shown to significantly reduce incontinence episodes and is included in the current S2E guideline as recommendation C, level of evidence IV1–3.
It is the aim of the ongoing study to evaluate the efficacy of pelvic floor training through biofeedback methods using ACTICORE1 for the improvement of urinary incontinence and associated symptoms and quality of life.
Materials and methods
A multicenter randomized controlled clinical pilot study, the ACU-1 trial, was performed to evaluate the efficacy of pelvic floor training through biofeedback methods using ACTICORE1 for the improvement of urinary incontinence. The study was conducted between October 2021 and January 2022. Patient enrollment took place at the German Pelvic Floor Center at Alexianer Hospital St. Hedwig Berlin (Germany) and the University Hospital Brandenburg an der Havel (Germany).
The study was approved by the Ethics Committee of Berlin (Eth-14/21) and Brandenburg (S12(bB)/2021) in 2021 in accordance with the Declaration of Helsinki.
This trial was registered in the German Clinical Trials Register (DRKS00024913). All the participants taking part in the study signed a standard written consent form. The study design was controlled by the CONSORT checklist (Supplemental Digital Content 1, http://links.lww.com/MS9/A218) for randomized clinical trials4.
Follow-up data were obtained by sending questionnaires (ICIQ+EG-5D-3L) by e-mail at 4, 8, and 12 weeks after enrollment5–7.
Study population
The intervention group was instructed to use ACTICORE1 for pelvic floor training for 6 min daily for 12 weeks. The control group was instructed not to perform pelvic floor training for 12 weeks.
Inclusion criteria
Over 18-year-old men and women with urinary incontinence and an ICIQ score of ≥5 were included in the study.
Exclusion criteria
Exclusion criteria primarily included health conditions likely to preclude adequate ACTICORE1 training due to insufficient muscle tissue, poor nerve response, and pain.
Patients were excluded if they refused to participate in the study, were unable to understand the content and scope of the study, had vulvodynia, were paraplegic due to traumatic injury to the cervical, thoracic, lumbar, and sacral nerves and spinal cord, and had an open sacral or perineal secretory wound after rectal resection or amputation, or who had rectal resection or amputation, thoracic, lumbar, and sacral levels, congenital paraplegia, an open sacral or perineal secretory wound after rectal resection or amputation, rectal resection and amputation, an ICIQ score <4, and with an ASA score >III.
Patients in the control group who desired another form of pelvic floor muscle training were excluded from the study.
Primary endpoint
The primary endpoint was the ICIQ score 12 weeks after enrollment.
Secondary endpoints
The secondary endpoints were the ICIQ score and quality of life using the EG-5D-3L questionnaire 4, 8, and 12 weeks after patient enrollment.
ACTICORE1
The ACTICORE1 (ACTICORE AG) is a Conformité Européenne (CE) certified approved medical device. The ACTICORE1 app is a digital home training program for strengthening the pelvic floor muscles. The patient must sit on a sensor, which does not have to be inserted (Figure S1, Supplemental Digital Content 2, http://links.lww.com/MS9/A219). The muscle movements are visualized via Bluetooth on a mobile device. Muscle activities facilitate the game movement of the avatar, called ANI – a digital personal coach (Jump ‘n’ Run/platform games). The ACTICORE training program was developed according to the P.E.R.F.E.C.T Scheme by Dr Jo Laycock8.
Statistical analysis
For nominally scaled variables, absolute and relative frequencies were calculated per group. Demographic variables were described. All continuous biometric variables were tested for normal distribution using a Q–Q plot. For normally distributed variables, the mean, SD, minimum, and maximum were calculated. For non-normally distributed variables median, interquartile range, minimum, and maximum were calculated per group. For the visual representation boxplots were chosen for nonparametric calculated variables. For the calculation of non-normally distributed dependent variables (intraindividual progression for the primary endpoint), the Wilcoxon test (two-tailed) between two time points and the Mann–Whitney U test for independent variables for group comparison of two groups (two-sided) was used. Since conservatively, it is not assumed that the values of the ICIQ are normally distributed, a nonparametric test procedure (Wilcoxon rank sum test) was performed.
The evaluation data set was the intention-to-treat population. This data set included all individuals who were enrolled and randomized to the clinical trial. Missing values were not replaced in this analysis. Statistical significance is assumed if the null hypothesis is accepted with a significance level of P≤0.05 (two-sided). For the calculation, we used the program RStudio with R version 4.0.5, and the package WMWssp version 0.4.0 were used.
As a pilot study, no power calculation took place. A sample size of 30 subjects for each study arm was considered sufficient to allow sample size calculation for a subsequent study. Due to the COVID pandemic and slow patient recruitment, an amendment was made that decided to reduce the sample size from 60 to 40. Rapid implementation of a follow-up study was sought.
Randomization
Randomization lists were used and pseudorandom numbers were generated in R (ver. 4.0.2). The principal investigator performed randomization after patients gave informed consent. No stratification took place.
The groups are not balanced in terms of sample size (ACTICORE group, n=21 vs. control group, n=19). Because the sample size was reduced due to COVID, the order of the original randomization list resulted in a small imbalance.
Results
A total of 40 individuals were recruited for the present study, 35 of whom were female and 5 male. In 30 cases, patients suffered from stress incontinence. The average initial ICIQ score was 11.5. Twenty-one patients were enrolled in the ACTICORE group and 19 in the control group. Figure S2 (Supplemental Digital Content 3, http://links.lww.com/MS9/A220) depicts the CONSORT 2010 (Supplemental Digital Content 1, http://links.lww.com/MS9/A218) flow diagram. The rate of lost to follow-up was 40%. These individuals did not complete the questionnaires sent to them and did not give any reasons for not doing so.
Univariate analysis of baseline characteristics
The baseline information of all enrolled individuals (n=40) is summarized in Table S1 (Supplemental Digital Content 4, http://links.lww.com/MS9/A226). Both groups were not statistically significantly different from each other after univariate analysis.
The baseline data for patients who met the primary endpoint (n=24; per protocol analysis) are summarized in Table 1. Both groups were not statistically significantly different from each other after univariate analysis.
Table 1.
Patient characteristics (per protocol analysis)
| ACTICORE group | Control group | ||
|---|---|---|---|
| Basic data | n=10 | n=14 | P a |
| Age | 42.5 [35.5–54.5] | 41.0 [35.5–48.5] | >0.9 |
| Sex | |||
| Female | 9 (90%) | 13 (92.9%) | >0.9 |
| Male | 1 (10%) | 1 (7.1%) | |
| ASA Score | |||
| I | 6 (60%) | 9 (64.3%) | 0.6 |
| II | 3 (30%) | 5 (35.7%) | |
| III | 1 (10%) | 0 ( 0%) | |
| BMI | |||
| kg/m2 | 24.6 [23.0–26.8] | 23.6 [22.3–24.8] | 0.3 |
| Cause of UI | |||
| Stress incontinence | 8 (80.0%) | 12 (85.7%) | 0.7 |
| Urge incontinence | 2 (20.0%) | 1 (7.1%) | |
| Mixed incontinence | 0 (0%) | 1 (7.1%) | |
| Amount of transvaginal births | |||
| 0 | 3.0 (30.0%) | 5 (35.7%) | 0.7 |
| 1 | 5.0 (50.0%) | 4 (28.6%) | |
| 2 | 2.0 (20.0%) | 3 (21.4%) | |
| 3 | 0 ( 0%) | 2 (14.3%) | |
| Perineal tears | |||
| 0 | 6 (60.0%) | 5 (35.7%) | 0.5 |
| 1 | 4 (40.0%) | 8 (57.1%) | |
| 3 | 0 ( 0%) | 1 (7.1%) | |
| Perineal cut | 4 (40.0%) | 2 (14.3%) | 0.2 |
| Wearing of incontinence inserts | 5 (50.0%) | 6 (42.9%) | >0.9 |
| Conduct of heavy physical work | 1 (10.0%) | 2 (14.3%) | >0.9 |
| No feeling of prolapse | 8 (80.0%) | 8 (57.1%) | 0.9 |
| Negative impact of UI on sexuality (NAS) | 10 (2.0–20.0) | 32.1 (0.0–60.0) | 0.6 |
| Nycturia | |||
| No suffering of nycturia | 4 (40.0%) | 4 (28.6%) | >0.9 |
| 1 UPN | 4 (40.0%) | 6 (42.9%) | |
| 1–2 UPN | 1 (10.0%) | 2 (14.3%) | |
| 2–3 UPN | 1 (10.0%) | 1 (7.1%) | |
| 3 UPN | 0 ( 0%) | 1 (7.1%) | |
Continuous measurements are presented as mean (SD), median [IQR]; ASA, American Society of Anaesthesiologists physical status classification; NAS, numeric analog scale; UI, urinary incontinence; UPN, urination per night.
Fisher’s exact test; Wilcoxon Mann–Whitney test; χ 2test for independence.
Table 1 Univariate analysis of patients who met the primary endpoint (per protocol analysis).
Univariate analysis of endpoints
The initial ICIQ score of patients in the ACTICORE group was 12 (9.0–13.0), and that of the control group was 11.0 (8.0–13.5). The ICIQ score of those in the intervention group decreased to 7.5 at 12 weeks. The ICIQ score in the control group decreased from 11.0 to 10.5. Univariate analysis showed a statistically significant difference between the 2 groups at 8 weeks (intervention group, ICIQ 8; control group, ICIQ 11.5; P=0.033). Significance at 12 weeks (primary endpoint) was not reached (lost to follow-up in the ACTICORE group, n=11). Table 2 summarizes information on ICIQ data.
Table 2.
Summarized ICIQ data
| ACTICORE group | Control group | ||
|---|---|---|---|
| n=21 | n=19 | P * | |
| Initial ICIQ | 12.0 (9.0–13.0) | 11.0 (8.0–13.5) | 0.5 |
| ICIQ after 4 weeks | 10.0 (7.0–12.5) | 11.0 (10.0–14.5) | 0.2 |
| Missing: 6 | Missing: 8 | ||
| ICIQ after 8 weeks | 8.0 (6.5–9.5) | 11.5 (10.0–14.2) | 0.033 |
| Missing: 10 | Missing: 7 | ||
| ICIQ after 12 weeks | 7.5 (6.2–10.2) | 10.5 (8.2–13.8) | 0.2 |
| Missing: 11 | Missing: 5 |
(P<0.05) value bold.
Continuous measurements are presented as mean (SD), median [IQR]; ICIQ, international consultation on incontinence questionnaire.
Mann–Whitney U test.
Regarding quality of life using the EG-5D-3L, no significant differences were found between both groups and intraindividually (Table 3).
Table 3.
Summarized EQ-5D-3L data
| Initial data on EQ-5D-3L | Data on EQ-5D-3L after 12 weeks | |||||
|---|---|---|---|---|---|---|
| Characteristics | ACTICORE group, N=10a | Control group, N=14a | P-Wertb | ACTICORE, N=10a | Control group, N=14a | P b |
| mobility | >0.9 | 0.14 | ||||
| 0 | 0.0 (0.0%) | 0.0 (0.0%) | 2.0 (20.0%) | 0.0 (0.0%) | ||
| 1 | 8.0 (80.0%) | 12.0 (85.7%) | 6.0 (60.0%) | 13.0 (92.9%) | ||
| 2 | 2.0 (20.0%) | 2.0 (14.3%) | 2.0 (20.0%) | 1.0 (7.1%) | ||
| self_care | >0.9 | 0.2 | ||||
| 0 | 0.0 (0.0%) | 0.0 (0.0%) | 2.0 (20.0%) | 0.0 (0.0%) | ||
| 1 | 10.0 (100.0%) | 14.0 (100.0%) | 8.0 (80.0%) | 13.0 (92.9%) | ||
| 2 | 0.0 (0.0%) | 0.0 (0.0%) | 0.0 (0.0%) | 1.0 (7.1%) | ||
| usual_activity | 0.3 | 0.3 | ||||
| 0 | 0.0 (0.0%) | 0.0 (0.0%) | 2.0 (20.0%) | 0.0 (0.0%) | ||
| 1 | 9.0 (90.0%) | 9.0 (64.3%) | 6.0 (60.0%) | 10.0 (71.4%) | ||
| 2 | 1.0 (10.0%) | 5.0 (35.7%) | 2.0 (20.0%) | 4.0 (28.6%) | ||
| pain_discomfort | 0.4 | 0.092 | ||||
| 0 | 0.0 (0.0%) | 0.0 (0.0%) | 2.0 (22.2%) | 0.0 (0.0%) | ||
| 1 | 7.0 (70.0%) | 7.0 (50.0%) | 5.0 (55.6%) | 6.0 (42.9%) | ||
| 2 | 3.0 (30.0%) | 7.0 (50.0%) | 2.0 (22.2%) | 8.0 (57.1%) | ||
| NA | 1 | 0 | ||||
| anxiety_depression | 0.2 | 0.075 | ||||
| 0 | 0.0 (0.0%) | 0.0 (0.0%) | 2.0 (20.0%) | 0.0 (0.0%) | ||
| 1 | 7.0 (70.0%) | 6.0 (42.9%) | 6.0 (60.0%) | 6.0 (42.9%) | ||
| 2 | 3.0 (30.0%) | 8.0 (57.1%) | 2.0 (20.0%) | 8.0 (57.1%) | ||
| Health status | 77.5 (9.5) | 78.6 (8.6) | 0.8 | 76.5 (11.3) | 72.1 (11.9) | 0.4 |
| NA | 0 | 1 | ||||
n (%); continuous measurements are presented as mean (SD).
Fisher’s exact test; Wilcoxon Mann–Whitney U test.
NA, not applied.
Study participants in the ACTICORE group showed a statistically significant intraindividual improvement in urinary incontinence symptoms (ICIQ score from 12 to 7.5). An improvement of 4.5 ICIQ score points was considered a clinically important difference, according to Nyström et al.6 (2015; minimal clinically important difference 2.52). A box plot thereon is depicted in Figure 1.
Figure 1.
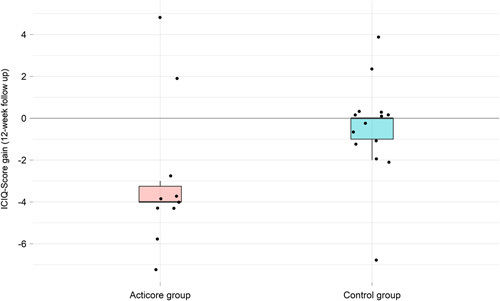
The results of the primary endpoint are shown in box plotting.
Discussion
Although scientific research projects existed before, the term ʻbiofeedbackʼ was not used until 1969, when the Biofeedback Research Society (now the Association for Applied Psychophysiology and Biofeedback) was founded9. From the beginning, research was conducted in a variety of areas, focusing on visceral learning as well as the development of neurofeedback. The use of biofeedback to treat urinary incontinence, described, for example by Cardozo et al.10, also began in the early 1970s. Unfortunately, from today’s perspective, some research projects have even caused harm, for example, the attempt to influence homosexuality through biofeedback11. The effectiveness of feedback and biofeedback was confirmed in a meta-analysis of 17 studies, where pelvic floor muscle training took place in the control group (RR: 0.75; 95% CI: 0.66–0.86)12,13.
In general, ACTICORE1 is not the only biofeedback trainer using an app. But this device can increase adherence due to its nature as a jump ‘n’ run/platform game. Several devices are available for biofeedback training of the pelvic floor. For example, the so-called Elvie-Trainer, Perifit, or the TensCare Perfect PFE Trainer are devices that also enable home training as a digital health application. Unfortunately, to our knowledge, a long-term evaluation with PubMed-indexed and registered clinical studies on this has not taken place. Only short-term evaluation was performed on the Elvie-Trainer without using validated questionnaires on incontinence14. This development naturally reflects the digitization of healthcare worldwide. However, clinical evaluation of these digital health applications should be mandatory to prescribe (or purchase) devices that have been shown to contribute to clinical improvement.
ACTICORE1 has already been evaluated with a pilot trial on fecal incontinence. The results show that pelvic floor training with ACTICORE1 provides adequate pelvic floor training. Noninferiority of ACTICORE1 training compared to Kegel exercises was found15. Following the evaluation of ACTICORE1 in fecal incontinence, the present study aimed to investigate the benefit of pelvic floor muscle training with this device in urinary incontinence.
In this study, at 4, 8, and 12 weeks, the ICIQ score of those in the ACTICORE1 group decreased from 12.9 to 7.5 and in the control group from 11.0 to 10.5. The lack of intraindividual improvement in the control group was expected because participants were asked not to exercise the pelvic floor. The slight increase in ICIQ may reflect a spontaneous improvement in incontinence symptoms. On the other hand, a value of 0.5 ICIQ scoring points does not seem to be considered a minimal clinically important difference6. Both groups’ statistical significance differed only after 8 weeks and not after 12 weeks (primary endpoint). That can be explained by the high rate of lost to follow-up in the ACTICORE group (52%). Nevertheless, participants in the intervention group achieved an improvement in urinary incontinence symptoms (ICIQ scoring points of 5.4 after 12 weeks) that exceeded the published minimal clinically significant difference of 2.526.
The intraindividual improvement of patients in the ACTICORE group was statistically significant and may reflect the positive effect of biofeedback training demonstrated in the literature12,13.
ACTICORE1 has the advantage that the sensor does not have to be inserted in comparison to other devices. The gaming aspect may increase the adherence of patients and can also be considered a benefit. On the other hand, adherence was not sufficient among the participants of the ACTICORE group (lost to follow-up rate of 52%). One explanation could be the fact that ACTICORE1 was provided free of charge to the patients. This might have led to a lower motivation to complete the questionnaires in comparison to those of the control group. They received ACTICORE1 free of charge after 12 weeks (lost to follow-up rate of 26%). Another explanation could be that individuals of older age may not have as much affinity for digital health applications as younger patients. When analyzing the per protocol analysis population (Table 1), it is notable that these participants are almost 10 years younger compared with the overall study population (42.5 [35.5–54.5] years vs. 52 [39–60] years). These results may be due to a higher affinity for ACTICORE1.
ACTICORE1 also has further disadvantages. As a digital health application, it is only usable for patients who own a smartphone. Some people just do not like smartphone games at all.
In this study, quality of life was assessed during the same periods as the ICIQ score. No statistically significant interindividual and intraindividual improvements were measured when using the EQ-5D-3L questionnaire. The fact that the improvement of urinary incontinence symptoms in the ACTICORE group did not lead to an improvement in quality of life is notable. It might be explained that the EQ-5D-3L questionnaire does not focus on incontinence symptoms. Therefore, future studies should use, for example, the validated King’s Health Questionnaire from 1997, as it addresses patients’ incontinence16,17. Another explanation could be that urinary incontinence does not affect the quality of life to the extent that therapists and physicists may assume. Thus, although incontinence is widespread, its impact on quality of life might be overestimated.
The inclusion of both sexes and all forms of urinary incontinence may be considered a limitation of the study. However, after univariate analysis, the groups did not differ regarding these variables. The small sample size can be criticized. However, the trial aimed to facilitate a power calculation for a subsequent project. A total of 40 individuals seems to be an appropriate sample size. As a further limitation, there was no adherence measurement and no stratified randomization.
Conclusion
Biofeedback training with ACTICORE1 significantly reduces symptoms of urinary incontinence after 12 weeks.
Ethical approval
The study was approved by the Ethics Committee of Berlin (Eth-14/21) and Brandenburg (S12(bB)/2021) in 2021. It was conducted in accordance with the Declaration of Helsinki.
consent
All patients gave their written consent prior to study enrollment.
Sources of funding
ACTICORE AG.
Authors contribution
S.S. and E.L.: writing of the paper and patients recruitment (Berlin); J.O., M.G., and H.B.: patients recruitment (Department of Urology and Gynecology, Brandenburg an der Havel); S.H.: patients recrutiment (Department of General and Visceral Surgery, Brandenburg an der Havel); M.H. and R.M.: supervision of manuscript writing, study designing, and conduction; R.H.: statistical analysis; C.P.: manuscript writing, data analysis, and study designing.
Conflicts of interest disclosure
C.P., R.H., and S.S. received payment for conducting the study from ACTICORE AG.
Research registration unique identifying number (UIN)
This trial was registered in the German Clinical Trials Register (DRKS00024913).
Guarantor
Christoph Paasch.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Data availability statement
The data are available on reasonable request.
Supplementary Material
Footnotes
S.S. shared first authorship.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, http://www.lww.com/annals-of-medicine-and-surgery.
Published online 18 August 2023
Contributor Information
Christoph Paasch, Email: chpaasch@web.de.
Sonja Soeder, Email: s.soeder@alexianer.de.
Eric Lorenz, Email: e.lorenz@alexianer.de.
Sophie Heisler, Email: sophie.heisler@klinikum-brandenburg.de.
Matthias Götze, Email: goetze@klinikum-brandenburg.de.
Hendrik Borgmann, Email: hendrik.borgmann@klinikum-brandenburg.de.
Julia Olthoff, Email: j.olthoff@klinikum-brandenburg.de.
Michael Hünerbein, Email: michael.huenerbein@oberhavel-kliniken.de.
Richard Hunger, Email: hungerlpz@web.de.
René Mantke, Email: mantke.mhb@klinikum-brandenburg.de.
References
- 1.Beutel ME, Hessel A, Schwarz R, et al. Prevalence of urinary incontinence in the German population. Urologe A 2005;44:232–238. [DOI] [PubMed] [Google Scholar]
- 2.McLean L, Varette K, Gentilcore-Saulnier E, et al. Pelvic floor muscle training in women with stress urinary incontinence causes hypertrophy of the urethral sphincters and reduces bladder neck mobility during coughing. Neurourol Urodyn 2013;32:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DgfGuGeV (DGGG). S2k-Leitlinie Harninkontinenz der Frau. AWMF online, 2022; Registernummer 015 – 091.
- 4.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–343. [DOI] [PubMed] [Google Scholar]
- 6.Nyström E, Sjöström M, Stenlund H, et al. ICIQ symptom and quality of life instruments measure clinically relevant improvements in women with stress urinary incontinence. Neurourol Urodyn 2015;34:747–751. [DOI] [PubMed] [Google Scholar]
- 7.Uren AD, Cotterill N, Pardoe M, et al. The international consultation on incontinence questionnaires (ICIQ): an update on status and direction. Neurourol Urodyn 2020;39:1889–1896. [DOI] [PubMed] [Google Scholar]
- 8.Laycock JJD. Pelvic floor muscle assessment: the PERFECT scheme. Physiotherapy 2001;87:631–642. [Google Scholar]
- 9.Moss D. Chapter 9 Biofeedback, mind–body medicine, and the higher limits of human nature. Humanistic and Transpersonal Psychology: a Historical and Biographical Sourcebook. Greenwood; 1999.
- 10.Cardozo L, Stanton SL, Hafner J, et al. Biofeedback in the treatment of detrusor instability. Br J Urol 1978;50:250–254. [DOI] [PubMed] [Google Scholar]
- 11.Barlow DH, Agras WS, Abel GG, et al. Case histories and shorter communications. Biofeedback and reinforcement to increase heterosexual arousal in homosexuals. Behav Res Ther 1975;13:45–50. [DOI] [PubMed] [Google Scholar]
- 12.Herderschee R, Hay-Smith EJ, Herbison GP, et al. Feedback or biofeedback to augment pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev 2011;7:Cd009252. [DOI] [PubMed] [Google Scholar]
- 13.Kopańska M, Torices S, Czech J, et al. Urinary incontinence in women: biofeedback as an innovative treatment method. Ther Adv Urol 2020;12:1756287220934359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czyrnyj CS, Bérubé M, Brooks K, et al. Reliability and validity of a mobile home pelvic floor muscle trainer: the Elvie trainer. Neurourol Urodyn 2020;39:1717–1731. [DOI] [PubMed] [Google Scholar]
- 15.Paasch C, Bruckert L, Soeder S, et al. The effect of biofeedback pelvic floor training with ACTICORE1 on fecal incontinence: a prospective multicentric cohort pilot study. Int J Surg 2022;101:106617. [DOI] [PubMed] [Google Scholar]
- 16.Kelleher CJ, Cardozo LD, Khullar V, et al. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstetr Gynaecol 1997;104:1374–1379. [DOI] [PubMed] [Google Scholar]
- 17.Luz R, Pereira I, Henriques A, et al. King’s Health Questionnaire to assess subjective outcomes after surgical treatment for urinary incontinence: can it be useful? Int Urogynecol J 2017;28:139–145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available on reasonable request.


