Abstract
Background
Whiplash associated disorders (WAD) are the most common non-hospitalised injuries resulting from a motor vehicle crash. Half of individuals with WAD experience ongoing pain and disability. Furthermore, individuals with persistent WAD have lower levels of aerobic capacity and isometric strength compared with age-matched controls. It is not known whether these differences are associated with increased levels of pain and disability, or with reduced physical activity (PA) participation.
Objective
Our primary aim was to compare PA levels in individuals with persistent WAD with healthy controls. Secondary aims were to: compare objective and subjective measurements of PA; explore factors that may influence PA; and describe proportions of these populations meeting World Health Organisation PA guidelines.
Methods
Objective (ActiGraph accelerometer; seven days) and subjective (International Physical Activity Questionnaire (IPAQ)) PA data were collected for n = 53 age-matched participants (WAD n = 28; controls n = 25).
Results
Independent sample t-tests showed no significant difference in objectively measured PA (p>0.05) between WAD and controls. For the subjective measure (IPAQ), controls reported more overall weekly PA (t = 0.219, p<0.05), while WAD participants reported more weekly walking minutes (t = -0.712, p<0.05). Linear regression showed mental health quality-of-life predicted objectively measured moderate intensity PA (R2 = 0.225, F (2, 44) = 6.379, p<0.004) and subjectively reported overall PA (R2 = 0.132, F (1, 41) = 6.226, p<0.017). Bland-Altman analyses indicated that subjects over-reported MVPA and under-reported sedentary time using the IPAQ.
Conclusions
Individuals with WAD had levels of physical and mental health quality-of-life significantly lower than controls and below population norms yet participated in similar levels of PA. Given that increased perceptions of mental health quality-of-life were positively associated with objectively measured MVPA and subjectively reported overall PA, strategies to help people with WAD achieve adequate doses of MVPA may be beneficial. ActiGraph-measured and IPAQ-reported PA were discordant. Hence, IPAQ may not be a reliable measure of habitual PA in WAD.
Introduction
Whiplash associated disorders (WAD) is the term used to describe a cluster of symptoms, including neck pain and disability, that typically result from an acceleration/deceleration movement of the neck following a motor vehicle crash (MVC). WAD are the most common non-hospitalised injuries resulting from a MVC, estimated to account for approximately 75% of all survivable MVC injuries in Australia [1], and over 95% in the USA [2]. Over the past few decades, recovery rates have remained unchanged with approximately 50% of individuals experiencing on-going pain, disability and psychological distress [3, 4]. Additionally, individuals with persistent WAD have lower levels of aerobic capacity and isometric strength compared with age-matched healthy controls [5, 6]. It is not known if this reduced physical fitness is associated with increased levels of pain and disability, or with reduced physical activity (PA) participation.
Most intervention trials in WAD have focussed on therapeutic exercise and have not addressed the need for promoting sustained PA participation in WAD [7]. Thus, clinical practice guidelines for treating WAD focus largely on neck specific exercises including range of motion and low load isometric exercises to optimise function and prevent disability [8–10]. Information about the inclusion of habitual physical activity to improve physical and psychological health is lacking.
While the benefits of participation in regular PA are numerous and accepted [11], the relationship between participation in regular PA and musculoskeletal pain is less clear. In individuals with chronic non-traumatic neck pain, reduced PA is associated with an increased risk of ongoing neck pain [12]. Some data suggest that individuals with chronic non-traumatic neck pain may be less physically active than age-matched healthy controls [12, 13], whereas other data suggest that PA levels are similar [14, 15]. Given that individuals with WAD have higher neck disability [16, 17] and pain [17], as well as lower health-related quality of life (HRQoL) [16, 18] compared with individuals with non-traumatic neck pain, it may be that individuals with persistent WAD are insufficiently active for good health, thereby, increasing their risk of preventable morbidity and mortality, and compounding the effects of the WAD.
Current World Health Organisation (WHO) guidelines recommend that adults should accumulate 150 to 300 minutes of moderate intensity PA or 75 to 150 minutes of vigorous intensity PA, or an equivalent combination of both, each week [11]. It is not known whether individuals with WAD meet the WHO recommendations. Habitual PA can be measured using objective (e.g., accelerometry) or subjective (e.g., self-report) measures. Objective tools provide increased reliability and accuracy since they do not rely on recall and thereby avoid biases associated with self-reporting [19]. However, accelerometers can be expensive and data analyses oftentimes require research expertise [19]. Self-report tools that have shown acceptable measurement properties and may be a more feasible way to assess habitual PA in adults [20]. The International Physical Activity Questionnaire (IPAQ) is the most widely used self-report PA questionnaire with the long version (IPAQ-L) shown to be valid for both research purposes and clinical practice [20, 21].
Our primary aim was to compare PA levels in individuals with persistent WAD and aged-matched healthy controls. The secondary aims were to compare objective (ActiGraph accelerometer) and subjective (IPAQ-L) measurements of PA, to describe the proportions of participants meeting WHO guidelines for PA, and to explore factors that may influence PA.
Materials and methods
Participants
Participants were individuals with persistent WAD and aged-matched healthy controls.
Inclusion criteria were: aged 18–65 years; ambulatory; and able to wear an accelerometer for seven days. Additional inclusion criteria for individuals with WAD: Quebec task force WAD grade II or III of at least three months duration [22] and reported presence of neck pain on a numeric rating scale of ≥ 3/10 over the past week [23]. Participants with WAD were excluded if they had: a serious spinal pathology; undergone spinal surgery in the past 12 months; a confirmed fracture or dislocation at time of injury (e.g., WAD Grade IV); or nerve root compromise. Additional exclusion criteria for healthy controls were: a previous whiplash injury, or history of neck pain.
Procedure
Participants were invited to attend two sessions in Brisbane, Queensland, Australia. At session one, participants completed a baseline questionnaire and were provided with the ActiGraph GT9X Link (ActiGraph LLC, Pensacola, FL, USA) accelerometer. Participants were asked to continue with their usual routine for seven days. Seven to 10 days after the first session, participants returned the accelerometer and completed the IPAQ-L [21].
The baseline questionnaire included demographic data (age, body height, body weight, working status, and compensation status), and the following validated tools:
the neck disability index (NDI) (0–100%) to assess neck pain and disability [24] with scores ≥ 30% indicating moderate to severe neck disability [25];
the Medical Outcomes Study Short Form Health Survey (SF12) to assess perceived quality of life related to physical functioning and mental health [26] with composite scores < 50 indicating lower than normal quality of life [27]; and
the pain self-efficacy questionnaire to assess confidence in performing tasks despite pain [28] with scores < 20 indicating low pain self-efficacy [29].
Objective measure of PA
Participants were asked to wear the ActiGraph GT9X Link, a commonly used research-grade accelerometer, on their non-dominant wrist continually for seven days. Data were processed using ActiLife software (v6.13.3) with the devices initialised to record accelerations at 30 Hz and vector magnitude counts integrated over 60 second epochs. Data were exported to MATLAB R2017b (The MathWorks, Inc., 2017, Natick, MA) for analysis of non-wear time, valid wear time and conversion to intensity of PA. Non-wear time was defined as ≥ 90 consecutive minutes of 0 vector magnitude counts, with an allowance for up to two minutes of nonzero counts to allow for detection of movement artifacts. Aligned with recommendations, a valid wear day was defined as ≥ 10 hours of wear time, and a valid week was ≥ 4 valid wear days [30]. The outcome for total habitual PA was ActiGraph counts per day. Additional outcomes were minutes per day (mpd) spent sedentary, and in light, moderate, vigorous and moderate/vigorous intensity PA (MVPA). Counts were converted to PA intensity levels using wrist-developed cut-points for ActiGraph accelerometers (sedentary ≤ 2860 cpm, light = 2861–4835 cpm, moderate = 4836–8452 cpm, vigorous = ≥ 8453 cpm) [31, 32].
Subjective measure of PA
The IPAQ-L is a validated, self-report survey used to subjectively measure habitual PA [20, 21]. The amount of time spent walking and participating in moderate or vigorous PA is reported for the previous seven days across various domains (e.g., transport, work, home, and leisure time). Time spent sitting is also estimated. In the present study, participants were asked to respond to the questions for the seven-day period in which they wore the ActiGraph. To optimise the accuracy of participant’s reflection of their PA, only IPAQ-L data completed within three days of the ActiGraph wear-week were included in the analyses. The IPAQ scoring manual (www.ipaq.ki.se) provided the information to compute volume of PA by weighting each type and intensity of PA by its energy requirement defined in metabolic equivalents (MET). For example, while one MET represents the amount of energy expended while sitting quietly, moderate intensity PA was defined as four METs. MET-minutes were calculated by multiplying the MET score of an activity by the number of activity minutes. The outcome measure for total PA was total MET–minutes of PA per week, calculated by summing total walking, moderate, and vigorous MET-minutes. Additional outcomes were mpd spent sitting, walking, and participating in MVPA.
Sample size
Since there are no published PA data for individuals with persistent WAD, data from a population study of ActiGraph measured PA in adults was used to calculate sample size [33]. Based on the difference of predicted means of 25% and standard deviation equal to 30% of the mean, with significance at p < 0.05 and power of 80% (β = 0.20), a sample size of 23 participants was needed in each group. A sample size of 46 participants was deemed sufficient to test the prediction of objectively or subjectively measured PA using four baseline measures based on the assumption that 10 cases are needed per predictor variable.
Data analysis
Baseline measures (e.g., sex, age, body mass index (BMI), SF12PCS, SF12MCS), and objective/subjective measures of PA and sedentary time were tested for normality using the Kolmogorov-Smirnov test. Comparisons were conducted using t-tests for normally distributed data, or Mann Whitney U otherwise. The proportion of WAD/control participants meeting WHO PA guidelines [11] was assessed by chi-squared analysis. To test whether baseline measures were associated with objective and subjective measures of PA, we used univariate linear regression followed by multivariate linear regression with those variables with a relationship of p < 0.1 from the corresponding univariate analysis.
The relationship between the objective and subjective measures of total PA, MVPA and sedentary time were assessed using Pearson-product moment correlation. In addition, the Bland-Altman method was used to describe agreement between objectively and subjectively measured MVPA and sedentary time with 95% limits of agreement used to explain total error [34]. The difference between the objective and subjective methods was assessed with a paired t test. SPSS (version 28.0) was used for all statistical analyses. Cohen’s criteria (r = 0.1, 0.3, 0.5 small, medium and large respectively) [35] were used to interpret effect size.
Ethics
Ethical approval was granted by the University of Queensland (#2019000085). The study was conducted in accordance with Declaration of Helsinki, and all participants provided written informed consent prior to participating in the study.
Results
We recruited 28 individuals with WAD and 25 controls. Most were female (WAD n = 23 (82%); controls n = 21 (84%)). Most participants were currently employed (WAD n = 23 (82%); controls n = 25 (100%)), though 11 (39%) WAD participants were not working or working reduced hours because of neck pain. Twenty-four (86%) WAD participants had submitted a third-party compensation claim. WAD participants had moderate levels of neck disability [25] and mildly reduced levels of confidence to participate in activities while in pain [29] (Table 1). There were no significant differences between the groups in age or BMI (Table 1). However, compared with controls, WAD had significantly lower mental (U = 127.0, p<0.000) and physical (U = 2.0, p<0.000) HRQoL (Table 1). There were insufficient males in the sample to allow any assessment by sex.
Table 1. The median [interquartile range], and results of Mann Whitney U for baseline measures.
| WAD (n = 28) | Controls (n = 25) | U, z | |
|---|---|---|---|
| NDI (%) | 40 [12, 58] | ||
| PSEQ | 30 [4, 56] | ||
| Age | 43 [35, 52] | 45 [35, 54] | 339.5, -0.187 |
| BMI | 26.7 [21.7, 30.5] | 23.8 [22.2, 26.0] | 417.5, 1.496 |
| SF12MCS | 44.3 [35.5, 51.3] | 57.8 [55.0, 57.9] | 127.0, -3.857** |
| SF12PCS | 37.5 [33.3, 44.2] | 56.6 [55.5, 57.0] | 2.0, -6.148** |
BMI (body mass index), NDI (neck disability index), PSEQ (pain self-efficacy questionnaire), SF12MCS (the medical outcomes short form mental component score), SF12PCS (the medical outcomes short form physical component score), WAD (whiplash associated disorder)
** p<0.000
Objectively measured PA
A valid wear week was completed by 82% (n = 23) of WAD and 96% (n = 24) of control participants. ActiGraph wear time was not significantly different between the groups (Table 2). There were no significant differences between WAD and control in objectively measured total PA, or mpd of light, moderate, or vigorous intensity PA, MVPA, or sedentary time (Table 2). There was no significant difference between the number of WAD (n = 16 (70%)) and controls (n = 19 (79%)) meeting the WHO guidelines for PA (χ2 = 0.569, df = 1, p<0.517) [11].
Table 2. Mean (standard deviation), and results of independent samples t tests for ActiGraph and IPAQ measures.
| ActiGraph | WAD (n = 23) | Controls (n = 24) | t |
|---|---|---|---|
| Wear time mpd | 921.5 (79.3) | 916.0 (92.3) | -0.218 |
| PA counts per day | 238.2 x 104 (63.2 x 104) | 256.6 x 104 (70.3 x 104) | 0.946 |
| Light intensity mpd | 337.2 (71.4) | 332.6 (84.1) | -0.202 |
| Moderate intensity mpd | 16.5 (8.2) | 17.2 (7.6) | 0.322 |
| Vigorous intensity mpd | 27.5 (15.3) | 35.4 (19.5) | 1.556 |
| MVPA mpd | 43.9 (22.8) | 52.6 (25.3) | 0.985 |
| Sedentary mpd | 540.3 (102.4) | 530.7 (108.4) | -0.312 |
| IPAQ-L | WAD (n = 20) | Controls (n = 24) | |
| MET-minutes per week | 2906.2 (2302.2) | 3030.4 (1431.0) | 0.219* |
| Walking mpd | 44.7 (45.7) | 36.5 (26.3) | -0.712* |
| Moderate intensity mpd | 51.1 (49.7) | 51.8 (37.0) | -0.053 |
| Vigorous intensity mpd | 9.4 (17.2) | 16.3 (19.5) | 1.220 |
| MVPA mpd | 60.5 (61.4) | 68.0 (48.6) | 0.454 |
| Sedentary mpd | 379.7 (182.5) | 378.6 (152.0) | -0.022 |
mpd (minute per day), MVPA (moderate-vigorous intensity physical activity), PA (physical activity), WAD (whiplash associated disorder)
*p < 0.05
The results of univariate linear regressions are shown in Table 3. While SF12PCS was a predictor of ActiGraph measured PA counts per day, this variable explained only 9% of the variance (R2 = 0.090, F (1, 45) = 4.453, p<0.040). A standard multiple regression showed that MVPA mpd was predicted by age and SF12MCS (R2 = 0.225, F (2, 44) = 6.379, p<0.004) where SF12MCS was the only variable to contribute significantly to the model (B = 0.972, p<0.004).
Table 3. Univariate linear regression analyses assessing the relationship between objective and subjective PA measures and baseline variables.
| Age | BMI | SF12PCS | SF12MCS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Β | t | p | β | t | p | β | t | p | β | t | p | |
| ActiGraph | ||||||||||||
| PA counts per day | -2854.86 | -0.307 | 0.761 | -23485.99 | -1.422 | 0.162 | 19175.37 | 2.110 | 0.040* | 12547.09 | 1.284 | 0.206 |
| MVPA mpd | -0.573 | -0.252 | 0.087* | -0.455 | -0.747 | 0.459 | 0.515 | 1.526 | 0.134 | 0.981 | 2.973 | 0.005* |
| Sedentary mpd | 0.177 | 0.121 | 0.904 | 3.926 | 1.526 | 0.134 | -1.372 | -0.930 | 0.357 | -2.331 | -1.538 | 0.131 |
| IPAQ-L | ||||||||||||
| MET-minutes per week | 2.193 | 0.082 | 0.935 | -77.363 | -1.645 | 0.108 | 38.950 | 1.441 | 0.157 | 67.245 | 2.495 | 0.017* |
| MVPA mpd | -0.168 | -0.214 | 0.831 | -1.418 | -1.011 | 0.318 | 1.116 | 1.411 | 0.166 | 1.220 | 1.481 | 0.146 |
| Sedentary mpd | -7.290 | -3.454 | 0.001* | -0.147 | -0.034 | 0.973 | 0.707 | 0.285 | 0.777 | -0.133 | -0.051 | 0.959 |
BMI (body mass index), mpd (minute per day), MVPA (moderate-vigorous intensity physical activity), PA (physical activity), SF12MCS (the medical outcomes short form mental component score), SF12PCS (the medical outcomes short form physical component score), WAD (whiplash associated disorder)
*P<0.1 and therefore eligible to be included in multivariate analyses
Subjectively measured PA
The IPAQ-L questionnaire was completed between days 7 and 10 post-baseline by 71% (n = 20) of WAD and 96% (n = 24) of control participants. Controls reported to participate in significantly more overall PA per week than WAD participants (t = 0.219, p<0.024) (Table 2). While there were no significant differences between the groups in terms of reported MVPA, WAD participants reported significantly more walking mpd than controls (t = -0.712, p<0.025) (Table 2). There was no significant difference between the number of WAD participants (n = 13 (65%)) and controls (n = 19 (79%)) meeting the WHO guidelines for PA (χ2 = 1.104, p<0.329) [11].
The results of univariate linear regressions showed that SF12MCS was a predictor of IPAQ-L total weekly MET-minutes, explaining 13% of the variance (R2 = 0.132, F (1, 41) = 6.226, p<0.017) (Table 3). Furthermore, age was a predictor of reported daily sedentary minutes, explaining 22% of the variance (R2 = 0.225, F (1, 41) = 11.928, p<0.001).
Relationship of objective and subjective PA measures
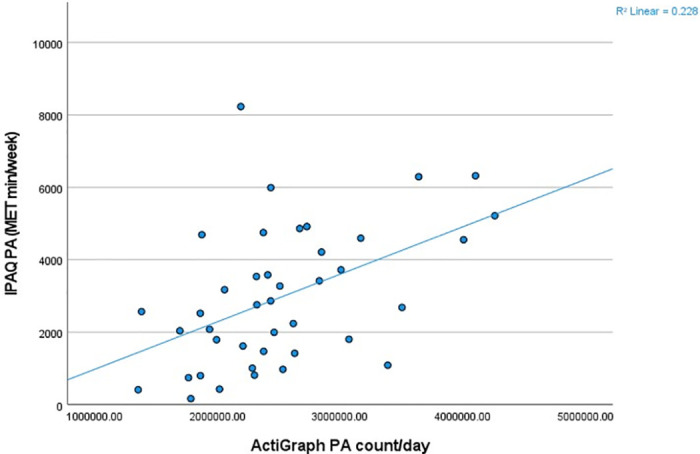
Pearson-product moment correlational analysis showed a moderate correlation between the objective (Actigraph PA counts/day) and subjective (IPAQ-L MET-minutes/week) measures of overall PA (r = 0.477, p<0.002) (Fig 1).
Fig 1. Scatterplot of total PA measured objectively (ActiGraph total PA counts/day) and subjectively (IPAQ-L reported MET-minutes/week).
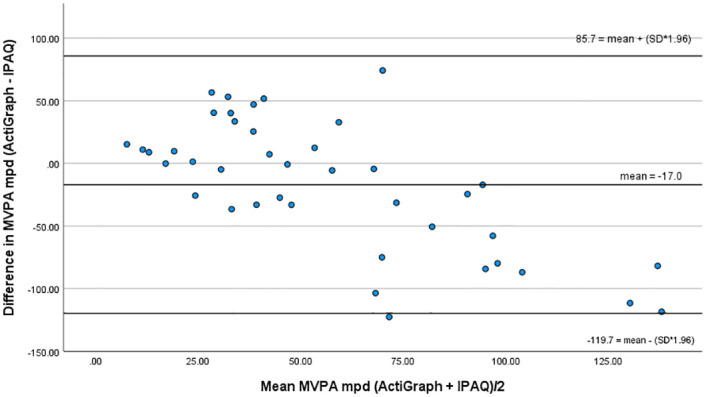
A small correlation was found between objectively and subjectively measured MVPA (r = 0.362, p<0.020), though Bland-Altman analysis highlighted the over-reporting (IPAQ) of MVPA by a mean of 17 mpd and showed wide limits of agreement (-119.7 and 85.7 mpd) (Fig 2). Paired t- test showed a significant difference between the measures (ActiGraph mean = 48.4 mpd; IPAQ-L mean = 65.4 mpd; t = -2.077, p<0.022).
Fig 2. Bland-Altman plot for MVPA.
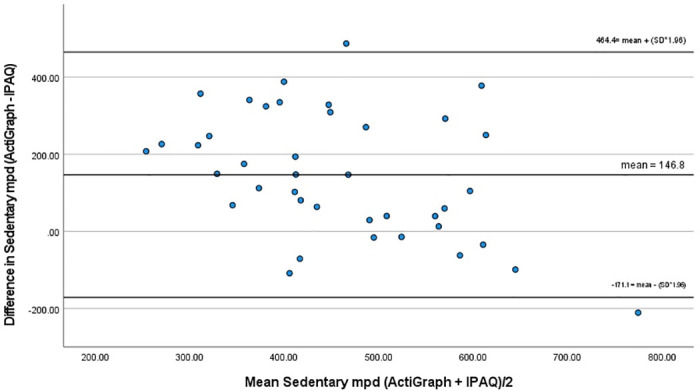
While a small correlation was also found between objective and subjective measures of sedentary time (r = 0.371, p<0.019), Bland-Altman analysis highlighted the under-reporting (IPAQ) of sedentary time by a mean of 147 mpd and showed wide limits of agreement (-171 and 464 mpd) (Fig 3). Paired t- test showed a significant difference between the measures (ActiGraph mean = 532.2 mpd; IPAQ-L mean = 385.4 mpd; t = 5.722, p<0.000).
Fig 3. Bland-Altman plot for sedentary time.
Discussion
Objectively measured habitual PA, MVPA and sedentary time were similar for WAD and controls. On the other hand, there were some differences in the subjective reporting of weekly PA: controls reported significantly more overall weekly PA, and participants with WAD reported significantly more walking minutes. For WAD participants, levels of physical and mental health-related quality of life were below population norms, and significantly lower than controls with increased perceptions of mental health quality of life positively associated with objectively measured MVPA and subjectively reported overall PA. Given the very low physical and mental health quality of life perceived by WAD participants and the well accepted benefits of participation in MVPA for health and quality of life, it may be beneficial to include strategies within clinical guidelines to help people with WAD achieve adequate doses of MVPA.
Participation in any level of physical activity is better than no participation though the health benefits are optimal if weekly PA includes 150–300 minutes of moderate intensity PA or 75 to 150 minutes of vigorous intensity PA, or an equivalent combination of both [11]. We found no significant difference in the percentage of WAD or controls who met these WHO guidelines. Moreover, participants appeared to engage in more habitual MVPA than the general Australian population. Seventy percent of WAD and 79% of HC met WHO guidelines for MVPA. Whereas, recent Australian data showed only 59% of Australian adults completed 30 minutes of aerobic PA on five or more days per week (https://www.abs.gov.au/statistics/health/health-conditions-and-risks/physical-activity/latest-release, accessed 22 August, 2022). Wearing a monitor may have motivated participants to increase their activity, though to minimise extrinsic motivation, the monitor only displayed time and date. While persistent neck disability from a whiplash injury did not appear to inhibit PA participation for many participants, 30% of WAD participants were not meeting current PA guidelines.
Both median physical and mental HRQoL for WAD were below population norms, levels consistently shown in WAD [18, 36]. While physical HRQoL had a small positive association with objectively measured overall PA, increased mental HRQoL predicted increased objectively measured MVPA. Previous research has shown physical, but not mental, HRQoL to be significantly associated with objective and self-reported MVPA in older Europeans (>65 years) [37] and self-reported weekly PA in individuals with rheumatic and musculoskeletal diseases [38]. It may be that high symptom levels of depression, anxiety, and posttraumatic stress disorder reported in WAD [39] affect not only mental health quality of life but also participation in MVPA. Participation in PA improves perceptions of quality of life. Previous research by our group showed SF12PCS and SF12MCS scores increased to levels above population norms in participants with persistent WAD following participation in a 12-week PA intervention [36]. Similarly, in general populations, both physical and mental HRQoL scores have been shown to improve following increased frequency of moderate PA [40], with a positive dose-response relationship between weekly MVPA and HRQoL reported across age groups [41]. While therapeutic exercise is an important component of clinical guidelines and research with WAD, including information about the importance of habitual PA and strategies to help achieve adequate doses of MVPA would be valuable for this population.
Based on our results, it is difficult to recommend the IPAQ-L as a self-report measure to assess habitual PA in WAD. Small to moderate correlations were found between objective and subjective PA data though further analysis of MVPA and sedentary time indicated that neither were concordant. Participants over-reported MVPA and under-reported sedentary time with older participants reporting significantly less sedentary time. While WAD participants reported significantly more walking time and less overall PA compared with controls, no significant differences were found for overall objectively measured habitual PA between WAD and controls. No statistical comparisons of objective versus subjective light activity were performed since the IPAQ-L uses walking as a proxy for light intensity PA. A similar lack of agreement has been reported in systematic reviews with weak correlations between subjective self-report measures and accelerometry [19, 42]. Although self-report measures are inexpensive and convenient and the IPAQ-L has been shown to be valid and reliable [20, 21], subjective measures rely on an individual’s ability to estimate PA intensity and recall all types of activity. On the other hand, accelerometers such as the ActiGraph provide less overall measurement error and more acute estimates of PA, yet data processing is complex with outcomes such as accelerometer counts requiring valid conversions to meaningful measures such as PA intensity [19, 42]. While accelerometer devices such as the ActiGraph may not be feasible for all research or clinical purposes, the growth of PA measurement devices and wearables provides alternatives that may be more cost effective and require less expertise in analysing the data.
Study limitations
This study provides much needed data about PA levels in individuals with WAD. Nevertheless, limitations exist. Firstly, neither the IPAQ nor ActiGraph accurately collect information about participation in muscular strength activities, an important component of WHO PA recommendations [11]. Further research is needed to determine if people with WAD achieve recommended doses of muscular strength activities. Secondly, compared with hip-worn ActiGraph devices, wrist-worn ActiGraph devices have better wear compliance but may overestimate MVPA [43]. However, to minimise potential overestimations, wrist-developed cut-points were used to convert ActiGraph proprietary counts to PA intensities [31]. Finally, the study was powered to assess differences in ActiGraph measured PA. It is not known if these numbers are adequate to detect PA differences using the IPAQ-L.
Conclusions
This was the first study to objectively and subjectively measure habitual PA in WAD. Individuals with a persistent moderate disability from a whiplash injury had levels of physical and mental health-related quality of life significantly lower than controls and below population norms yet participated in similar levels of PA. Given that increased perceptions of mental health quality of life were positively associated with objectively measured MVPA and subjectively reported overall PA, it may be beneficial to include strategies within clinical guidelines to help people with WAD achieve adequate doses of MVPA. Further research is needed to identify the most appropriate methods to assess PA in WAD given the lack of concordance in PA measured with the ActiGraph and reported through the IPAQ.
Supporting information
(XLSX)
Acknowledgments
The authors acknowledge and thank the Master of Physiotherapy students at the University of Queensland for assisting with data collection.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Connelly LB, Supangan R. The economic costs of road traffic crashes: Australia, states and territories. Accident Analysis & Prevention. 2006;38(6):1087–93. doi: 10.1016/j.aap.2006.04.015 [DOI] [PubMed] [Google Scholar]
- 2.Freeman MD, Leith WM. Estimating the number of traffic crash-related cervical spine injuries in the United States: An analysis and comparison of national crash and hospital data. Accid Anal Prev. 2020;142:105571. doi: 10.1016/j.aap.2020.105571 [DOI] [PubMed] [Google Scholar]
- 3.Carroll L, Holm L, Hogg-Johnston S. Course and prognostic factors for neck pain in Whiplash Associated Disorder (WAD): Results of the bone and joint decade 2000–2010 Task Force on neck pain and its associated disorders. Journal of Manipulative and Physiological Therapeutics Supplement. 2009;32(2):S97–107. [DOI] [PubMed] [Google Scholar]
- 4.Sterling M, Hendrikz J, Kenardy J. Compensation claim lodgement and health outcome developmental trajectories following whiplash injury: A prospective study. Pain. 2010;150(1):22–8. doi: 10.1016/j.pain.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 5.Smith A, Ritchie C, Pedler A, McCamley K, Roberts K, Sterling M. Exercise induced hypoalgesia is elicited by isometric, but not aerobic exercise in individuals with chronic whiplash associated disorders. Scandinavian Journal of Pain. 2017;15(Supplement C):14–21. [DOI] [PubMed] [Google Scholar]
- 6.Smith A, Ritchie C, Warren J, Sterling M. Exercise-induced Hypoalgesia Is Impaired in Chronic Whiplash-associated Disorders (WAD) With Both Aerobic and Isometric Exercise. The Clinical journal of pain. 2020;36(8):601–11. doi: 10.1097/AJP.0000000000000845 [DOI] [PubMed] [Google Scholar]
- 7.Griffin A, Leaver A, Moloney N. General Exercise Does Not Improve Long-Term Pain and Disability in Individuals With Whiplash-Associated Disorders: A Systematic Review. Journal of Orthopaedic & Sports Physical Therapy. 2017;47(7):472–80. doi: 10.2519/jospt.2017.7081 [DOI] [PubMed] [Google Scholar]
- 8.State Insurance Regulatory Authority. Guidelines for the management of acute whiplash-associated disorders–for health professionals. Sydney, NSW; 2014.
- 9.South Australian Centre for Trauma and Injury Recovery (TRACsa). Clinial guidelines for best practice management of acute and chronic whiplash associated disorders: clinical resource guide. South Australia; 2008.
- 10.Blanpied PR, Gross AR, Elliott JM, Devaney LL, Clewley D, Walton DM, et al. Neck Pain: Revision 2017, Clinical Practice Guidelines Linked to the International Classification of Functioning, Disability and Health From the Orthopaedic Section of the American Physical Therapy Association. Journal of Orthopaedic & Sports Physical Therapy. 2017;47(7):A1–A83. [DOI] [PubMed] [Google Scholar]
- 11.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. British Journal of Sports Medicine. 2020;54(24):1451. doi: 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallman DM, Ekman AH, Lyskov E. Changes in physical activity and heart rate variability in chronic neck–shoulder pain: monitoring during work and leisure time. International Archives of Occupational and Environmental Health. 2014;87(7):735–44. doi: 10.1007/s00420-013-0917-2 [DOI] [PubMed] [Google Scholar]
- 13.Mansfield M, Thacker M, Spahr N, Smith T. Factors Associated With Physical Activity Participation In Adults With Chronic Cervical Spine Pain. A Systematic Review. Physiotherapy. 2017;104(1):54–60. [DOI] [PubMed] [Google Scholar]
- 14.Cheung J, Kajaks T, MacDermid JC. The Relationship Between Neck Pain and Physical Activity. The Open Orthopaedics Journal. 2013;7:521–9. doi: 10.2174/1874325001307010521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan F, Byrne KS, Ramakrishnan R, Ferreira M, Dwyer T, Jones G. Association between musculoskeletal pain at multiple sites and objectively measured physical activity and work capacity: Results from UK Biobank study. Journal of science and medicine in sport. 2019;22(4):444–9. doi: 10.1016/j.jsams.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 16.Coppieters I, De Pauw R, Kregel J, Malfliet A, Goubert D, Lenoir D, et al. Differences Between Women With Traumatic and Idiopathic Chronic Neck Pain and Women Without Neck Pain: Interrelationships Among Disability, Cognitive Deficits, and Central Sensitization. Physical Therapy. 2017;97(3):338–53. doi: 10.2522/ptj.20160259 [DOI] [PubMed] [Google Scholar]
- 17.Stenneberg MS, Scholten-Peeters GGM, den Uil CS, Wildeman ME, van Trijffel E, de Bie RA. Clinical characteristics differ between patients with non-traumatic neck pain, patients with whiplash-associated disorders, and pain-free individuals. Physiotherapy theory and practice. 2021;38(13):1–11. doi: 10.1080/09593985.2021.1962464 [DOI] [PubMed] [Google Scholar]
- 18.Ris I, Juul-Kristensen B, Boyle E, Kongsted A, Manniche C, Søgaard K. Chronic neck pain patients with traumatic or non-traumatic onset: Differences in characteristics. A cross-sectional study. Scand J Pain. 2016;14(1):1–8. doi: 10.1016/j.sjpain.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 19.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int J Behav Nutr Phys Act. 2008;5(1):56-. doi: 10.1186/1479-5868-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silsbury Z, Goldsmith R, Rushton A. Systematic review of the measurement properties of self-report physical activity questionnaires in healthy adult populations. BMJ Open. 2015;5(9):e008430–e. doi: 10.1136/bmjopen-2015-008430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig LC, Marshall LA, Sjöström EM, Bauman LA, Booth EM, Ainsworth FB, et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Medicine & Science in Sports & Exercise. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 22.Spitzer W, Skovron M, Salmi L, Cassidy JD, Duranceau J, Suissa S, et al. Scientific Monograph of Quebec Task Force on Whiplash Associated Disorders: redefining "Whiplash" and its management. Spine. 1995;20(8S):1–73. [PubMed] [Google Scholar]
- 23.Dijkers M. Comparing Quantification of Pain Severity by Verbal Rating and Numeric Rating Scales. The Journal of Spinal Cord Medicine. 2010;33(3):232–42. doi: 10.1080/10790268.2010.11689700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vernon H, Moir S. The Neck Disability Index: A study of reliability and validity. Journal of Manipulative Physiological Therapeutics. 1991;14(7):409–15. [PubMed] [Google Scholar]
- 25.Young IA, Dunning J, Butts R, Mourad F, Cleland JA. Reliability, construct validity, and responsiveness of the neck disability index and numeric pain rating scale in patients with mechanical neck pain without upper extremity symptoms. Physiother Theory Pract. 2019;35(12):1328–35. doi: 10.1080/09593985.2018.1471763 [DOI] [PubMed] [Google Scholar]
- 26.Ware EJ, Kosinski DM, Keller DS. A 12- Item Short- Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Medical Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 27.Díaz-Arribas JM, Fernández-Serrano MM, Royuela SA, Kovacs SF, Gallego-Izquierdo ST, Ramos-Sánchez SM, et al. Minimal Clinically Important Difference in Quality of Life for Patients With Low Back Pain. Spine. 2017;42(24):1908–16. doi: 10.1097/BRS.0000000000002298 [DOI] [PubMed] [Google Scholar]
- 28.Asghari KA, Nicholas KM. Pain self-efficacy beliefs and pain behaviour. A prospective study. Pain. 2001;94(1):85–100. doi: 10.1016/S0304-3959(01)00344-X [DOI] [PubMed] [Google Scholar]
- 29.Suzuki H, Aono S, Inoue S, Imajo Y, Nishida N, Funaba M, et al. Clinically significant changes in pain along the Pain Intensity Numerical Rating Scale in patients with chronic low back pain. PloS one. 2020;15(3):e0229228. doi: 10.1371/journal.pone.0229228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Migueles JH, Cadenas-Sanchez C, Ekelund U, Delisle Nyström C, Mora-Gonzalez J, Löf M, et al. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Medicine. 2017;47(9):1821–45. doi: 10.1007/s40279-017-0716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhudy MB, Dreisbach SB, Moran MD, Ruggiero MJ, Veerabhadrappa P. Cut points of the Actigraph GT9X for moderate and vigorous intensity physical activity at four different wear locations. J Sports Sci. 2020;38(5):503–10. doi: 10.1080/02640414.2019.1707956 [DOI] [PubMed] [Google Scholar]
- 32.Montoye AHK, Clevenger KA, Pfeiffer KA, Nelson MB, Bock JM, Imboden MT, et al. Development of cut-points for determining activity intensity from a wrist-worn ActiGraph accelerometer in free-living adults. Journal of Sports Sciences. 2020;38(22):2569–78. doi: 10.1080/02640414.2020.1794244 [DOI] [PubMed] [Google Scholar]
- 33.Hagstromer M, Ainsworth BE, Oja P, Sjostrom M. Comparison of a subjective and an objective measure of physical activity in a population sample. Journal of physical activity & health. 2010;7(4):541–50. [DOI] [PubMed] [Google Scholar]
- 34.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60. doi: 10.1177/096228029900800204 [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. ed. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- 36.Ritchie C, Clanchy K, Sterling M, Tate R, Smits EJ, Day M, et al. Evaluation of a physical activity promotion intervention for adults with whiplash associated disorders: a single-case experimental design study. Disabil Rehabil. 2021;44(23):7255–68. doi: 10.1080/09638288.2021.1989062 [DOI] [PubMed] [Google Scholar]
- 37.Sansano-Nadal O, Giné-Garriga M, Rodríguez-Roca B, Guerra-Balic M, Ferri K, Wilson JJ, et al. Association of self-reported and device-measured sedentary behaviour and physical activity with health-related quality of life among european older adults. Int J Environ Res Public Health. 2021;18(24):13252. doi: 10.3390/ijerph182413252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courbalay A, Jobard R, Descarreaux M, Bouvard B. Direct and indirect relationships between physical activity, fitness level, kinesiophobia, and health-related quality of life in patients with rheumatic and musculoskeletal diseases: A network analysis. J Pain Res. 2021;14:3387–99. doi: 10.2147/JPR.S323424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craig A, Tran Y, Guest R, Gopinath B, Jagnoor J, Bryant RA, et al. Psychological impact of injuries sustained in motor vehicle crashes: systematic review and meta-analysis. BMJ Open. 2016;6(9):e011993. doi: 10.1136/bmjopen-2016-011993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Im J-S, Choi Y-H. Objectively measured sedentary behavior and moderate-to-vigorous physical activity on the health-related quality of life in US adults: The National Health and Nutrition Examination Survey 2003–2006. Qual Life Res. 2017;26(5):1315–26. doi: 10.1007/s11136-016-1451-y [DOI] [PubMed] [Google Scholar]
- 41.Alzahrani H. Dose–Response Association between Physical Activity and Health-Related Quality of Life in General Population: A Population-Based Pooled Study. Healthcare (Basel). 2022;10(1460):1460. doi: 10.3390/healthcare10081460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skender S, Ose J, Chang-Claude J, Paskow M, Brühmann B, Siegel EM, et al. Accelerometry and physical activity questionnaires—A systematic review. BMC Public Health. 2016;16(1):515. doi: 10.1186/s12889-016-3172-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gall N, Sun R, Smuck M. A Comparison of Wrist- Versus Hip-Worn ActiGraph Sensors for Assessing Physical Activity in Adults: A Systematic Review. Journal for the Measurement of Physical Behaviour. 2022;5(4):252–62. [Google Scholar]


