Abstract
Background
Post-hepatectomy bile leakage (PHBL) is a potentially fatal complication that can arise after hepatectomy. Previous studies have identified obesity as a risk factor for PHBL. In this study, we investigated the impact of sarcopenic obesity on PHBL in hepatocellular carcinoma (HCC) patients.
Methods
In total, we enrolled 409 patients who underwent hepatectomy without bilioenteric anastomosis for HCC between January 2010 and August 2021. Patients were grouped according to the presence or absence of PHBL. Patient characteristics, including body mass index and sarcopenic obesity, were then analyzed for predictive factors for PHBL.
Results
Among the 409 HCC patients included in the study, 39 developed PHBL. Male sex, hypertension, cardiac disease, white blood cell counts, the psoas muscle area, and visceral fat area, and intraoperative blood loss were significantly increased in the PHBL (+) group compared with the PHBL (−) group. Multivariate analysis showed that the independent risk factors for the occurrence of PHBL were intraoperative blood loss ≥370 mL and sarcopenic obesity.
Conclusions
Our results show that it is important to understand whether a patient is at high risk for PHBL prior to surgery and to focus on reducing intraoperative blood loss during surgery for patients with risk factors for PHBL.
Introduction
Post-hepatectomy bile leakage (PHBL) is one of the most common and notable complications after hepatectomy, occurring in approximately 5−10% of patients [1, 2], and can lead to surgical site infection (SSI) or post-hepatectomy liver failure (PHLF) [3]. According to previous reports, factors such as liver cirrhosis, non-anatomical hepatectomy, and obesity have been identified as risk factors for PHBL [4, 5].
Recently, sarcopenic obesity has been reported to be associated with postoperative outcomes in various carcinomas. Kim et al. [6] showed that sarcopenic obesity was an independent risk factor for increased mortality in patients with gastric cancer. Kobayashi et al. [7] reported that sarcopenic obesity was a significant prognostic factor for poor overall survival and relapse-free survival after hepatectomy for hepatocellular carcinoma (HCC). However, few studies have reported the relationship between short-term outcomes, especially PHBL, and sarcopenic obesity after hepatectomy for HCC.
The purpose of this study was to evaluate the impact of sarcopenic obesity on the occurrence of PHBL after hepatectomy for HCC.
Materials and methods
In total, 409 patients who underwent hepatectomy without bilioenteric anastomosis for HCC between January 2010 and August 2021 were enrolled in this study. Patients were categorized into the following two groups based on the presence or absence of PHBL: PHBL (+) and PHBL (−). Patient characteristics and relevant clinicopathological variables, surgical details, and short-term outcomes were recorded. The study was approved by the Biological and Medical Research Ethics Committee of Shinshu University School of Medicine (approval no. 5701) and was conducted in accordance with the principles of the Declaration of Helsinki. Owing to the retrospective nature of the study and the absence of invasive interventions, the requirement for written consent was waived by the review board, and consent was obtained through an opt-out method. The data were collected between December 2022 and March 2023 and analyzed anonymously by several physicians on the basis of medical records.
Perioperative management
In this study, the hepatectomy procedures were conducted by several surgeons. Parenchymal transection was performed using an ultrasonic dissector and/or a clamp-crushing technique. The intermittent Pringle maneuver (PM) was routinely used to control intraoperative blood loss for 15 min, followed by 5 min of reperfusion; this process was repeated as needed. Abdominal drains were routinely placed along the cut surface of the liver. Postoperatively, bilirubin concentrations in the drainage fluid were routinely measured twice per week for surveillance of bile leakage. In addition, for several days after surgery, the presence or absence of fluid collection around the hepatectomy site was checked every day by ultrasonography. When PHBL was detected, additional percutaneous drainage or exchange of drainage tubes was performed as needed. The end of follow‐up was December 2021 or the time of discharge.
Definitions
Pathological findings were evaluated in accordance with the American Joint Committee on Cancer (AJCC) Staging Manual, 7th edition [8]. Liver cirrhosis was defined as a fibrosis score of 4 using the new Inuyama classification [9]. Postoperative complications were graded using the Clavien–Dindo classification [10]. PHLF and PHBL were diagnosed and graded according to the criteria of the International Study Group of Liver Surgery [11, 12]. Major hepatectomy was defined as resection of three or more Couinaud segments of the liver. Anatomical resection included Couinaud segmentectomy, sectionectomy, hemihepatectomy, and trisectionectomy.
Multidetector-row computed tomography (CT) was performed within 4 weeks before surgery for diagnostic and staging purposes and was used to evaluate sarcopenia, which was defined in accordance with the international consensus [13] as a skeletal muscle index (SMI) of <52.4 cm2/m2 for men and <38.9 cm2/m2 for women. SMI was defined as the total muscle area measured on an axial section through the third lumbar vertebra (L3) where both pedicles were visible with a preestablished density threshold of −29 to +150 Hounsfield units normalized for stature (Fig 1). The visceral fat area (VFA) and subcutaneous fat area were also measured, and sarcopenic obesity was defined as the VFA/SMI ratio [14, 15]. The cut-off value for sarcopenic obesity was defined in accordance with the maximum sensitivity and specificity for predicting PHBL in a receiver operating characteristic (ROC) curve analysis; the cut-off value was 1.65. The CT scans were checked by two qualified physicians using a Synapse Vincent FN-7941 (Fujifilm, Tokyo, Japan).
Fig 1. Computed tomography image analysis of a third lumbar vertebra.
Green, muscle area; red, visceral fat area; blue, subcutaneous fat area. (a) A patient without sarcopenic obesity. (b) A patient with sarcopenic obesity.
Statistical analysis
All data were collected by a research assistant and stored in a computer database. Statistical analyses were performed by the chi-square test or Fisher’s exact test to compare categorical variables and by the Mann–Whitney U test to compare continuous variables. ROC curve analyses were carried out for the predictive parameters to evaluate the associations with PHBL, and the Youden index was used to determine the cut-off values. Multivariate analysis using a logistic regression model was conducted to identify significant predictive factors for the occurrence of PHBL. All statistical analyses were performed using JMP Pro 16.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The study involved 409 patients who underwent hepatectomy. Among the total patients, 39 (9.5%) developed PHBL. The background characteristics and comorbidities of the patients according to the presence or absence of PHBL are summarized in Table 1. The ratio of male sex and the prevalences of hypertension and cardiac disease were higher in the PHBL (+) group than in the PHBL (−) group (89.7% vs. 74.3%; 74.4% vs. 53.0%; 28.2% vs. 13.0%; P = 0.020, 0.009, and 0.018, respectively). The indocyanine green retention rate at 15 min was lower in the PHBL (+) group than in the PHBL (−) group (9.7% vs. 12.0%; P = 0.026). No significant differences were observed between the two groups for other variables, including age, etiology, and Child–Pugh classification.
Table 1. Patient characteristics and surgical outcomes.
| Variables | PHBL (+) (n = 39) | PHBL (−) (n = 370) | P Value | ||
|---|---|---|---|---|---|
| Characteristics | |||||
| Age, years a | 71 | (44–82) | 71 | (36–89) | 0.977 |
| Sex, male/female | 35 | /4 | 275 | /95 | 0.020 |
| Body mass index, kg/m2 a | 23.1 | (20.2–31.6) | 22.9 | (13.4–45.2) | 0.168 |
| Etiology | |||||
| HBsAg positive | 5 | (13) | 80 | (22) | 0.175 |
| HCVAb positive | 12 | (31) | 151 | (41) | 0.216 |
| Social history | |||||
| Smoking | 29 | (74) | 236 | (64) | 0.179 |
| Drinking | 32 | (82) | 281 | (76) | 0.379 |
| Comorbidity | |||||
| Hypertension | 29 | (74) | 196 | (53) | 0.009 |
| Diabetes mellitus | 14 | (36) | 121 | (33) | 0.688 |
| Cardiac disease | 11 | (28) | 48 | (13) | 0.018 |
| Pulmonary disease | 2 | (5) | 40 | (11) | 0.227 |
| Child-Pugh classification | 0.203 | ||||
| Grade A | 39 | (100) | 362 | (98) | |
| Grade B | 0 | (0) | 8 | (2) | |
| Laboratory data | |||||
| Albumin, g/dl a | 4.1 | (3.3–5.1) | 3.9 | (2.9–5.2) | 0.161 |
| T-Bil, mg/dl a | 0.79 | (0.3–2.22) | 0.80 | (0.2–2.81) | 0.384 |
| AST, units/l a | 24 | (11–74) | 30 | (11–142) | 0.005 |
| ALT, units/l a | 29 | (6–149) | 30 | (7–215) | 0.278 |
| PT% a | 86.0 | (53.4–109.7) | 87.1 | (28.1–146.6) | 0.322 |
| White blood cell, /μl a | 5090 | (2450–11220) | 4275 | (1770–10610) | 0.032 |
| Platelet, ×104 /μl a | 15.3 | (4.7–25.2) | 14.3 | (4.0–45.6) | 0.158 |
| Tumor markers | |||||
| AFP > 20 ng/ml | 15 | (38) | 135 | (36) | 0.808 |
| PIVKA-II > 40 mAU/ml | 20 | (51) | 178 | (48) | 0.706 |
| Modified ALBI grade, 1 / 2a / 2b / 3 | 25 | / 10 / 4 / 0 | 184 | / 124 / 62 / 0 | 0.213 |
| ICGR15, % a | 9.7 | (2.9–26.3) | 12.0 | (2.6–46.4) | 0.026 |
| Sarcopenia | 16 | (41) | 141 | (38) | 0.723 |
| Sarcopenic obesity | 35 | (90) | 221 | (60) | < 0.001 |
| Parameters associated with muscle and fat area at the level of third lumbar vertebra | |||||
| Skeletal muscle index, cm2/m2 a | 53.3 | (38.1–71.4) | 51.2 | (32.2–85.4) | 0.477 |
| Total muscle area, cm2 a | 143.8 | (83.5–221.2) | 135.8 | (77.3–234.0) | 0.150 |
| Psoas muscle area, cm2 a | 16.6 | (4.1–27.4) | 14.0 | (4.9–31.4) | 0.016 |
| Visceral fat area, cm2 a | 135.3 | (40.8–254.5) | 96.8 | (2.6–330.9) | 0.003 |
| Subcutaneous fat area, cm2 a | 107.4 | (46.9–251.9) | 100.2 | (3.6–347.4) | 0.141 |
| Surgical outcomes | |||||
| Type of hepatectomy | 0.373 | ||||
| Partial hepatectomy | 16 | (41) | 187 | (51) | |
| Segmentectomy | 8 | (21) | 84 | (23) | |
| Sectionectomy | 11 | (28) | 61 | (16) | |
| Hemihepatectomy or more | 4 | (10) | 38 | (10) | |
| Primary hepatectomy | 30 | (77) | 272 | (74) | 0.641 |
| Major hepatectomy | 4 | (10) | 48 | (13) | 0.619 |
| Anatomical hepatectomy | 23 | (59) | 183 | (49) | 0.257 |
| Surgical duration, min a | 368 | (202–890) | 334 | (82–990) | 0.109 |
| Inflow occlusion time, min a | 66 | (15–150) | 59 | (0–247) | 0.207 |
| Blood loss, ml a | 430 | (25–2100) | 278 | (0–5500) | 0.002 |
| Intraoperative blood transfusion | 10 | (26) | 51 | (14) | 0.065 |
Values in parentheses are percentages unless otherwise indicated.
Abbreviations: PHBL, post-hepatectomy bile leakage; HBsAg, hepatitis B surface antigen; HCVAb, hepatitis C virus antibody; Bil, bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PT, prothrombin time; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist II; ICGR15, indocyanine green retention rate at 15 min.
aValues are median (range).
Preoperative muscle and fat areas
The differences in the muscle and fat areas between the two groups are shown in Table 2. At the L3 level, the psoas muscle area and VFA were significantly larger in the PHBL (+) group than in the PHBL (−) group (16.6 vs. 14.0 cm2; 135.3 vs. 96.8 cm2; P = 0.016 and 0.003, respectively). In contrast, the SMI values were comparable between the two groups. Although the ratios of patients with sarcopenia were comparable, the ratio of patients with sarcopenic obesity was significantly higher in the PHBL (+) group than in the PHBL (−) group (89.7% vs. 59.7%; P < 0.001).
Table 2. Histopathological findings.
| Variables | PHBL (+) (n = 39) | PHBL (−) (n = 370) | P value | ||
|---|---|---|---|---|---|
| Liver cirrhosis a | 13 | (33) | 115 | (31) | 0.774 |
| Maximum tumor diameter, cm b | 2.8 | (0.9–15.0) | 2.5 | (0.1–17.0) | 0.523 |
| Number of tumors | 0.097 | ||||
| Solitary | 33 | (85) | 270 | (73) | |
| Multiple | 6 | (15) | 100 | (27) | |
| Milan criteria | 0.930 | ||||
| Within | 30 | (77) | 280 | (76) | |
| Beyond | 9 | (23) | 87 | (24) | |
| Histological grade c | 0.542 | ||||
| GX | 0 | (0) | 0 | (0) | |
| G1 | 4 | (10) | 61 | (17) | |
| G2 | 24 | (62) | 204 | (55) | |
| G3 | 11 | (28) | 104 | (28) | |
| G4 | 0 | (0) | 0 | (0) | |
| Microvascular invasion c | 7 | (18) | 101 | (27) | 0.191 |
| Portal vein invasion | 5 | (13) | 81 | (22) | 0.164 |
| Hepatic vein invasion | 3 | (8) | 36 | (10) | 0.672 |
| Pathological AJCC staging c | 0.071 | ||||
| Stage I | 10 | (26) | 103 | (28) | |
| Stage II | 19 | (48) | 145 | (39) | |
| Stage III | 10 | (26) | 91 | (25) | |
| Stage IV | 0 | (0) | 31 | (8) | |
| Residual tumor | 0.293 | ||||
| R0 | 35 | (90) | 349 | (94) | |
| R1 | 4 | (10) | 21 | (6) | |
Values in parentheses are percentages unless otherwise indicated.
Abbreviations: AJCC, American Joint Committee on Cancer.
aFibrosis score of 4 using the new Inuyama classification.
bValues are presented as median (range).
cIn accordance with the definition in the American Joint Committee on Cancer Staging Manual, 7th edition.
Surgical outcomes
Among the total cohort, the median surgical duration time was 340 min (range: 82–990 min), the median inflow occlusion time was 60 min (range: 0–247 min), and the median intraoperative blood loss was 300 mL (range: 0–5500 mL). The surgical outcomes are shown in Table 1. No significant differences were observed between the two groups with regards to the type of hepatectomy, the ratio of primary hepatectomy, or the ratio of anatomical hepatectomy. There were also no significant differences in the ratios of medial sectionectomy and central bisectionectomy. Furthermore, the surgical duration and inflow occlusion times were comparable between the two groups. However, intraoperative blood loss was greater in the PHBL (+) group than in the PHBL (−) group (430 vs. 278 mL; P = 0.002). As a result, the percentage of intraoperative blood transfusions tended to be higher in the PHBL (+) group (25.6% vs. 13.8%; P = 0.065).
Histopathological findings
The histopathological findings are summarized in Table 2. The prevalences of liver cirrhosis were comparable between the two groups (P = 0.074). In contrast, the ratio of multiple tumors tended to be lower in the PHBL (+) group than in the PHBL (−) group (15.4% vs. 27.0%; P = 0.097). Furthermore, the pathological AJCC staging tended to be lower in the PHBL (+) group (P = 0.071). There were no significant differences between the two groups in the maximum tumor diameter, the ratio of microvascular invasion, or the R0 resection rate.
Short-term outcomes
Regarding the short-term outcomes (Table 3), 225 patients (55.0%) developed postoperative complications, including 70 (17.1%) with complications of grade III or higher [10]. One patient (0.2%) in the total cohort died due to grade C PHLF. In the PHBL (+) group, 12 patients (30.8%) developed grade B PHBL and 27 (69.2%) developed grade A PHBL, while no patients developed grade C PHBL. Regarding PHLF, all cases of PHLF in the PHBL group were grade A, and there was no significant difference in the occurrence of PHLF between the two groups. Moreover, the incidences of intra-abdominal infection and pleural effusion were comparable between the two groups. However, the postoperative hospital stay was longer in the PHBL (+) group (14 vs. 12 d; P = 0.036).
Table 3. Short-term outcomes.
| Variables | PHBL (+) (n = 39) | PHBL (−) (n = 370) | P value | ||
|---|---|---|---|---|---|
| 90-day Mortality | 0 | (0) | 1 | (0.2) | 0.654 |
| Morbidity | 39 | (100) | 186 | (50) | < 0.001 |
| Clavien–Dindo classification ≥III | 12 | (31) | 58 | (16) | 0.027 |
| Post-hepatectomy bile leakage | < 0.001 | ||||
| Grade A | 27 | (69) | 0 | (0) | |
| Grade B | 12 | (31) | 0 | (0) | |
| Grade C | 0 | (0) | 0 | (0) | |
| Post-hepatectomy liver failure | 0.321 | ||||
| Grade A | 5 | (13) | 48 | (13) | |
| Grade B | 0 | (0) | 16 | (4) | |
| Grade C | 0 | (0) | 1 | (1) | |
| Incisional surgical site infection | 3 | (8) | 23 | (6) | 0.727 |
| Intra–abdominal infection | 4 | (10) | 15 | (4) | 0.122 |
| Pleural effusion | 7 | (18) | 37 | (10) | 0.156 |
| Postoperative hospital stay, days a | 14 | (7–130) | 12 | (4–117) | 0.036 |
Values in parentheses are percentages unless otherwise indicated.
Abbreviations: PHBL, post-hepatectomy bile leakage.
aValues are median (range).
Risk factors for PHBL
The cut-off value for intraoperative blood loss was determined using a ROC curve analysis. The independent risk factors for the occurrence of PHBL were intraoperative blood loss ≥370 mL (odds ratio [OR]: 2.25; 95% confidence interval [CI]: 1.10–4.59; P = 0.026) and sarcopenic obesity (OR: 4.08; 95% CI: 1.37–12.1; P = 0.011) (Table 4). Furthermore, when the analysis was restricted to patients who underwent primary hepatectomy, sarcopenic obesity (OR: 3.61; 95% CI: 1.21–10.8; P = 0.022) was the only independent risk factor for the occurrence of PHBL.
Table 4. Univariate and multivariate analyses of risk factors for the occurrence of post-hepatectomy bile leakage.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value |
| Age, ≥ 70 years | 1.02 (0.52–1.98) | 0.956 | ||
| Sex, male | 3.02 (1.05–8.73) | 0.041 | 1.80 (0.18–1.68) | 0.298 |
| Body mass index, ≥ 25 kg/m2 | 1.14 (0.53–2.43) | 0.737 | ||
| Hypertension, yes | 2.57 (1.22–5.43) | 0.013 | 1.80 (0.83–3.91) | 0.138 |
| Diabetes mellites, yes | 1.15 (0.58–2.30) | 0.687 | ||
| Surgical duration, ≥ 340 min | 1.41 (0.73–2.74) | 0.310 | ||
| Inflow occlusion time, ≥ 60 min | 1.62 (0.82–3.18) | 0.164 | ||
| Blood loss, ≥ 370 ml | 3.00 (1.51–5.97) | 0.002 | 2.25 (1.10–4.59) | 0.026 |
| Anatomical hepatectomy, yes | 1.47 (0.75–2.87) | 0.261 | ||
| Multiple tumors, yes | 0.49 (0.20–1.21) | 0.121 | ||
| Sarcopenia, yes | 1.13 (0.58–2.21) | 0.722 | ||
| Sarcopenic obesity, yes | 5.90 (2.05–16.9) | 0.001 | 4.08 (1.37–12.1) | 0.011 |
| Liver cirrhosis, yes | 1.11 (0.55–2.24) | 0.773 | ||
Abbreviations: CI, confidence interval.
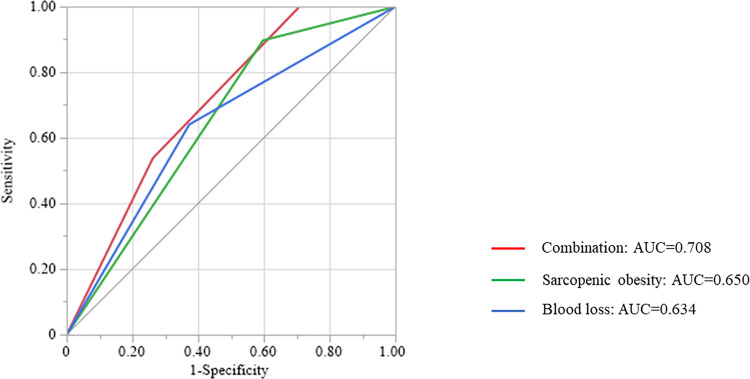
A scatter diagram of the correlation between intraoperative blood loss and sarcopenic obesity (VFA/SMI) was created (Fig 2). Spearman’s rank correlation analysis showed a low correlation between the amount of intraoperative blood loss and the VFA/SMI value (ρ = 0.201; P < 0.001). No patients with intraoperative blood loss <370 mL and without sarcopenic obesity developed PHBL (0 of 108 patients). In contrast, patients with intraoperative blood loss ≥370 mL and sarcopenic obesity had the highest ratio of the occurrence of PHBL among all combinations of these two parameters (17.8%; 21 of 118 patients). Moreover, a ROC curve analysis of combined intraoperative blood loss and sarcopenic obesity had a higher area under the curve value (0.708; P = 0.013) than either parameter alone (Fig 3).
Fig 2. Scatter diagram of the correlation between visceral fat area (VFA)/skeletal muscle index (SMI) and intraoperative blood loss.
Coefficients (ρ) and P-values were calculated using Spearman’s rank correlation analysis. Red circles, patients with post-hepatectomy bile leakage (PHBL); blue circles, patients without PHBL.
Fig 3. Receiver operating characteristic (ROC) curve analysis for the occurrence of post-hepatectomy bile leakage.
Red, ROC curve of the combination of sarcopenic obesity and intraoperative blood loss; green, ROC curve of sarcopenic obesity; blue, ROC curve of intraoperative blood loss.
Discussion
Recently, the targets for hepatectomy have been expanding, to liver metastases of various carcinomas for example, and thus hepatectomy has been increasing in importance despite advances in drug therapy. PHBL is one of the most common postoperative complications after hepatectomy. Although most cases of PHBL can be resolved by conservative treatment, PHBL can lead to PHLF or sepsis from an infection. Shehta et al. [16] reported that PHBL occurred in 5.8% of hepatectomy patients, and their PHBL (+) group had higher grades of PHLF. Lo et al. [17] showed that biliary complications including PHBL developed in 8.1% of hepatectomy patients, and that these complications carried high risks for PHLF and mortality. In contrast, Okabayashi et al. [18] revealed that high body mass index (BMI), high intraoperative blood loss, presence of PHBL, and poor postoperative glucose control were risk factors for the occurrence of SSI in a multivariate analysis. In the present study, no significant differences were observed in the occurrences of PHLF and SSI between the PHBL (+) and PHBL (−) groups. These findings may be attributed to the close monitoring and early intervention and appropriate usage of prophylactic antibiotics at our institute. The results of many previous studies have indicated that PHBL is associated with other postoperative complications after hepatectomy; hence, it is desirable and reasonable to try and preoperatively identify the patients at a high risk for PHBL to improve their postoperative outcomes.
Obesity is one of the main health concerns that needs to be resolved, not only in Japan, but also in developed countries in particular. Obesity is clearly related to lifestyle diseases, such as hypertension, cardiovascular diseases, HCC from nonalcoholic steatohepatitis, and colorectal cancer [19]. A large database retrospective study [20] found that obesity was an independent risk factor for colorectal cancer across all age groups compared with the general population. BMI is the most common and simple indicator of obesity. Almost all previous reports that have shown a relationship between obesity and postoperative outcomes have been based on BMI. However, BMI can sometimes be inaccurate because it depends on muscle mass as well as fat mass. Therefore, we focused on sarcopenic obesity as a more objective and accurate indicator. Sarcopenic obesity was identified as an independent risk factor for the occurrence of PHBL in this study, whereas BMI was not.
Sarcopenic obesity, characterized by the depletion of lean body mass alongside the preservation or even augmentation of fat mass, serves as an indicator for assessment of patient nutrition [21]. Previous investigations suggested that sarcopenic obesity is a risk factor for both short- and long-term surgical outcomes. For example, Runkel et al. [22] reported that sarcopenic obesity was an independent risk factor for overall complications after hepatectomy for colorectal liver metastases. Regarding the association between fat and PHBL, our previous study [15] identified sarcopenic obesity as an independent risk factor for the occurrence of postoperative pancreatic fistula after pancreaticoduodenectomy. The rationale behind this observation was considered to arise from the potential complication posed by adipose tissue surrounding the pancreatic duct. The presence of this adipose tissue may complicate the anastomosis process, diminish the local blood flow essential for wound healing, and generate inflammatory cytokines that hinder the healing process. The surplus production of inflammatory cytokines by excessive adipose tissue has the potential to cause delayed healing of biliary wounds, consequently giving rise to the occurrence of PHBL. Another possible reason is that sarcopenic obesity may be associated with inadequate exposure to the incision of the liver. Underexposure of the operating field may affect the blood loss and bile leakage during the operation. Therefore, the results of the present study fit with existing data.
There are several reports on the relationship between intraoperative bleeding and PHBL. Wang et al. [23] found that tumor size, type of tumor, surgical duration time, blood loss, and blood transfusion were independent risk factors for PHBL. Intraoperative blood loss may cause liver damage and reduced blood flow around the bile duct, resulting in bile leakage. Another possibility is that surgery with a wide surface area for the incision exposes more bile ducts at the surface [24]. Hepatectomy with such a wide surface area of the incision may increase intraoperative blood loss, and the present findings may reflect this possibility. However, the present study was a retrospective study, and the area of the cut surface could not be measured accurately. Furthermore, as mentioned in the Results section, no significant difference was observed in the type of hepatectomy, such as medial sectionectomy or central bisectionectomy.
Occlusion of the hepatic inflow pedicle, also known as the PM, is a widely accepted method for reducing intraoperative blood loss. However, the PM results in ischemia-reperfusion changes [25]. According to several animal studies, ischemia-reperfusion injury caused by hilar vascular clamping can accelerate tumor growth, stimulate tumor cell adhesion, and promote metastasis [26]. Furthermore, studies have shown that PM duration for HCC was associated with postoperative long-term outcomes [27, 28]. However, because of recent advances in surgical techniques, the PM is not necessary for all operations. Maurer et al. [29] reported that major resection without the PM is feasible and safe and may reduce liver damage and failure. However, the decision to perform the PM during hepatectomy should be based on risk assessment and operative difficulties. Selection of the appropriate intraoperative supportive techniques required to complete the scheduled operation with minimal intraoperative blood loss is important.
Several limitations were present in this study. First, it was a single-center retrospective study, and thus the potential for selection bias exists. Second, the determined cut-off thresholds for sarcopenic obesity and intraoperative blood loss were established based on optimal sensitivities and specificities derived from ROC curve analyses for predicting PHBL. As a result, these cut-off values may not necessarily apply universally across different medical institutions. Notwithstanding these shortcomings, we maintain that our findings hold significance for surgeons because this study stands as a pioneering attempt to explore the correlation between sarcopenic obesity and PHBL in HCC patients.
Conclusion
Sarcopenic obesity and intraoperative blood loss were identified as significant risk factors for the occurrence of PHBL. It is important to preoperatively understand whether a patient is at high or low risk for PHBL for early therapeutic intervention.
Supporting information
(DOCX)
Acknowledgments
We thank James P. Mahaffey, PhD, and Alison Sherwin, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Data Availability
Data cannot be shared publicly as it contains personal information. Data are available from the Shinshu Institutional Data Access / Ethics Committee (contact via shinhp@shinshu-u.ac.jp) for researchers who meet the criteria for access to confidential data.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Kaibori M, Shimizu J, Hayashi M, Nakai T, Ishizaki M, Matsui K, et al. Late-onset bile leakage after hepatic resection. Surgery 2015;157:37–44. doi: 10.1016/j.surg.2014.05.026 [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto K, Tamesa T, Yukio T, Tokuhisa Y, Maeda Y, Oka M. Risk Factors and Managements of Bile Leakage After Hepatectomy. World J Surg 2016;40:182–189. doi: 10.1007/s00268-015-3156-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadamori H, Yagi T, Shinoura S, Umeda Y, Yoshida R, Satoh D, et al. Risk factors for major morbidity after liver resection for hepatocellular carcinoma. Br J Surg 2013;100:122–129. doi: 10.1002/bjs.8957 [DOI] [PubMed] [Google Scholar]
- 4.Panaro F, Hacina L, Bouyabrine H, Al-Hashmi AW, Herrero A, Navarro F. Risk factors for postoperative bile leakage: a retrospective single-center analysis of 411 hepatectomies. Hepatobiliary Pancreat Dis Int 2016;15:81–86. doi: 10.1016/s1499-3872(15)60424-6 [DOI] [PubMed] [Google Scholar]
- 5.Nakano R, Ohira M, Kobayashi T, Imaoka Y, Mashima H, Yamaguchi M, et al. Independent risk factors that predict bile leakage after hepatectomy for hepatocellular carcinoma: Cohort study. Int J Surg 2018;57:1–7. doi: 10.1016/j.ijsu.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Han SH, Kim HI. Detection of sarcopenic obesity and prediction of long-term survival in patients with gastric cancer using preoperative computed tomography and machine learning. J Surg Oncol 2021;124:1347–1355. doi: 10.1002/jso.26668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Yao S, et al. Impact of Sarcopenic Obesity on Outcomes in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Ann Surg 2019;269:924–931. doi: 10.1097/SLA.0000000000002555 [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 9.Yamada G. Histopathological characteristics and clinical significance of New Inuyama Classification in chronic hepatitis B. Nihon Rinsho 2004;62 Suppl 8:290–292. [PubMed] [Google Scholar]
- 10.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 11.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 12.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 13.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7 [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Yamada S, Sonohara F, Takami H, Hayashi M, Kanda M, et al. Pancreatic Fat and Body Composition Measurements by Computed Tomography are Associated with Pancreatic Fistula After Pancreatectomy. Ann Surg Oncol 2021;28:530–538. doi: 10.1245/s10434-020-08581-9 [DOI] [PubMed] [Google Scholar]
- 15.Hayashi H, Shimizu A, Kubota K, Notake T, Masuo H, Yoshizawa T, et al. A new fistula risk score using sarcopenic obesity and subcutaneous fat area for predicting postoperative pancreatic fistula after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2022. doi: 10.1002/jhbp.1283 [DOI] [PubMed] [Google Scholar]
- 16.Shehta A, Farouk A, Said R, Nakeeb AE, Aboelenin A, Elshobary M, et al. Bile Leakage After Hepatic Resection for Hepatocellular Carcinoma: Does It Impact the Short- and Long-term Outcomes? J Gastrointest Surg 2022;26:2070–2081. doi: 10.1007/s11605-022-05433-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo CM, Fan ST, Liu CL, Lai EC, Wong J. Biliary complications after hepatic resection: risk factors, management, and outcome. Arch Surg 1998;133:156–161. doi: 10.1001/archsurg.133.2.156 [DOI] [PubMed] [Google Scholar]
- 18.Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Yatabe T, Maeda H, et al. Risk factors and predictors for surgical site infection after hepatic resection. J Hosp Infect 2009;73:47–53. doi: 10.1016/j.jhin.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 19.Kim DS, Scherer PE. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metab J 2021;45:799–812. doi: 10.4093/dmj.2021.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elangovan A, Skeans J, Landsman M, Ali SMJ, Elangovan AG, Kaelber DC, et al. Colorectal Cancer, Age, and Obesity-Related Comorbidities: A Large Database Study. Dig Dis Sci 2021;66:3156–3163. doi: 10.1007/s10620-020-06602-x [DOI] [PubMed] [Google Scholar]
- 21.Trouwborst I, Verreijen A, Memelink R, Massanet P, Boirie Y, Weijs P, et al. Exercise and Nutrition Strategies to Counteract Sarcopenic Obesity. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Runkel M, Diallo TD, Lang SA, Bamberg F, Benndorf M, Fichtner-Feigl S. The Role of Visceral Obesity, Sarcopenia and Sarcopenic Obesity on Surgical Outcomes After Liver Resections for Colorectal Metastases. World J Surg 2021;45:2218–2226. doi: 10.1007/s00268-021-06073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Zhao JP, Wang JJ, Chai SS, Zhang YX, Zhang ZG, et al. The impact of bile leakage on long-term prognosis in primary liver cancers after hepatectomy: A propensity-score-matched study. Asian J Surg 2020;43:603–612. doi: 10.1016/j.asjsur.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 24.Nagano Y, Togo S, Tanaka K, Masui H, Endo I, Sekido H, et al. Risk factors and management of bile leakage after hepatic resection. World J Surg 2003;27:695–698. doi: 10.1007/s00268-003-6907-x [DOI] [PubMed] [Google Scholar]
- 25.Ezaki T, Seo Y, Tomoda H, Furusawa M, Kanematsu T, Sugimachi K. Partial hepatic resection under intermittent hepatic inflow occlusion in patients with chronic liver disease. Br J Surg 1992;79:224–226. doi: 10.1002/bjs.1800790311 [DOI] [PubMed] [Google Scholar]
- 26.van der Bilt JD, Kranenburg O, Borren A, van Hillegersberg R, Borel Rinkes IH. Ageing and hepatic steatosis exacerbate ischemia/reperfusion-accelerated outgrowth of colorectal micrometastases. Ann Surg Oncol 2008;15:1392–1398. doi: 10.1245/s10434-007-9758-0 [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Li X, Li H, Guo L, Zhang B, Gong Z, et al. Longer duration of the Pringle maneuver is associated with hepatocellular carcinoma recurrence following curative resection. J Surg Oncol 2016;114:112–118. doi: 10.1002/jso.24271 [DOI] [PubMed] [Google Scholar]
- 28.Hao S, Chen S, Yang X, Wan C. Adverse impact of intermittent portal clamping on long-term postoperative outcomes in hepatocellular carcinoma. Ann R Coll Surg Engl 2017;99:22–27. doi: 10.1308/rcsann.2016.0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer CA, Walensi M, Kaser SA, Kunzli BM, Lotscher R, Zuse A. Liver resections can be performed safely without Pringle maneuver: A prospective study. World J Hepatol 2016;8:1038–1046. doi: 10.4254/wjh.v8.i24.1038 [DOI] [PMC free article] [PubMed] [Google Scholar]