Abstract
BACKGROUND:
Focused ultrasound (FUS-T) and stereotactic radiosurgery thalamotomy (SRS-T) targeting the ventral intermediate nucleus are effective incisionless surgeries for essential tremor (ET). However, their efficacy for tremor reduction and, importantly, adverse event incidence have not been directly compared.
OBJECTIVE:
To present a comprehensive systematic review with network meta-analysis examining both efficacy and adverse events (AEs) of FUS-T vs SRS-T for treating medically refractory ET.
METHODS:
We conducted a systematic review and network meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, using the PubMed and Embase databases. We included all primary FUS-T/SRS-T studies with approximately 1-year follow-up, with unilateral Fahn-Tolosa-Marin Tremor Rating Scale or Clinical Rating Scale for Tremor scores prethalamotomy/post-thalamotomy and/or AEs. The primary efficacy outcome was Fahn-Tolosa-Marin Tremor Rating Scale A+B score reduction. AEs were reported as an estimated incidence.
RESULTS:
Fifteen studies of 464 patients and 3 studies of 62 patients met inclusion criteria for FUS-T/SRS-T efficacy comparison, respectively. Network meta-analysis demonstrated similar tremor reduction between modalities (absolute tremor reduction: FUS-T: −11.6 (95% CI: −13.3, −9.9); SRS-T: −10.3 (95% CI: −14.2, −6.0). FUS-T had a greater 1-year adverse event rate, particularly imbalance and gait disturbances (10.5%) and sensory disturbances (8.3%). Contralateral hemiparesis (2.7%) often accompanied by speech impairment (2.4%) were most common after SRS-T. There was no correlation between efficacy and lesion volume.
CONCLUSION:
Our systematic review found similar efficacy between FUS-T and SRS-T for ET, with trend toward higher efficacy yet greater adverse event incidence with FUS-T. Smaller lesion volumes could mitigate FUS-T off-target effects for greater safety.
KEY WORDS: HIFU, FUS, MRgFUS, SRS, Thalamotomy, Essential tremor
ABBREVIATIONS:
- AEs
adverse events
- CRST
Clinical Rating Scale for Tremor
- ET
essential tremor
- FTM-TRS
Fahn-Tolosa-Marin Tremor Rating Scale
- FUS-T
focused ultrasound thalamotomy
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SDR
skull density ratio
- SRS-T
stereotactic radiosurgery thalamotomy.
Essential tremor (ET) is the most common movement disorder, with a prevalence of 0.5% to 5%.1,2 Although neuromodulation surgery such as deep brain stimulation (DBS) of the thalamus can be highly effective to reduce symptoms of medically refractory ET,3-7 incisionless lesion-based approaches may be needed in cases where patients have contraindications to DBS. Circumstances include medical comorbidities that increase complication risks for implantation surgery, limited access to DBS programming, and patient preference.8,9 Two common incisionless thalamotomy surgeries are stereotactic radiosurgery thalamotomy (SRS-T), also known as Gamma Knife thalamotomy, and more recently, focused ultrasound thalamotomy (FUS-T).
SRS-T and FUS-T are both permanently ablative surgeries, most commonly targeting the ventral intermediate nucleus (Vim) of the thalamus. In SRS-T, multiple radiation beams are delivered to create the lesion,10 although real-time monitoring of symptom improvement is precluded because of delay of symptom improvement by up to 6 months.11 By contrast, for FUS-T, ultrasound waves are delivered to heat and ablate tissue with real-time monitoring of tissue temperature using MR thermometry and clinical assessment for tremor improvement.12,13
Given these methodological differences, it is important to compare the relative efficacy of these incisionless modalities. Prior systematic reviews and meta-analyses comparing the 2 modalities have reported percent tremor reduction14 and composite efficacy.11 However, neither of these measures directly compares tremor reduction using the same rating scale, limiting conclusions. In addition, no study has complemented analysis of tremor reduction with direct comparison of adverse effect profiles of SRS-T and FUS-T. Thus, we performed a systematic review and network meta-analysis to directly compare SRS-T and FUS-T efficacy in tremor reduction for patients with medically refractory ET, with comprehensive characterization of adverse effects for each therapy.
METHODS
Retrospective Review
Literature Search
The systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.15 This study answered the following questions: (1) What is the effect of FUS-T vs SRS-T on tremor reduction in patients with ET? (2) What are the long-term adverse effects of FUS-T vs SRS-T in patients with ET? The PubMed database search was conducted on October 6th, 2022, from inception of the database to October 2022 using the following search: “(((ET OR essential tremor) AND thalamotomy) AND ((SRS OR stereotactic radiosurgery OR GK OR gamma knife) OR (focused US OR focused ultrasound))).” We repeated this query on the Embase database on October 10th, 2022, with the same time period and search terms. In accordance with guidelines for meta-analyses comprising publicly available research reports, written informed consent from participants was not required. The data sets supporting the current study are available from the corresponding author upon reasonable request.
Study Selection
Two researchers (S.K. and A.B.S.) independently screened all titles and abstracts based on the following inclusion and exclusion criteria.16 The inclusion criteria were (1) the study population comprised patients with only ET, (2) the patients received either unilateral SRS-T or FUS-T, and (3) tremor severity in the study was scored using a validated tremor scoring scale. Exclusion criteria were (1) all articles not available in English; (2) studies that involved animals, without any human participants; (3) technical analyses; (4) editorials; (5) literature or systematic reviews; (6) case reports or series with <5 patients; and (7) follow-up ≤3 months (Figure 1). In the case of studies with mixed patient populations (eg, patients with either ET or Parkinson disease), studies were included only when ET patient data could be reliably separated from the larger cohort. In the case where multiple studies reported the same or overlapping patient data, the study with most recent and comprehensive patient population was included.
FIGURE 1.
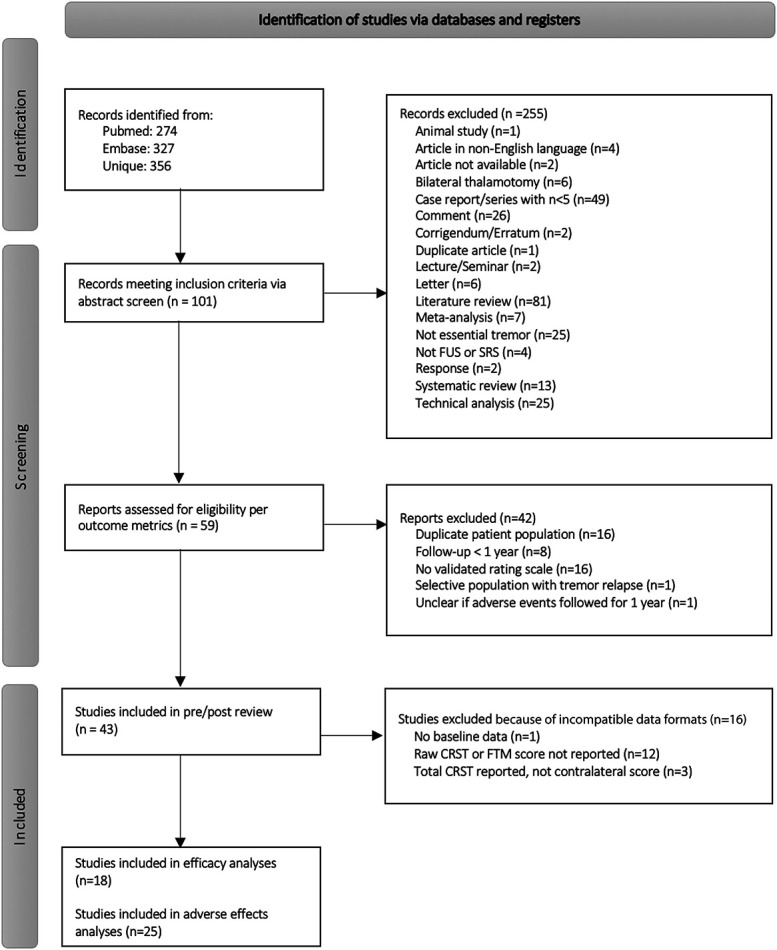
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of study identification, screening, and inclusion. CRST, Clinical Rating Scale for Tremor; FTM, Fahn-Tolosa-Marin; FUS, focused ultrasound; SRS, stereotactic radiosurgery.
Given the difficulty in directly comparing across different tremor scales, we then further subselected studies that used the unilateral tremor score (parts A and B) from the Fahn-Tolosa-Marin Tremor Rating Scale (FTM-TRS) or Clinical Rating Scale for Tremor (CRST), also noted as the tremor score for the treated hand (TSTH). Although referred to with different names, each of these scales record information about subcomponents of tremor on the same numerical scale; the maximum possible unilateral tremor score is 58. For inclusion in network meta-analysis, total score with standard deviation for each group needed to be reported. If a study included the median and interquartile range, the data were converted to mean and standard deviation.17 Studies that did not meet criteria for inclusion in the network meta-analysis of efficacy were still considered for analysis if there was characterization of adverse events (AEs) after 1 year of follow-up. The results of the full process for study selection are presented in Figure 1.
Data Extraction
Two reviewers (S.K. and A.B.S.) independently extracted data for the systematic review on study design, patient population (whether inclusion and exclusion criteria were met, lesion area, and number of patients), and outcomes (unilateral tremor scores). All reviewers (S.K., A.B.S., and D.D.W.) verified accuracy of study inclusion or exclusion, study metrics, and outcomes.
Data Bias
No study included in the network meta-analysis directly compared FUS-T vs SRS-T with 2 groups of participants, precluding assessment of bias during treatment allocation between the 2 modalities. There was also no bias due to selective reporting because we only included studies reporting the total unilateral FTM-TRS score. However, we have used the ROBINS-I risk of bias assessment18 for each study to examine quality of outcome assessment (Table 1).
TABLE 1.
Pooled Demographics for Prethalamotomy vs Post-Thalamotomy Studies with ∼12 Month Follow-up
| Authors | Year | FUS or SRS | n | F/M | Age at baseline, mean, ± SD (range) | Disease duration (y), mean ± SD (range) | FTM-TRS Baseline, mean ± SD |
Follow-up time (mo) | FTM-TRS at follow-up, mean ± SD | Post-treatment lesion size: volume, mm3 | ROBINS-I risk of bias assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S Kato, et al.27 | 2022 | FUS | 15 | 4/11 | 72.8 ± 5.39 (64-81) | 27.6 ± 20.9 (6-65) | 19 ± 3.4 | 6 | 7.7 ± 5.4 | ∼ | Confounding |
| C Pae, et al.28 | 2022 | FUS | 85 | 18/67 | 65.3 ± 8.7 (45-86) | 13.8 ± 10.6 (1-40) | 18.2 ± 5.3 | 6 | 5.1 ± 5.5 | 242 ± 91 | Confounding Selection of participants |
| V Purrer, et al.29 | 2022 | FUS | 37 | 12/25 | 69.4 ± 12.2 | 19.1 ± 12.0 | 19.2 ± 4.2 | 12 | 6.2 ± 5.0 | ∼ | Confounding |
| K Yamamoto, et al.26 | 2022 | FUS | 53 | 31/69a | 38.1 ± 20.6a | ∼ | 20.4 ± 5.1a | 12 | 10.7 ± 6.5 | 175.3 ± 93.4 | Confounding Missing data |
| K Fukutome, et al.32 | 2021 | FUS | 15 | 4/11 | 62.9 ± 11.3 | 21.5 ± 14.0 | 18.5 ± 5.8 | 12 | 4.6 ± 5.7 | 68.0 ± 29.8 | Confounding |
| P Wu, et al.30 | 2021 | FUS | 48 | 17/31 | 59.14 ± 13.5 | 19.2 ± 13.6 | 14.7 ± 4.9 | 12 | 7.0 ± 5.5 | ∼ | Confounding |
| G Zur, et al.31 | 2020 | FUS | 22 | 8/14 | 72 ± 6 | 13 ± 8 | 19.2 ± 6.9 | 6 | 4 ± 4 | ∼ | Confounding Selection of participants |
| A Sinai, et al.33 | 2019 | FUS | 24 | 17/27a | 70.5 (63-87)a | 16.3 ± 10.4a | 19.5 ± 7.2a | 12 | 7.0 ± 5.8 | 297.1 ± 128.0 | Confounding Missing data |
| YS Park, et al.34 | 2019 | FUS | 15 | 2/10 | 61.7 ± 8.1 | 17.8 ± 13.03 | 17.4 ± 3.8 | 12 | 5.3 ± 3.4 | 82.6 ± 29.0 | Confounding |
| C Gasca-Salas, et al.35 | 2019 | FUS | 23 | 6/17 | ∼ | ∼ | 16.6 ± 4.6 | 12 | 6.1 ± 4.1 | ∼ | Confounding Selection of participants |
| Q Tian, et al.36 | 2018 | FUS | 8 | ∼ | ∼ | ∼ | 18.9 ± 2.4 | 12 | 11.0 ± 4.8 | ∼ | Confounding |
| NY Jung, et al.37 | 2018 | FUS | 20 | 3/17 | 64.1 (47-77) | 21.2 (5-54) | 18.2 ± 4.0 | 12 | 5.8 ± 4.5 | ∼ | Confounding Selection of participants |
| WJ Elias, et al.38 | 2016 | FUS | 76 | 24/52 | 70.8 ± 8.7 | 16.4 ± 13.1 | 18.1 ± 4.8 | 12 | 10.9 ± 4.5 | ∼ | Confounding |
| WS Chang, et al.39 | 2015 | FUS | 8 | 1/7 | 66.1 ± 5.25 | 32.1 ± 16.1 | 18.1 ± 3.2 | 6 | 4.0 ± 3.5 | 97.9 ± 41.9 | Confounding |
| WJ Elias, et al.40 | 2013 | FUS | 15 | 5/10 | 66.6 ± 8.0 | 32.0 ± 21.3 | 20.4 ± 5.2 | 12 | 5.2 ± 4.8 | ∼ | Confounding |
| FUS aggregate | 464 | 152 (29.2%)/368 (70.8%)b | 63.3b | 59.9b | 18.3 ± 5.2a | 10.3 | 7.3 ± 5.7 | 200.2b | |||
| TAW Bolton, et al.41 | 2022 | SRS | 34 | 17/17 | 70.1 ± 9.1 | 35.5 ± 18.3 | 20.4 ± 5.5 | 12 | 6.3 ± 7.7 | 120 ± 130 | |
| C Tuleasca, et al.42 | 2017 | SRS | 17 | 12/5 | 70.1 ± 9.8 | 38 ± 19.5 | 18.6 ± 5.5 | 12 | 7.2 ± 4.1 | 125 ± 162 | |
| SY Lim, et al.43 | 2010 | SRS | 11 | 2/9 | 75.8 ± 5.9 | 21.1 ± 12.7 | 22.0 ± 5.7 | 24 | 20.1 ± 8.4 | ∼ | Confounding |
| SRS aggregate | 62 | 31 (50.0%)/31 (50.0%)b | 71.1b | 33.6 | 20.2 ± 5.6 | 14.1 | 9.0 ± 8.7 | 121.7b |
CRST, Clinical Rating Scale for Tremor; FTM-TRS, Fahn-Tolosa-Marin Tremor Rating Scale; FUS, focused ultrasound; SRS, stereotactic radiosurgery.
From initial enrolment, exact demographics of specific sample not available.
From data available. ∼not reported.
CRST scores were relabeled as FTM-TRS scores.
Data Ethics Characterization
We assessed each study for whether it met the criteria for ethical data collection per the Declaration of Helsinki. Twenty one studies provided explicit statements with institutional review board (IRB)/local ethics committee (LEC) approval and written consent. Seven studies stated IRB/LEC approval, and 5 studies stated informed consent for the procedure. Four studies implied consent through statements such as “enrolled” and “enrollment in clinical trials.” Finally, 6 studies had no mention as to whether the data were collected ethically or with patient consent.19-24
Data Synthesis and Statistical Analysis
Meta-analysis
First, we conducted independent meta-analyses for FUS-T and SRS-T tremor reduction. A pooled weighted mean distribution and 95% CI of FTM-TRS A+B scores relative to preprocedure baseline were determined for studies that had follow-up close to 12 months. We assessed heterogeneity using the I2 statistic. For meta-analysis calculations and visual result display, we used MetaXL (version 5.3, EpiGear).
Network meta-analysis
For comparing tremor reduction after FUS-T or SRS-T, a network meta-analysis was conducted to compute a pooled weighted mean distribution and 95% CI of FTM-TRS A+B tremor reduction. This was also compared with preprocedure baseline, using the same studies that were included in the prior meta-analyses. We assessed transitivity by examining age of cohorts at baseline, disease duration, difference in sex distributions, and average follow-up times. Network meta-analysis calculations and visual display of results were conducted using MetaInsight (version 3.1.14, NIHR CRSU), which uses frequentist models from the R package “netmeta.”25
Failure rate analysis
For each study that met inclusion criteria for either efficacy or adverse event comparison, we characterized whether authors explicitly described a subpopulation of treated patients where the outcome was below a prespecified efficacy threshold. Descriptions included “failure,” “nonresponse,” “poor response,” “relapse,” or “recurrence.” Cohorts were then descriptively pooled across FUS-T and SRS-T treatment modalities.
Adverse events analysis
We compiled AEs reported in the literature for all studies that included follow-up from FUS-T or SRS-T for at least 1 year. Incidences of AEs were estimated by summing the count of AEs across all included studies and dividing by the total number of patients summed over all studies. We additionally compared the difference in AEs between FUS patient populations with “classic” Vim targeting (ie, at the z-level of the intercommissural (AC-PC) plane) vs those with “modified” Vim targeting (ie, noted in the respective Methods sections to be superior to the AC-PC plane). In addition to each category of AEs, we included a “maximum AE rate” category, defined by the maximum AE rate of the categories included, for subanalyses given that multiple AEs could be present in a single patient. Statistical analysis was performed using the 2-sample z-test for proportions or linear regression where appropriate.
Data Availability
The data sets supporting the current study are available from the corresponding author on reasonable request.
RESULTS
Patient Demographics of Included Studies for Efficacy Comparison
The full study selection process for inclusion in this analysis is detailed in the PRISMA flow diagram (Figure 1). Fifteen studies (n = 464) with FUS-T26-40 and 3 studies (n = 62) with SRS-T41-43 met inclusion criteria for pre-thalamotomy vs post-thalamotomy efficacy analyses. The weighted average follow-up times were 10.3 months and 14.1 months for FUS-T and SRS-T groups, respectively. Full pooled patient demographics by paper are detailed in Table 1.
Assessment of Risk of Bias
Sixteen of 18 of the studies did not explicitly address confounding factors, such as age, duration of symptoms, or lesion volume, that could have compromised the results. Four of 18 studies had risk of bias in selection of participants in the study because they did not explicitly detail both inclusion and exclusion criteria. Two studies across all analyses demonstrated attrition bias from initial enrollment baseline statistics; these are marked by superscript a in Table 1. In addition, low skull density ratio (SDR) was an exclusion criterion for the FUS cohorts, whereas this was not the case for the SRS cohorts. As we subselected those studies that reported the contralateral FTM-TRS A+B raw scores at baseline and follow-up, there was no risk of bias arising from measurement of exposure or outcome or in selection of the reported result. Finally, as only studies that had FUS-T or SRS-T without additional intervention were included, there was no risk of bias due to postexposure intervention.
FUS-T and SRS-T Tremor Reduction vs Prethalamotomy Baseline
From the pairwise meta-analysis, FUS-T demonstrated significant tremor reduction as measured by the unilateral FTM-TRS A+B score reduction at follow-up closest to 12 months (Supplemental Figure 1, http://links.lww.com/NEU/D755, absolute tremor reduction: −11.13 [95% CI: −12.74, −9.52]; relative tremor reduction, 60.8%). SRS-T also demonstrated significant absolute tremor reduction (Supplemental Figure 2, http://links.lww.com/NEU/D756, absolute tremor reduction: −11.41 [95% CI: −17.39, −5.44]; relative tremor reduction 56.5%).
Network meta-analysis demonstrated a trend toward greater absolute tremor reduction with FUS-T (Figure 2). FUS-T demonstrated 1.31 (95% CI: −2.96, 5.99) greater weighted mean difference in FTM-TRS A+B reduction relative to SRS-T (Table 2, FUS-T: −11.57 [95% CI: −13.3, −9.93]; SRS-T: −10.26 [95% CI: −14.21, −5.96]), resulting in a 72.8% probability that FUS-T is the more efficacious treatment for tremor reduction (Table 3).
FIGURE 2.
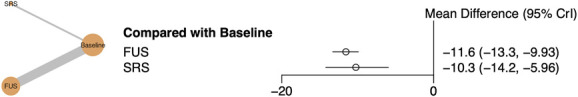
Network meta-analysis results comparing reduction in tremor score from baseline for FUS vs SRS thalamotomy. Left, Network plot of FUS vs SRS thalamotomy. Right, Forest plot of frequentist model for network meta-analysis. FUS, focused ultrasound; SRS, stereotactic radiosurgery.
TABLE 2.
Comparison of All Treatments for FTM-TRS A+B Tremor Reduction
| Condition | Baseline | FUS | SRS |
|---|---|---|---|
| Baseline | Baseline | −11.57 (−13.3, −9.93) | −10.26 (−14.21, −5.96) |
| FUS | 11.57 (9.93, 13.3) | FUS | 1.31 (−2.96, 5.99) |
| SRS | 10.26 (5.96, 14.21) | −1.31 (−5.99, 2.96) | SRS |
FTM-TRS, Fahn-Tolosa-Marin Tremor Rating Scale; FUS, focused ultrasound; SRS, stereotactic radiosurgery.
TABLE 3.
Ranking Table for All Studies, With Probability for Each Treatment to be the Best
| Condition | Rank 1 | Rank 2 | Rank 3 |
|---|---|---|---|
| Baseline | 0 | 7.5E-05 | 0.9999 |
| FUS | 0.7281 | 0.2719 | 0 |
| SRS | 0.2719 | 0.7280 | 7.5E-05 |
FUS, focused ultrasound; SRS, stereotactic radiosurgery.
For FUS-T and SRS-T, I2 was 79% and 84%, Q statistic was 62.29 and 12.40, and P < .01 and < .01, respectively, indicating significant heterogeneity in study results among both therapies. We also note that there are differences in the FUS-T and SRS-T baseline demographics, with a greater proportion of men, younger age, greater disease duration, shorter follow-up time, and greater lesion volume in the FUS-T combined cohort, leading to decreased transitivity for comparison in the network meta-analysis. We used descriptive comparison as none of the demographics were reported across all studies for direct quantitative comparison.
Failure Rate
We assessed all papers meeting either efficacy or adverse event inclusion criteria for whether failure rates were explicitly characterized by the authors. Although 9 SRS-T papers explicitly did so, only 4 FUS-T papers did, possibly because of real-time feedback regarding tremor reduction that is available with FUS-T (Table 4). In addition, the definition of failure was most commonly <50% contralateral FTM-TRS improvement with FUS-T, whereas with SRS-T it was more commonly little or no reduction in tremor. Thus, although the failure rate was numerically higher for FUS-T (37%) than SRS-T (13%), this was confounded by differences in failure rate definitions, as well as no reporting of 0 failure rate in the FUS-T studies.
TABLE 4.
Reported Failure Rates for Prethalamotomy vs Post-Thalamotomy Studies Included in Either Efficacy or Adverse Outcome Analyses
| Authors | Year | FUS or SRS | n | Failure definition | Failure rate |
|---|---|---|---|---|---|
| C Pae et al.28 | 2022 | FUS | 72 | <50% contralateral CRST improvement at 6 mo | 0.18 |
| J Torii et al.44 | 2021 | FUS | 61 | <50% contralateral CRST improvement at 3 mo | 0.51 |
| K Yamamoto et al.51 | 2019 | FUS | 6 | Recurrence at 3 mo | 0.33 |
| Y Meng et al.52 | 2018 | FUS | 35 | <50% tremor improvement at 1 y | 0.54 |
| FUS aggregate | 174 | 0.37b | |||
| C Tuleasca et al.42 | 2017 | SRS | 17 | ≤50% improvement in TSTH | 0.35 |
| A Niranjan et al.20 | 2017 | SRS | 91 | No improvement in any FTM scores | 0.12 |
| C Ohye et al.60 | 2012 | SRS | 53a | ≤50% improvement in tremor | 0.15a |
| SY Lim et al.43 | 2010 | SRS | 17 | Lack of tremor suppression | 0.12 |
| D Kondziolka et al.9 | 2008 | SRS | 27 | Lack of tremor improvement | 0.11 |
| C Ohye et al.61 | 2002 | SRS | 11 | <75% tremor reduction | 0.22 |
| RF Young et al.22 | 2000 | SRS | 51 | Criteria not described, “treatment failure” | 0.079 |
| A Niranjan et al.23 | 2000 | SRS | 8 | <50% tremor improvement | 0.00 |
| RF Young et al.24 | 1998 | SRS | 27 | Little or no reduction in tremor | 0.11 |
| SRS aggregate | 302 | 0.13b |
CRST, Clinical Rating Scale for Tremor; FTM, Fahn-Tolosa-Marin; FUS, focused ultrasound; SRS, stereotactic radiosurgery; TSTH, tremor score for the treated hand.
Includes both essential tremor and Parkinson disease patient populations.
From studies that explicitly reported failure rates, poor responses, or nonresponder cohort.
Adverse Events
Twenty three studies for FUS-T19,26,29,30,32-34,37,38,44-57 and 11 studies for SRS-T9,20-24,43,58-61 met inclusion criteria for comparison of AE incidence (Table 4). Seven hundred and twenty four FUS-T and 449 SRS-T cases were available for AE analysis. Long-term AEs for FUS-T in order of decreasing frequency were imbalance/gait disturbance (10.5%), sensory-related disturbance (8.3%), motor weakness (3.0%), dysmetria (2.5%), dysgeusia (2.3%), dysarthria (2.1%), and others (1.5%, including dyskinesia/dystonia, lethargy, and disequilibrium), as seen in Table 4. For SRS-T, adverse thalamotomy effects in order of decreasing frequency were motor weakness/hemiparesis (2.7%), dysarthria/speech impairment (2.5%), sensory disturbance (1.1%), headache (0.4%), and thalamic edema/hemorrhage (0.2%). Of note, many studies reported that different AEs could be present for the same patient; studies which either explicitly or implicitly (via frequency total) stated this are indicated in Table 5.
TABLE 5.
Pooled AEs for Thalamotomy Studies with ∼12 Month Follow-up
| Authors Year FUS or SRS |
Follow-up length maximum (mo) | Targeting method (classic or modified Vim, or other [coords: x, y, z])a | Post-treatment lesion size: volume (mm3) | n | Adverse effects | Imbalance/gait disturbance | Sensory disturbance | Dysmetria | Dysgeusia | Motor weakness | Dysarthria | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K Yamamoto26 2022 FUS |
24b | Modified x: 11 mm y: 1/4 AC-PC distance z: 2 mm |
175.3 ± 93.4d | 56 | 8 | 6 | n/a | n/a | 3 | 0 | n/a | |
| V Purrer29 2022 FUS |
12 | Classic | n/a | 37 | 4 | 8 | n/a | 10 | 1 | n/a | 6 (dyskinesia/dystonia) | |
| J Torii44 2022 FUS |
24b | Modified x: 11.5 to 12.0 mm y: 1/3 AC-PC distance minus 1.5 mm z: 1.5 to 2.0 mm |
n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a | None | |
| K Abe45 2021 FUS |
12 | Classic | n/a | 35 | 0 | 0 | n/a | n/a | 0 | 0 | None | |
| DJ Segar46 2021 FUS |
12 | Modified x: 11 mm y: 1/4 AC-PC distance minus 1.5 mm z: 1.5 to 2.0 mm |
288.98 ± 137.72d | 100 | 15c | 17c | 7c | 3c | 3c | 6c | N/A | |
| P Wu30 2021 FUS |
24b | Classic | n/a | 48 | 0 | 3 | n/a | n/a | 2 | 0 | 3 (not detailed) | |
| E Tommasino47 2021 FUS |
12 | Modified x: 11 mm y: 1/4 to 3/10 AC-PC distance z: 0 to 2 mm |
n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a | None | |
| H Ito48 2020 FUS |
24b | Classic | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a | None | |
| K Fukutome32 2020 FUS |
12 | Classic | 68.0 ± 29.8 | 15 | 0 | 1 | n/a | n/a | n/a | n/a | n/a | |
| MN Gallay49 2020 FUS |
12 | Other: cerebellothalamic tract between red nucleus and subthalamic nucleus at the level of the AC-PC plane | n/a | 10 | 3 | 1 | n/a | n/a | 1 | 0 | n/a | |
| AN Kapadia50 2020 FUS |
12 | n/a | 180.8 ± 91.5 | 94 | 27c | 8c | 9c | n/ac | 9c | 9c | n/a | |
| A Sinai33 2019 FUS |
60b | n/a | 297.1 ± 128.0d | 24 | 2 | 2 | n/a | 2 | n/a | n/a | n/a | |
| YS Park34 2019 FUS |
48b | Classic | 82.6 ± 29.023 | 15 | n/a | n/a | n/a | n/a | n/a | n/a | None | |
| K Yamamoto51 2019 FUS |
12 | n/a | n/a | 6 | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
| Y Meng52 2018 FUS |
24 | n/a | 220.2 ± 112.3 | 35 | 5c | 2c | 0 | n/a | 2c | 0 | 1 (lethargy)c | |
| M Harary53 2018 FUS |
12 | n/a | 300 ± 100 | 7 | 3 | 1 | n/a | n/a | n/a | n/a | n/a | |
| NY Jung37 2018 FUS |
12 | Classic | n/a | 20 | 0 | n/a | n/a | n/a | n/a | n/a | n/a | |
| DG Iacopino54 2018 FUS |
12 | Modified x: 12 to 14 mm laterally from the intercommissural plane y: 1/4 AC-PC distance z: 0 to 2 mm |
n/a | 13 | 1 | 0 | n/a | n/a | n/a | n/a | n/a | |
| M Zaaroor19 2018 FUS |
12 | Classic | n/a | 18 | 0 | 0 | n/a | n/a | n/a | n/a | n/a | |
| M Kim55 2017 FUS |
12 | Classic | n/a | 10 | n/a | 0 | n/a | n/a | n/a | 0 | none | |
| WJ Elias38 2016 FUS |
12 | n/a | n/a | 56 | 5 | 8 | 2 | 2 | 1 | 0 | 1 (disequilibrium) | |
| MN Gallay56 2016 FUS |
12 | Other: Cerebellothalamic tract x: 8 mm lateral to the thalamo-ventricular border y: 5 mm posterior to the midcommissural line z: 3 mm below the intercommissural plane |
n/a | 21 | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
| DS Huss57 2015 FUS |
24b | Classic | n/a | 15 | 0 | 3 | n/a | n/a | 0 | 0 | n/a | |
| FUS aggregate | 724 | 76 (10.5%) | 60 (8.3%) | 18 (2.5%) | 17 (2.3%) | 22 (3.0%) | 15 (2.1%) | 11 (1.5%) | ||||
| MH Khattab58 2022 SRS |
12 | Modified x: 11 mm y: 1/4 AC-PC distance z: 2 to 4 mm |
n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a | 2 (headache) | |
| A Niranjan20 2017 SRS |
152b | Modified x: 11 mm y: 1/4 AC-PC distance z: 2 mm |
n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| T Witjas59 2015 SRS |
12 | Modified x: 11 mm y: 3.9 to 9.9 mm z: 2.5 mm |
n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a | None | |
| C Ohye60 2012 SRS |
24b | Modified x: 15 to 17 mm from midsagittal plane y: 7 mm z: 4 mm |
n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a | None | |
| RF Young21 2010 SRS |
12 | n/a | n/a | 172 | n/a | 2 | n/a | n/a | 9a | 9a | n/a | |
| SY Lim63 2010 SRS |
30b | Modified x: 11 mm y: 1/4 AC-PC distance +1 mm z: 2 to 3 mm |
n/a | 11 | n/a | 2 (18, 19 mo) | n/a | n/a | n/a | n/a | 1 (thalamic hemorrhage with speech difficulty and right hemiparesis at 14 months) | |
| D Kondziolka9 2008 SRS |
96b | Modified x: 11 mm y: 1/4 AC-PC distance +1 mm z: 2 to 3 mm |
n/a | 26 | n/a | n/a | n/a | n/a | 1c (6 mo -) | 1c (6 mo -) | n/a | |
| C Ohye61 2002 SRS |
96b | n/a | (∼125-750, 1-10 mo) | 11 | n/a | n/a | n/a | n/a | n/a | n/a | None | |
| RF Young22 2000 SRS |
96b | n/a | 166 (0-523, 3 mo) | 25 | 0 | 1 | n/a | n/a | 1 | n/a | n/a | |
| A Niranjan23 2000 SRS |
11 | Modified x: 11 mm y: 1/4 AC-PC distance z: 2 mm |
n/a | 12 | n/a | n/a | n/a | n/a | 1c | 1c | n/a | |
| RF Young24 1998 SRS |
50b | n/a | 245 (30-910, 3 mo)d | 27 | n/a | n/a | n/a | n/a | n/a | n/a | None | |
| SRS aggregate | 449 | 0 (0.0%) | 5 (1.1%) | 0 (0.0%) | 0 (0.0%) | 12 (2.7%) | 11 (2.4%) | 3 (0.7%) |
AEs, adverse events; FUS, focused ultrasound; n/a, not discussed within the paper; none, paper explicitly stated no adverse effects with 1 year or greater follow-up; SRS, stereotactic radiosurgery.
Classic AC-PC = targeting at 0 mm in the z-direction relative to the AC-PC plane. Modified AC-PC = targeting at 1 to 2 mm above the AC-PC plane. Coordinates deviating from - x: 11 to 12 mm lateral to wall of third ventricle; y: 1/4 to 1/3 AC-PC distance anterior to the front of the PC; z: 0 mm above the intercommissural plane are noted.
If follow-up length exceeded 12 months, the adverse effects at close to 12 months were included here for comparison.
Multiple AEs within the same individual explicitly stated or deduced from total numbers.
Lesion volume of initially enrolled population, with adverse event population demonstrating attrition.
To address possible factors leading to high AE rate in the FUS cohorts, we compared lesion characteristics to AE rates in each study. First, we assessed contribution of initial lesion targeting coordinates (ie, classic at the level of the AC-PC plane or modified above the AC-PC plane). We found increased imbalance/gait disturbance (9.1% vs 1.9%, P = .00054, Bonferroni-corrected α = 0.0071) with modified coordinates (Table 6). We next assessed whether lesion volume and AE rate were correlated for imbalance/gait disturbance. Eight studies had both lesion volume and AEs, demonstrating increased sensory disturbance with increased lesion size (R2 = 0.52, P = .045), as well as trends toward increased imbalance/gait disturbance (R2 = 0.36, P = .12) and maximum AE rate (R2 = 0.31, P = .15) with increased lesion size (Figure 3). Subsequently, we found no correlation between rate of AEs and efficacy (n = 8 studies, P = .63 for imbalance/gait disturbance, P = .74 for sensory disturbance, P = .85 for maximum rate of any AE). Finally, we found no correlation between efficacy and lesion volume (n = 6 studies, P = .63).
TABLE 6.
FUS AE Rate by Thalamotomy Initial Coordinate Location with z-Test for Difference in Proportions
| Thalamotomy coordinate location | Imbalance/gait disturbance | Sensory disturbance | Dysmetria | Dysgeusia | Motor weakness | Dysarthria | Others |
|---|---|---|---|---|---|---|---|
| Modified (n = 263) | 24 (9.1%) | 23 (8.7%) | 7 (2.7%) | 3 (1.1%) | 6 (2.3%) | 6 (2.3%) | 0 (0.0%) |
| Classic (n = 223) | 4 (1.9%) | 15 (6.7%) | 0 (0.0%) | 10 (4.4%) | 3 (0.0028) | 0 (0.00) | 9 (4.0%) |
| P-value | .00054 | .41 | .014 | .023 | .45 | .023 | .001 |
AE, adverse event; CRST, Clinical Rating Scale for Tremor; FUS, focused ultrasound.
Significance indicated in bold, with a Bonferroni-corrected α of 0.0071.
FIGURE 3.
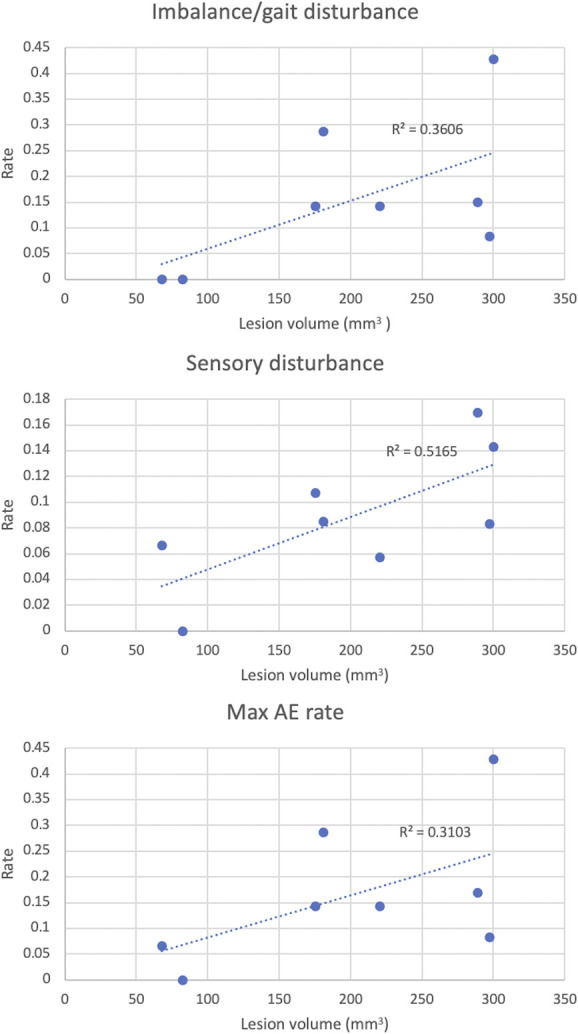
Linear regression of lesion volume vs rate of adverse events across FUS studies: imbalance/gait disturbance (top), sensory disturbance (middle), and maximum AE rate (bottom). AE, adverse event; FUS, focused ultrasound.
DISCUSSION
Our study presents the first network meta-analysis of studies directly comparing FUS-T and SRS-T as incisionless treatment modalities for patients with ET. We found similar absolute contralateral FTM-TRS tremor reduction between FUS-T and SRS-T (FUS-T, −11.6; SRS-T, −10.3) and relative tremor reduction (FUS: 60.8%, SRS-T: 56.5%). In addition, we estimated rates of AEs with both modalities. FUS-T demonstrated a greater rate of AEs, in particular imbalance and gait disturbance (10.5%). Importantly, we found that rate of AEs in FUS-T, such as sensory disturbance, was correlated with lesion size, echoing observations seen in individual studies.46,50,62 There was no relationship between lesion size and efficacy nor AE rate and efficacy, highlighting the importance of smaller, well-targeted lesions.
When examining the tremor reduction results further, we note that FUS-T trended toward greater efficacy than SRS-T, with FUS-T having a 72.8% probability of being the best treatment. The consistency of improvements with FUS-T was also greater, as evidenced by (1) the lower I2 value, which is the fraction of variance that is due to heterogeneity among studies and (2) a smaller CI for tremor reduction, although we recognize more studies with FUS-T met criteria for this analysis. Despite the low number of SRS-T studies that met inclusion criteria, 1 of the 3 SRS-T studies (Lim 2010)43 did not report significant improvements in a patient population blinded to treatment, demonstrating the greater variability with SRS-T in tremor reduction efficacy. Our result regarding SRS-T variability is consistent with a meta-analysis that included further subscore comparisons.14 The wider tremor response range could partially be explained by the variability in lesion size seen with SRS-T because there is no way to tailor or assess lesion size during treatment.63,64
Why might FUS-T have a higher rate of side effects compared with SRS-T? SRS-T lesions evolve over time63,64 and often can be quite small to undetectable on follow-up imaging65 thus limiting off-target effects. In addition, the lesion shape created with SRS-T tends to be more spherical9,21,66 because radiation energy can be emitted linearly to lesion at a precise target. By contrast, FUS-T lesions tend to be more ellipsoid,46,53 with the long axis extending from the medial superior direction to the inferior lateral position because of convergence of ultrasound transducer elements off of the midline of the transducer array.67 The resulting ellipsoid lesion may encroach onto the internal capsule, comprising corticospinal tract, resulting in off-target effects.46 To mitigate these off-target side effects, many treating physicians target above the intercommissural plane. Interestingly, we found that superior targeting with modified coordinates (1-2 mm above the intercommissural plane) resulted in greater gait disturbance than classical targeting (at the level of the intercommissural plane). This is likely due to the fact that classically targeted lesions were smaller in volume compared with modified targets (classic, n = 2. 75.3 mm3; modified, n = 2, 248.2 mm3). Thus, we suspect that the greater gait disturbance seen with superior targeting both in our analysis and a recent cohort68 could be due to greater lesion volume, extending below the AC-PC plane or inferolateral to the thalamus.53,62,64 Although FUS-T enables real-time monitoring of both magnetic resonance-based thermometry and treatment effect and during thalamotomy,12,53 further investigation into factors that could contribute to larger lesion volume, such as increased acceleration in power per sonication, higher skull density ratio, and increased distance between targets in multiple sonications, would improve an already efficacious incisionless thalamotomy option. Our analysis motivates future studies that model lesion size and use improved imaging techniques such as diffusion tractography imaging69 to optimize lesion location, limit off-target side effects, and improve efficacy with FUS-T.
Limitations
A main limitation of this network meta-analysis is that many noteworthy studies from the literature search only reported subscores or percentage change instead of total unilateral FTM-TRS scores, and another small subset did not report standard deviation of scores. In particular, many FUS-T studies reported bilateral FTM-TRS scores or only A or B subscores, whereas SRS-T studies focused on part A and B subcomponents, such as kinetic tremor and handwriting. Thus, transparent and standardized reporting of FTM-TRS total scores, in addition to the subcomponents, would improve the power of this and subsequent comparative studies. Of note, although many studies were excluded in this analysis, tremor reduction from FUS matched findings from a recent meta-analysis.70
CONCLUSION
Both SRS-T and FUS-T have been used as incisionless stereotactic ablative surgeries for treatment of medically refractory ET. Although there is significant heterogeneity between studies in each modality, our network meta-analysis found that FUS-T and SRS-T demonstrated similar efficacy in tremor reduction in ET, with FUS-T having both a higher probability of being more efficacious and a higher incidence of adverse effects, correlated with increased lesion volume.
Footnotes
Supplemental digital content is available for this article at neurosurgery-online.com.
Contributor Information
Sravani Kondapavulur, Email: sravani.kondapavulur@ucsf.edu.
Alexander B. Silva, Email: alexander.silva@ucsf.edu.
Annette M. Molinaro, Email: annette.molinaro@ucsf.edu.
Funding
This study did not receive any funding or financial support. Sravani Kondapavulur and Alexander B. Silva were supported by an NIH-funded fellowship award from the UCSF Medical Scientist Training Program (2T32GM007618-39).
Disclosures
Doris D. Wang is a consultant for Boston Scientific Inc, Iota Biosciences Inc, and Insightec Inc. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Visual abstract includes illustrations created with BioRender.com.
SUPPLEMENTAL DIGITAL CONTENT
Supplemental Figure 1. Meta-analysis results for difference in pre-FUS vs post-FUS thalamotomy total unilateral FTM-TRS tremor scores at ∼12 months of follow-up.
Supplemental Figure 2. Meta-analysis results for difference in pre-SRS vs post-SRS thalamotomy total unilateral FTM-TRS tremor scores at ∼12 months of follow-up.
COMMENTS
What's in a name? In the current days of DBS yet another systematic review of ventrolateral thalamic lesions for tremors may seem something of an anachronism. Is the same old Freiburg thalamotomy still with us1a—made sweeter once again by the allure of focused ultrasound?2a Perhaps not. New names and nuanced targets do make a difference.
This detailed comparison of modern stereotactic radiosurgery (SRS) and focused ultrasound (FUS) thalamotomy for ET asserts that there is a trend towards great efficacy with FUS. A brief glance at Supplemental Figures 1 and 2 (http://links.lww.com/NEU/D755 and http://links.lww.com/NEU/D756) shows that this may be a rosy claim. A well targeted thalamotomy by any other name is an effective thalamotomy.
But where is the safe target? By aiming a single 4 mm shot above the horizontal intercommissural plane—usually by about 4 mm—and contouring it to limit radiation exposure laterally towards the internal capsule, radiosurgeons have learned to minimize both gait disturbance and weakness. Although not mentioned by the authors, conforming a sonication centroid is also possible. Indeed early in sonication alignment phases an FUS case it is important to apply acoustic filters. These masks reshape the so-called “ellipsoid lesion” that will otherwise approach the internal capsule and cause weakness. Moving a focused ultrasound thalamotomy 2 to 4 mm above the horizontal plane, as with radiosurgery, will reduce (and not increase) the risk of gait disturbance. Energy dose within the target, as the authors note, is still something of an art for SRS and under ongoing refinement for FUS. Finally, it's worth remembering that the Vim is not spherical. Preplanning either procedure with tractography overlay can outline the nucleus as Krishna and others have elegantly shown.3a,4a Imaging the surrounding corticospinal and lemniscal fiber tracts may further mitigate risks that still accompany a thalamotomy of any sort.
As this paper nicely shows, the side effect profiles of these surgeries are quite different with SRS thalamotomy carrying less sensory and gait risk. Yet with either surgery the thornier risks of hemorrhage, infection, implant migration and failure are obviated. A rose by any other name... may yet be sweeter.
Travis Tierney
Nebraska City, Nebraska, USA
These authors provide a meaningful contribution to the discussion of treatment options for ET. SRS-T and focused ultrasound thalamotomy (FUS-T) can both be used to perform incisionless ablations within the ventral intermediate (VIM) nucleus of the thalamus to treat ET. However, the relative efficacy and rate of AEs remain under investigation. This article is well-written and thoughtfully discussed. Rigorous inclusion and exclusion criteria allow for thorough characterization of efficacy and AEs but limit the number of studies included, especially for SRS-T. It principally finds that the 2 techniques have comparable efficacy, albeit with a trend towards greater tremor reduction with FUS-T, while FUS-T has a higher rate of persistent AEs, particularly gait and sensory disturbances.
While the trend towards better tremor reduction found with FUS-T may be influenced by the small number of included SRS-T studies, the authors suggest that SRS-T is hampered by the inability to tailer lesion size and/or location to beneficial or AEs during the procedure, leading to inconsistencies between reported results. Furthermore, in characterizing the increased AE rate with FUS-T they found a positive correlation between the rate of AEs in FUS-T and lesion size, and no relationship between lesion size and efficacy. Larger lesion size has been associated with degree and durability of symptoms control1b; this would suggest that smaller, more precisely localized lesions may minimize AEs while still suppressing tremor. Intriguingly, the authors also find an almost 5-fold increase in the rate of gait disturbance with lesions targeted above the AC-PC plane, a modification suggested to reduce the likelihood of these AEs.2b,3b The current study would suggest that this modification may need to be revisited.
Overall, FUS-T is likely to continue gaining popularity as a noninvasive alternative to open surgery that does not require radiation, provides real-time feedback on target location, and offers instant benefit to patients. Determining the ideal target and lesion volume will become more important, with increased attention on lesion size informing targeting to optimize benefit while limiting AEs. Tractography and connectomics may be useful in this process.
Jay Kumar
Yarema B. Bezchlibnyk
Tampa, Florida, USA
REFERENCES
- 1.Findley LJ. Epidemiology and genetics of essential tremor. Neurology. 2000;54(11 suppl 4):S8-S13. [PubMed] [Google Scholar]
- 2.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25(5):534-541. [DOI] [PubMed] [Google Scholar]
- 3.Koller WC, Lyons KE, Wilkinson SB, Troster AI, Pahwa R. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord. 2001;16(3):464-468. [DOI] [PubMed] [Google Scholar]
- 4.Rehncrona S, Johnels B, Widner H, Törnqvist AL, Hariz M, Sydow O. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord. 2003;18(2):163-170. [DOI] [PubMed] [Google Scholar]
- 5.Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. 2011;10(2):148-161. [DOI] [PubMed] [Google Scholar]
- 6.Wharen RE, Okun MS, Guthrie BL, et al. Thalamic DBS with a constant-current device in essential tremor: a controlled clinical trial. Parkinsonism Relat Disord. 2017;40:18-26. [DOI] [PubMed] [Google Scholar]
- 7.Harary M, Segar DJ, Hayes MT, Cosgrove GR. Unilateral thalamic deep brain stimulation versus focused ultrasound thalamotomy for essential tremor. World Neurosurg. 2019;126:e144-e152. [DOI] [PubMed] [Google Scholar]
- 8.Elble RJ, Shih L, Cozzens JW. Surgical treatments for essential tremor. Expert Rev Neurother. 2018;18(4):303-321. [DOI] [PubMed] [Google Scholar]
- 9.Kondziolka D, Ong JG, Lee JYK, Moore RY, Flickinger JC, Lunsford LD. Gamma knife thalamotomy for essential tremor. J Neurosurg. 2008;108(1):111-117. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi Y, Matsuda S, Serizawa T. Gamma knife radiosurgery in movement disorders: indications and limitations. Mov Disord. 2017;32(1):28-35. [DOI] [PubMed] [Google Scholar]
- 11.Ravikumar VK, Parker JJ, Hornbeck TS, et al. Cost-effectiveness of focused ultrasound, radiosurgery, and DBS for essential tremor. Mov Disord. 2017;32(8):1165-1173. [DOI] [PubMed] [Google Scholar]
- 12.Wintermark M, Druzgal J, Huss DS, et al. Imaging findings in MR imaging–guided focused ultrasound treatment for patients with essential tremor. Am J Neuroradiol. 2014;35(5):891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harary M, Segar DJ, Huang KT, Tafel IJ, Valdes PA, Cosgrove GR. Focused ultrasound in neurosurgery: a historical perspective. Neurosurg Focus. 2018;44(2):E2. [DOI] [PubMed] [Google Scholar]
- 14.Dallapiazza RF, Lee DJ, De Vloo P, et al. Outcomes from stereotactic surgery for essential tremor. J Neurol Neurosurg Psychiatry. 2019;90(4):474-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondapavulur S, Silva AB, Wang DD. Ventral intermediate nucleus of the thalamus versus posterior subthalamic area: network meta-analysis of DBS target site efficacy for essential tremor. Stereotactic Funct Neurosurg. 2022;100(4):224-235. [DOI] [PubMed] [Google Scholar]
- 17.Estimating the Mean and Variance from the Median, Range, and the Size of a Sample—PubMed. Accessed August 7, 2022. https://pubmed.ncbi.nlm.nih.gov/15840177/ [Google Scholar]
- 18.ROBINS-I Tool | Cochrane Methods. Accessed October 31, 2022. https://methods.cochrane.org/methods-cochrane/robins-i-tool [Google Scholar]
- 19.Zaaroor M, Sinai A, Goldsher D, Eran A, Nassar M, Schlesinger I. Magnetic resonance-guided focused ultrasound thalamotomy for tremor: a report of 30 Parkinson’s disease and essential tremor cases. J Neurosurg. 2018;128(1):202-210. [DOI] [PubMed] [Google Scholar]
- 20.Niranjan A, Raju SS, Kooshkabadi A, Monaco E, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for essential tremor: retrospective analysis of a 19-year experience. Mov Disord. 2017;32(5):769-777. [DOI] [PubMed] [Google Scholar]
- 21.Young RF, Li F, Vermeulen S, Meier R. Gamma knife thalamotomy for treatment of essential tremor: long-term results. J Neurosurg. 2010;112(6):1311-1317. [DOI] [PubMed] [Google Scholar]
- 22.Young RF, Jacques S, Mark R, et al. Gamma knife thalamotomy for treatment of tremor: long-term results. J Neurosurg. 2000;93(suppl_3):128-135. [DOI] [PubMed] [Google Scholar]
- 23.Niranjan A, Kondziolka D, Baser S, Heyman R, Lunsford LD. Functional outcomes after gamma knife thalamotomy for essential tremor and MS-related tremor. Neurology. 2000;55(3):443-446. [DOI] [PubMed] [Google Scholar]
- 24.Young RF, Shumway-Cook A, Vermeulen SS, et al. Gamma knife radiosurgery as a lesioning technique in movement disorder surgery. J Neurosurg. 1998;89(2):183-193. [DOI] [PubMed] [Google Scholar]
- 25.Owen RK, Bradbury N, Xin Y, Cooper N, Sutton A. MetaInsight: an interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res Synth Methods. 2019;10(4):569-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto K, Sarica C, Elias GJB, et al. Ipsilateral and axial tremor response to focused ultrasound thalamotomy for essential tremor: clinical outcomes and probabilistic mapping. J Neurol Neurosurg Psychiatry. 2022;93(10):1049-1058. [DOI] [PubMed] [Google Scholar]
- 27.Kato S, Maesawa S, Bagarinao E, et al. Magnetic resonance-guided focused ultrasound thalamotomy restored distinctive resting-state networks in patients with essential tremor. J Neurosurg. 2023;138(2):306-317. [DOI] [PubMed] [Google Scholar]
- 28.Pae C, Kim MJ, Chang WS, et al. Differences in intrinsic functional networks in patients with essential tremor who had good and poor long-term responses after thalamotomy performed using MR-guided ultrasound. J Neurosurg. 2023;138(2):318-328. [DOI] [PubMed] [Google Scholar]
- 29.Purrer V, Borger V, Pohl E, et al. Transcranial high-intensity magnetic resonance-guided focused ultrasound (tcMRgFUS)—safety and impacts on tremor severity and quality of life. Parkinsonism Relat Disord. 2022;100:6-12. [DOI] [PubMed] [Google Scholar]
- 30.Wu P, Lin W, Li KH, et al. Focused ultrasound thalamotomy for the treatment of essential tremor: a 2-year outcome study of Chinese people. Front Aging Neurosci. 2021;13:697029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zur G, Lesman-Segev OH, Schlesinger I, et al. Tremor relief and structural integrity after mri-guided focused us thalamotomy in tremor disorders. Radiology. 2020;294(3):676-685. [DOI] [PubMed] [Google Scholar]
- 32.Fukutome K, Kuga Y, Ohnishi H, Hirabayashi H, Nakase H. What factors impact the clinical outcome of magnetic resonance imaging-guided focused ultrasound thalamotomy for essential tremor? J Neurosurg. 2021;134(5):1618-1623. [DOI] [PubMed] [Google Scholar]
- 33.Sinai A, Nassar M, Eran A, et al. Magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: a 5-year single-center experience. J Neurosurg. 2020;133(2):417-424. [DOI] [PubMed] [Google Scholar]
- 34.Park YS, Jung NY, Na YC, Chang JW. Four-year follow-up results of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Mov Disord. 2019;34(5):727-734. [DOI] [PubMed] [Google Scholar]
- 35.Gasca-Salas C, Guida P, Piredda R, et al. Cognitive safety after unilateral magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. J Neurol Neurosurg Psychiatry. 2019;90(7):830-831. [DOI] [PubMed] [Google Scholar]
- 36.Tian Q, Wintermark M, Jeffrey Elias W, et al. Diffusion MRI tractography for improved transcranial MRI-guided focused ultrasound thalamotomy targeting for essential tremor. NeuroImage Clin. 2018;19:572-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung NY, Park CK, Chang WS, Jung HH, Chang JW. Effects on cognition and quality of life with unilateral magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Neurosurg Focus. 2018;44(2):E8. [DOI] [PubMed] [Google Scholar]
- 38.Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730-739. [DOI] [PubMed] [Google Scholar]
- 39.Chang WS, Jung HH, Kweon EJ, Zadicario E, Rachmilevitch I, Chang JW. Unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor: practices and clinicoradiological outcomes. J Neurol Neurosurg Psychiatry. 2015;86(3):257-264. [DOI] [PubMed] [Google Scholar]
- 40.Elias WJ, Khaled M, Hilliard JD, et al. A magnetic resonance imaging, histological, and dose modeling comparison of focused ultrasound, radiofrequency, and Gamma Knife radiosurgery lesions in swine thalamus: laboratory investigation. J Neurosurg. 2013;119(2):307-317. [DOI] [PubMed] [Google Scholar]
- 41.Bolton TAW, Van De Ville D, Régis J, et al. Graph theoretical analysis of structural covariance reveals the relevance of visuospatial and attentional areas in essential tremor recovery after stereotactic radiosurgical thalamotomy. Front Aging Neurosci. 2022;14:873605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuleasca C, Najdenovska E, Régis J, et al. Pretherapeutic functional neuroimaging predicts tremor arrest after thalamotomy. Acta Neurol Scand. 2018;137(5):500-508. [DOI] [PubMed] [Google Scholar]
- 43.Lim SY, Hodaie M, Fallis M, Poon YY, Mazzella F, Moro E. Gamma knife thalamotomy for disabling tremor: a blinded evaluation. Arch Neurol. 2010;67(5):584-588. [DOI] [PubMed] [Google Scholar]
- 44.Torii J, Maesawa S, Nakatsubo D, et al. Cutoff values for the best management strategy for magnetic resonance-guided focused ultrasound ablation for essential tremor. J Neurosurg. 2023;138(1):38-49. [DOI] [PubMed] [Google Scholar]
- 45.Abe K, Horisawa S, Yamaguchi T, et al. Focused ultrasound thalamotomy for refractory essential tremor: a Japanese multicenter single-arm study. Neurosurgery. 2021;88(4):751-757. [DOI] [PubMed] [Google Scholar]
- 46.Segar DJ, Lak AM, Lee S, et al. Lesion location and lesion creation affect outcomes after focused ultrasound thalamotomy. Brain. 2021;144(10):3089-3100. [DOI] [PubMed] [Google Scholar]
- 47.Tommasino E, Bruno F, Catalucci A, et al. Prognostic value of brain tissues’ volumes in patients with essential tremor treated with MRgFUS thalamotomy. J Clin Neurosci. 2021;92:33-38. [DOI] [PubMed] [Google Scholar]
- 48.Ito H, Yamamoto K, Fukutake S, Odo T, Kamei T. Two-year follow-up results of magnetic resonance imaging-guided focused ultrasound unilateral thalamotomy for medication-refractory essential tremor. Intern Med. 2020;59(20):2481-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallay MN, Moser D, Jeanmonod D. MR-guided focused ultrasound cerebellothalamic tractotomy for chronic therapy-resistant essential tremor: anatomical target reappraisal and clinical results. J Neurosurg. 134(2), 2021:376-385. [DOI] [PubMed] [Google Scholar]
- 50.Kapadia AN, Elias GJB, Boutet A, et al. Multimodal MRI for MRgFUS in essential tremor: post-treatment radiological markers of clinical outcome. J Neurol Neurosurg Psychiatry. 2020;91(9):921-927. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto K, Ito H, Fukutake S, Kamei T, Yamaguchi T, Taira T. Ventralis intermedius thalamotomy with focused ultrasound for patients with low skull density ratio. Mov Disord. 2019;34(8):1239-1240. [DOI] [PubMed] [Google Scholar]
- 52.Meng Y, Solomon B, Boutet A, et al. Magnetic resonance-guided focused ultrasound thalamotomy for treatment of essential tremor: a 2-year outcome study. Mov Disord. 2018;33(10):1647-1650. [DOI] [PubMed] [Google Scholar]
- 53.Harary M, Essayed WI, Valdes PA, McDannold N, Cosgrove GR. Volumetric analysis of magnetic resonance-guided focused ultrasound thalamotomy lesions. Neurosurg Focus. 2018;44(2):E6. [DOI] [PubMed] [Google Scholar]
- 54.Iacopino DG, Gagliardo C, Giugno A, et al. Preliminary experience with a transcranial magnetic resonance-guided focused ultrasound surgery system integrated with a 1.5-T MRI unit in a series of patients with essential tremor and Parkinson’s disease. Neurosurg Focus. 2018;44(2):E7. [DOI] [PubMed] [Google Scholar]
- 55.Kim M, Jung NY, Park CK, Chang WS, Jung HH, Chang JW. Comparative evaluation of magnetic resonance-guided focused ultrasound surgery for essential tremor. Stereotact Funct Neurosurg. 2017;95(4):279-286. [DOI] [PubMed] [Google Scholar]
- 56.Gallay MN, Moser D, Rossi F, et al. Incisionless transcranial MR-guided focused ultrasound in essential tremor: cerebellothalamic tractotomy. J Ther Ultrasound. 2016;4(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huss DS, Dallapiazza RF, Shah BB, Harrison MB, Diamond J, Elias WJ. Functional assessment and quality of life in essential tremor with bilateral or unilateral DBS and focused ultrasound thalamotomy. Mov Disord. 2015;30(14):1937-1943. [DOI] [PubMed] [Google Scholar]
- 58.Khattab MH, Cmelak AJ, Sherry AD, et al. Noninvasive thalamotomy for refractory tremor by frameless radiosurgery. Int J Radiat Oncol Biol Phys. 2022;112(1):121-130. [DOI] [PubMed] [Google Scholar]
- 59.Witjas T, Carron R, Krack P, et al. A prospective single-blind study of gamma knife thalamotomy for tremor. Neurology. 2015;85(18):1562-1568. [DOI] [PubMed] [Google Scholar]
- 60.Ohye C, Higuchi Y, Shibazaki T, et al. Gamma knife thalamotomy for Parkinson disease and essential tremor: a prospective multicenter study. Neurosurgery. 2012;70(3):526-536; discussion 535-536. [DOI] [PubMed] [Google Scholar]
- 61.Ohye C, Shibazaki T, Zhang J, Andou Y. Thalamic lesions produced by gamma thalamotomy for movement disorders. J Neurosurg. 2002;97(5 suppl):600-606. [DOI] [PubMed] [Google Scholar]
- 62.Boutet A, Ranjan M, Zhong J, et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain. 2018;141(12):3405-3414. [DOI] [PubMed] [Google Scholar]
- 63.De Salles AAF, Melega WP, Laćan G, Steele LJ, Solberg TD. Radiosurgery performed with the aid of a 3-mm collimator in the subthalamic nucleus and substantia nigra of the vervet monkey. J Neurosurg. 2001;95(6):990-997. [DOI] [PubMed] [Google Scholar]
- 64.Iorio-Morin C, Yamamoto K, Sarica C, et al. Bilateral focused ultrasound thalamotomy for essential tremor (BEST-FUS Phase 2 Trial). Mov Disord. 2021;36(11):2653-2662. [DOI] [PubMed] [Google Scholar]
- 65.Moosa S, Elias WJ. Essential tremor: lesions. In: Pouratian N, Sheth SA, eds. Stereotactic and Functional Neurosurgery: Principles and Applications. Springer International Publishing; 2020:297-310. [Google Scholar]
- 66.Frighetto L, Bizzi J, Oppitz P. Stereotactic radiosurgery for movement disorders. In: De Salles AAF, Gorgulho A, Agazaryan N, et al., eds. Shaped Beam Radiosurgery: State of the Art. Springer; 2011:209-218. [Google Scholar]
- 67.Miller TR, Guo S, Melhem ER, et al. Predicting final lesion characteristics during MR-guided focused ultrasound pallidotomy for treatment of Parkinson’s disease. J Neurosurg. 2021;134(4):1083-1090. [DOI] [PubMed] [Google Scholar]
- 68.Jackson LM, Kaufmann TJ, Lehman VT, et al. Clinical characteristics of patients with gait instability after mr-guided focused ultrasound thalamotomy. Tremor Other Hyperkinet Mov. 2021;11(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feltrin FS, Chopra R, Pouratian N, et al. Focused ultrasound using a novel targeting method four-tract tractography for magnetic resonance–guided high-intensity focused ultrasound targeting. Brain Commun. 2022;4(6):fcac273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller WK, Becker KN, Caras AJ, et al. Magnetic resonance-guided focused ultrasound treatment for essential tremor shows sustained efficacy: a meta-analysis. Neurosurg Rev. 2022;45(1):533-544. [DOI] [PubMed] [Google Scholar]
References
- 1a.Hassler R, Riechert T. A special method of stereotactic brain operation. Proc R Soc Med. 1955;48(6):469-470. [PMC free article] [PubMed] [Google Scholar]
- 2a.Elias WJ, Huss D, Voss T, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013;369(7):640-648. [DOI] [PubMed] [Google Scholar]
- 3a.Sammartino F, Krishna V, King NKK, et al. Tractography-based ventral intermediate nucleus targeting: novel methodology and intraoperative validation. Mov Disord. 2016;31(8):1217-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Krishna V, Sammartino F, Agrawal P, et al. Prospective tractography-based targeting for improved safety of focused ultrasound thalamotomy. Neurosurgery. 2019;84(1):160-168. [DOI] [PubMed] [Google Scholar]
References
- 1b.Young RF, Li F, Vermeulen S, Meier R. Gamma Knife thalamotomy for treatment of essential tremor: long-term results. J Neurosurg. 2010;112(6):1311-1317. [DOI] [PubMed] [Google Scholar]
- 2b.Boutet A, Ranjan M, Zhong J, et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain. 2018;141(12):3405-3414. [DOI] [PubMed] [Google Scholar]
- 3b.Segar DJ, Lak AM, Lee S, et al. Lesion location and lesion creation affect outcomes after focused ultrasound thalamotomy. Brain. 2021;144(10):3089-3100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Meta-analysis results for difference in pre-FUS vs post-FUS thalamotomy total unilateral FTM-TRS tremor scores at ∼12 months of follow-up.
Supplemental Figure 2. Meta-analysis results for difference in pre-SRS vs post-SRS thalamotomy total unilateral FTM-TRS tremor scores at ∼12 months of follow-up.
Data Availability Statement
The data sets supporting the current study are available from the corresponding author on reasonable request.



