According to the Center for Disease Control (CDC)’s yearly Behavioral Risk Factor Surveillance System (BRFSS) report, approximately 25 million individuals worldwide, including 1–1.4 million adults in the United States identify as transgender (Flores et al., 2020; Meerwijk & Sevelius, 2017). Estimated to comprise roughly 0.4–0.6% of the population, these numbers have been rising consistently, with a reported two-fold increase between the years 2011–2016 (Flores et al., 2020; Meerwijk & Sevelius, 2017). In recent years, decreased social stigmatization, improved insurance coverage, and expanded access to gender-affirmation surgery (GAS) (through the Affordable Care Act and commercial insurance plans (Baker, 2017; Wiegmann et al., 2021)) have resulted in an increase in the number of transgender individuals pursuing medical and surgical interventions (Berli et al., 2017; Wiegmann et al., 2021). For appropriately selected transgender individuals, GAS is safe, effective, and medically necessary (Coleman et al., 2012; Wierckx et al., 2011).
Transgender women (TGW) may select from a variety of well-established therapeutic options which range from counseling and social role modifications, medical management in the form of gender-affirmation hormone therapy (GAHT), and a variety of GAS procedures. Among TGW treated with GAHT, approximately half will eventually undergo GAS (Kailas et al., 2017; Sineath et al., 2016). This includes genital surgery such as orchiectomy, penectomy, and vaginoplasty. In a recent study, Nolan et al. (2019) reported that 28% of TGW had undergone GAS procedures, 5–13% of these surgeries involved genital surgery. Of the TGW who had not yet undergone genital GAS (gGAS), 45–54% of respondents expressed interest in doing so in the future.
Infertility is often a consequence of both GAHT (temporary) and GAS (permanent), and fertility preservation should be discussed prior to medical and/or surgical interventions (Defreyne et al., 2020; Jones et al., 2021). Current fertility preservation options for TGW including sperm cryopreservation, surgical sperm extraction, and surgical biopsy of testicular tissue. However, a relatively small percentage of TGW pursue fertility preserving options reportedly due to financial barriers and the reluctance to delay transition (Chen et al., 2017; Nahata et al., 2017). Studies demonstrate that approximately half of TGW desire biological children (De Sutter et al., 2002). For some TGW, the inability to fulfill their reproductive aspirations may lead to an enduring sense of incompleteness and grief (Jones et al., 2021). Additionally, while current fertility options provide TGW the ability to contribute in production of an embryo with sperm donation, they do not allow for the opportunity to experience pregnancy and/or live birth. The ability to bear children may help TGW fulfill both physical and psychological goals.
Absolute uterine factor infertility (AUFI)
Absolute uterine factor infertility (AUFI) refers to a condition in which a cisgender woman cannot carry a pregnancy due to either lack of a uterus or a malfunctioning uterus (Johannesson et al., 2018). Causes of AUFI include congenital absence of the uterus, such as in Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome (1/5000 cisgender women) or acquired absence of the uterus following hysterectomy. In the US alone, 600,000 hysterectomies are performed annually. Globally, around 1.5 million women are affected by AUFI (Dahm-Kähler et al., 2016).
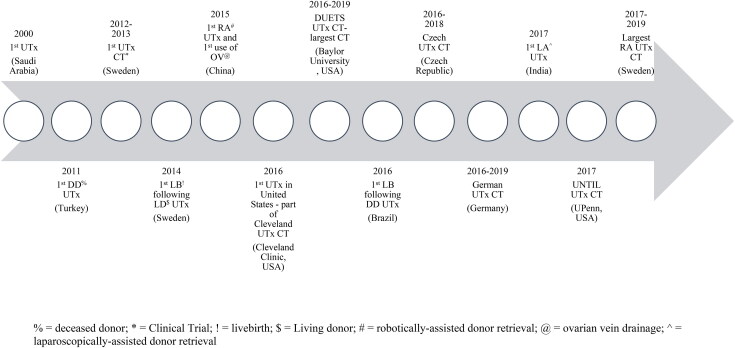
Uterine transplantation (UTx) has been proposed as a unique solution for AUFI. Though still considered experimental by many, multiple institutions have demonstrated that UTx can be performed safely and successfully. Since the first UTx performed in Saudi Arabia in 2000 (Fageeh et al., 2002), over 10 countries have performed UTx, including large clinical trials in Sweden (Brännström et al., 2020; Johannesson et al., 2015), Dallas, TX (Testa et al., 2020), and the Czech Republic (Fronek et al., 2021). Currently, more than 70 UTx have been performed worldwide resulting in more than 23 livebirths (Jones et al., 2019). While UTx in cisgender women has been performed, to our knowledge, UTx in TGW has not been successfully performed. Perhaps the first and only recorded attempt at UTx in a TGW occurred in Germany in 1931. Lili Elbe, portrayed in the film “The Danish Girl,” was a TGW who underwent UTx. Due to organ rejection and infection, Elbe died three months after surgery (Worthen, 2019).
UTx proof of concept for transgender recipients
The clinical and technical aspects of UTx were first tested in non-primates; subsequent tests in primates then followed. In 2012, the first human trial of UTx from living donors was performed at the University of Gothenburg in Sweden (Brännström et al., 2014; Johannesson et al., 2015). The Gothenburg University Group described their surgical procedure as:
Uterine Donor: total abdominal hysterectomy and upper vaginectomy with preservation of the major feeding arteries and veins.
Uterine Recipient: implantation of the donor uterus in the pelvis, including anastomosis of the donor vaginal rim to the recipient’s vaginal vault.
Vascular Anastomoses: two major uterine arteries and uterine veins (or ovarian veins) of the donor uterus are anastomosed to the external iliac vessels of the recipient using microsurgical techniques.
In 2014, the first live birth from a woman who received a living donor UTx in the Gothenburg trial was announced (Brännström et al., 2015). Subsequently, Baylor University Medical Center in Dallas, Texas, USA reported a 55% live-birth rate per attempted UTx and a 79% live-birth rate per technically successful UT (Johannesson et al., 2021). In 2016, a Brazilian team reported a successful UTx from a deceased donor (Ejzenberg et al., 2019). In 2017, The Cleveland Clinic reported their successful UTx transplant also using a deceased donor. The use of deceased donors expanded the potential pool of uterine donors (Flyckt et al., 2020) (see Figure 1).
Figure 1.
Historic timeline of the uterine transplant (UTx).
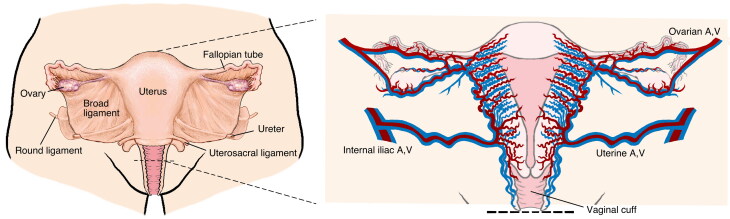
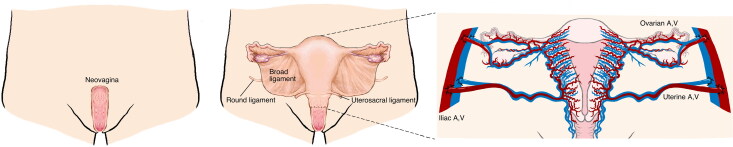
Given the success of UTx in cisgender women, the question as to whether TGW are candidates for this procedure has been raised (Jones et al., 2019). Technical considerations in women who are genetically XY include: (1) an android pelvis (narrow) rather than a gynecoid pelvis (wide); (2) the presence of neovagina (i.e., skin-lined or intestinal) rather than vaginal epithelium; (3) potential differences in vascular anatomy; and (4) the lack of ligamentous support for the uterus. While some degree of surgical adaptation is necessary, none of these barriers seem insurmountable (see Figures 2 and 3).
Figure 2.
Uterine transplant (UTx) donor anatomy.
Figure 3.
Uterine transplant (UTx) recipient anatomy.
In 2019, Jones et al. published a report discussing issues pertaining to the vascular anastomoses, the vaginal anastomosis, and the ligamentous support of the uterus in TGW (Jones et al., 2019). In terms of the pelvic vasculature, the external iliac arteries (recipient vessels) are similar size in both TGW and cisgender women. In addition, the vaginal anastomosis would be possible in TGW following construction of a neovagina. In fact, some of the previous cisgender UTx recipients were diagnosed with vaginal agenesis/MRKH syndrome. These individuals required either dilation of the vestigial vaginal remnants or vaginoplasty using skin grafts or intestinal segments prior to UTx (Herlin et al., 2020). The anastomosis of the neovaginal skin grafted lining (McIndoe vaginoplasty) to the donor’s vaginal cuff has been successfully reported (Herlin et al., 2020). In addition, ligamentous support of the donor uterus was obtained by fixation of the donor uterine round ligaments to the recipient’s pelvic sidewall (Bretschneider et al., 2018). While there were concerns regarding pregnancy in UTx patients with a neovagina, at least five of the UTx recipients underwent an initial vaginoplasty and successfully carried a pregnancy and gave birth (Brucker et al., 2020; Herlin et al., 2020; Johannesson et al., 2015; Puntambekar et al., 2018). This includes two transplants in the Swedish trial (cases with surgically created neovagina for vaginal agenesis). In terms of fertility and obstetric considerations, TGW would undergo similar protocols and monitoring to that of cisgender women. This editorial represents the first in what we anticipate will be a series of detailed publications on this topic.
Ethical considerations in UTx for transgender recipients
Ethical considerations regarding UTx in TGW have been raised (Alghrani, 2016, 2018; Robertson, 2017). The 2013 updated Montreal Criteria (Lefkowitz et al., 2013), which provide one ethical framework for UTx, require recipients to be “genetically female.” While these guidelines aim to “protect” patients (non-maleficence), others argue that excluding TGW violates their right to gestate (autonomy) (Mookerjee & Kwan, 2020). Furthermore, technical considerations such as vascular anastomoses, immunosuppression, fertility hormonal therapy, and the mode of child delivery (cesarean section), differ only slightly, if at all, between cisgender and transgender women (Mookerjee & Kwan, 2020). Balayla et al. (2021) state that the exclusion of TGW from the Montreal Criteria stems from “Moore’s criteria” pertaining to requirements for surgical innovation (Moore, 2000). The implication is that UTx should not be offered to TGW until: (1) experimental research in non-human species has been performed; and (2) enough time following successful UTx in cisgender women has elapsed (what constitutes “enough time” is not clear). We believe, as do other experts, that the necessary criteria have been met, and TGW should not be excluded from receiving a uterine transplant (Balayla et al., 2021; Murphy, 2015). Similarly, TGM should not be excluded from serving as UTx donors. The principles of medical ethics provide for the autonomy of individuals to make decisions regarding their health and to prevent individuals from being unfairly disadvantaged when accessing care (Beauchamp & Childress, 2009).
TGM as living uterine donors
Transgender men (TGM) may undergo hysterectomy (often with oophorectomy and/or partial or full colpectomy with colpocleisis) as part of their transition (Coleman et al., 2012; Safa et al., 2019). While hysterectomies for TGM comprise 1% of hysterectomies performed annually, it is a safe procedure with similar or lower rate of complications compared with cisgender women (Bretschneider et al., 2018). As many TGM seeking hysterectomy are young and healthy, they represent a possible group of volunteers for uterus donation. Currently, following hysterectomy-oophorectomy, the organs are discarded after routine pathologic evaluation. Considering the number of individuals who identify as transgender, both TGW and TGM may represent a significant population of potential UTx recipients and donors.
TGM are aware of the challenges of surgical transition and may be motivated to participate as altruistic uterine donors. In a study assessing the interest of uterine donation in TGM undergoing hysterectomy and bilateral salpingo-oophorectomy, respondents were interviewed regarding their attitudes toward uterus donation. Of 31 TGM respondents, 96.7% had positive attitudes initially and 84% wanted to volunteer for uterus donation after hearing detailed procedural information (Api et al., 2017). Prior to hysterectomy/oophorectomy, TGM will have undergone various preoperative assessments related to their surgical transition. As such, TGM may be more prepared for uterine donation as compared to previous cisgender women who served as altruistic donors in earlier studies.
As part of the informed consent process, it is important to consider additional risks posed to TGM who are acting as altruistic donors. While the hysterectomy performed to procure a uterus is more surgically complex (potential increased risk of ureteral injury) compared to that of a hysterectomy performed as part of GAS, this discussion is considered in the informed consent process. Additionally, the successful use of the ovarian or utero-ovarian veins (as opposed to the uterine veins) helps to mitigate the risk of ureteral injury (Testa et al., 2018; Wei et al., 2017). As many TGM will undergo simultaneous oophorectomy, retrieval of an elongated ovarian venous pedicle facilitates vascular anastomoses and should reduce the risk of ureteral injury.
TGM donors may be nulliparous and/or taking androgen medications prior to uterine donation. These issues raise the concern of transplanting a uterus of “unproven function.” Hysteroscopy prior to donation can exclude anatomic issues such as intracavity malformations (i.e., Asherman syndrome) or a septate uterus. Additionally, a team from the Czech Republic demonstrated that uteri from nulliparous, deceased donors can result in successful live births (Fronek et al., 2020). Also, in a survey of 41 TGM who became pregnant and gave birth, 61% had or were currently using testosterone (Light et al., 2014). The impact of androgens on endometrial function is not permanent, and endometrial function should normalize following cessation of the androgens (Light et al., 2014).
Institutional planning for UTx program
Institutional and programmatic requirements for vascularized composite allotransplantation (VCA) have been well-described (Gordon & Siemionow, 2009; Meyer-Marcotty et al., 2010; Pomahac, 2012; Siemionow & Gordon, 2010a, 2010b). Many of these (same) considerations may be applied to a UTx program. The specifics pertaining to uterine transplantation will be detailed in future publications.
Assess interest
At inception, our team assessed community and patient interest regarding UTx. Our efforts were motivated from overwhelmingly positive feedback from both the cis- and transgender communities. In a recent study, Jones et al. (2021) found that more than 90% of TGW who were polled regarding UTx, indicated that an UTx would improve their quality of life, enhance feelings of femininity, and alleviate dysphoric symptoms. Other institutions (Baylor, Cleveland Clinic, and a German UTx group) reported that of interested UTx recipients, 0.5%, 2%, and 1.4% of interested respondents were TGW (Arian et al., 2017; Johannesson et al., 2018; Taran et al., 2019).
Subsequent to this, informal discussions were undertaken with transgender individuals (both TGW and TGM) who expressed interest in serving as UTx recipients and donors, respectively. Discussions with professionals in transgender health (physicians, mental health professionals, legal experts, ethicists, and other experts) as well as members of the transgender community were also undertaken. Various forums were arranged (e.g., journal clubs, zoom meetings, conference calls) to facilitate discussion and communication between the participants.
Multidisciplinary team
UTx is a multidisciplinary effort that requires collaboration across specialties (see Table 1).
Table 1.
Essential UTx team members.
| Teams | Skills/Roles |
|---|---|
| Plastic and reconstructive surgeon(s) |
|
| Transplant surgeon(s) | |
| Urological surgeon(s) | |
| Obstetric/gynecological surgeon(s) | |
| Obstetric/gynecologist(s) |
|
| IVF specialists | |
| Fertility psychiatrists | |
| Maternal fetal medicine | |
| Neonatologists | |
| Pediatricians | |
| Institutional psychiatrist, psychologist |
|
| Institutionally approved psychologist | |
| Social worker | |
| Transplant research coordinator |
|
| Transplant immunologist | |
| Pathologist | |
| Transplant tissue lab technician | |
| Infectious disease expert | |
| Radiologist | |
| Anesthesia team |
|
| ICU staff | |
| Critical care physicians | |
| Physical therapy and rehabilitation | |
| Ancillary staff: |
|
| Nurses, hospital workers, clinical research staff |
This includes plastic and reconstructive surgeon(s) with experience in both microsurgery and GAS, transplant surgeon(s), urological surgeon(s), and obstetric/gynecological surgeon(s) experienced in robotic and/or laparoscopic hysterectomy-oophorectomy. Fertility specialists and endocrinologists help to develop a plan to verify uterine functionality, gamete selection, and embryo transfer. As all pregnancies in recipients of UTx are considered high-risk, maternal fetal medicine (MFM) physicians, neonatologists, and pediatricians are required. Radiologists assist with donor and recipient evaluation, both prior to and following transplantation. Psychiatrists and psychologists with expertise in transplantation, transgender healthcare, and fertility are also required. The mental health team participates in the pre-operative assessment process as well as provides weekly or bi-weekly counseling to support the UTx recipients and to identify potential stressors which may affect care.
A transplant immunologist, pathologist, infectious disease physician, and lab technicians experienced in donor/recipient compatibility assessment, immunosuppression protocols, and rejection/infection monitoring are required. Additionally, a transplant research coordinator and clinical research staff who assist with education and support for the donor and recipient are also recommended. Additional team members include anesthesiology, critical care physicians, social work, physical therapy, and rehabilitation. These specialists comprise the core team, however, additional support will undoubtedly be required.
Literature review
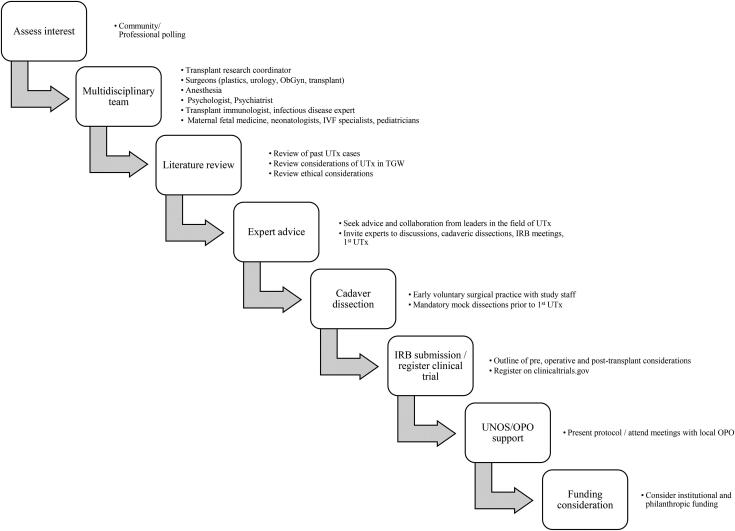
Prior to proceeding, a literature review was conducted. Relevant issues identified included: the surgical approach to organ procurement and insetting of the uterus (i.e., open incision, laparoscopic, and/or robotic), operative details (i.e., ischemia time, length of surgery, selection of recipient and donor vessels), donor characteristics (i.e., living/deceased, nulliparous/multiparous, pre/post-menopausal), recipient characteristics (i.e., age, indication, medical and/or mental health co-occurring conditions, social stability, etcetera), operative risks (i.e., ureter injury), potential postoperative complications (i.e., rejection, thrombosis), pregnancy/fetal outcomes (i.e., maternal complications, fetal complications, etcetera), ethical issues, and mental health considerations. Team members evaluated the success and failure of the greater than 70 previous UTx cases. While no reports of UTx in TGW were identified, many publications opine on the technical and ethical considerations that inform decision-making (see Figure 4, algorithm for institutional requirements).
Figure 4.
Algorithm for institutional planning of a uterine transplant (UTx) program.
Expert advice
The Rush University team contacted colleagues at Baylor University (Baylor Dallas Uterine Transplant Study (DUETS)) to serve as expert consultants. Advice and consultation were sought regarding the Baylor experience (including institutional review board (IRB) protocols). The Baylor physicians were invited to attend and assist with the first UTx performed by our team.
Cadaver dissection
Cadaver dissections were performed so as to review surgical anatomy and define the surgical roles of various team members. Members of the surgical team were invited to attend cadaver dissections so as to develop interdisciplinary coordination, optimize surgical planning, and address potential procedural concerns (Siemionow & Gordon, 2010a, 2010b). The program leader also met with surgical, nursing, and ICU staff, including the OR nurse manager(s), surgical intensivist(s), and anesthesiologists so as to discuss the procedure and define roles.
IRB submission
When starting an UTx program, IRB approval is recommended. The IRB application included a description of the following: pre-transplant evaluation (screening/work-up), operative details (donor and recipient procedural steps), post-transplant care (patient monitoring, immunosuppression, follow-up), and fertility and pregnancy plans (embryo transfer (ET)/pregnancy monitoring). In order to delineate and communicate the role of team members, the IRB was divided into recipient, donor and ET/IVF/pregnancy protocols. These sections included the following: the consent process, the participant inclusion/exclusion criteria, and the risks/benefits for the subjects involved. As discussed by Siemionow, depending upon the transplant status/designation of the institution, preparation may require a 1–2-year timeline (Siemionow & Gordon, 2010a, 2010b). Following IRB approval, the UTx study should be registered as a new clinical trial on clinicaltrials.gov website.
UNOS/OPO support
Support from United Network of Organ Sharing (UNOS) and a local Organ Procurement Organization (OPO) is required when instituting an UTx program. In Chicago, the regional OPO is “Gift of Hope.” An OPO may require meetings in order to discuss the program and review uterus allocation. UTx recipients who are identified and selected are placed on the OPO list by the institution’s manager of quality and regulation. The OPO process includes blood group analysis, tissue compatibility testing, and assessment of donor characteristics. Waiting time for a compatible organ (uterus) from a cadaveric, living-related, or altruistic donor depends upon the recipient’s prior requested organ type and the availability of a suitable organ. Currently, a system for uterus allocation has not been established. As the field of UTx expands and as more allocating systems (i.e., regional, national) are available, the issue of prioritization must be re-visited.
There are efforts to establish a “United States Uterine Transplant Consortium” in order to facilitate access and ensure proper regulation (Johannesson et al., 2020). Participation in this consortium and collaboration with other active centers is highly encouraged. UTx requires specific and unique educational initiatives as well as regulatory policies which are likely distinct from other VCAs (Johannesson et al., 2020).
Funding considerations
Significant institutional support, including financial, is required to start an UTx program. Currently, UTx is considered experimental and is not reimbursed by third party carriers. While the UTx surgery and subsequent immunosuppression may not be covered by third party payers, the pregnancy, delivery, and neonatal care may be reimbursable. The resulting financial burden may be borne by the institution or through philanthropic funding. Logistical and administrative supports (sharing published or unpublished data, literature search, previously submitted IRB, protocols, consent forms, etcetera) could be implemented through support of organizations like VCA, UNOS, or potentially under a unified umbrella of “United States Uterine Transplant Consortium.”
Conclusion
While current fertility options provide transgender women the ability to produce an embryo, they do not allow for the opportunity of experiencing pregnancy and live birth. Based upon our clinical consideration, UTx in transgender women, and uterine donation from transgender men, are feasible. UTx may address the fertility and reproductive goals of transgender women while also providing a further extension of GAS (i.e., allowing for the experience of pregnancy and live birth). A multidisciplinary approach encouraging collaboration with other active UTx centers are important steps for initiating and establishing an UTx program.
Conflict of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
Hamidian Jahromi A Division of Plastic & Reconstructive Surgery Rush University Medical Center, Chicago, IL, USAalirezahamidian@yahoo.com
Sydney R. Horen Division of Plastic & Reconstructive Surgery Rush University Medical Center, Chicago, IL, USAsydney_r_horen@rush.edu
Amir H. Dorafshar Division of Plastic & Reconstructive Surgery Rush University Medical Center, Chicago, IL, USAamir_dorafshar@rush.edu
Michelle Y. Seu Division of Plastic & Reconstructive Surgery Rush University Medical Center, Chicago, IL, USAmseu@luc.edu
Asa Radix Callen-Lorde Community Health CenterDepartment of Medicine, New York, NY, USAARadix@callen-lorde.orgErica AndersonUCSF Pediatric Endocrinology, Child and Adolescent Gender Center, San Francisco, CA, USA;President USPATH; United States affiliate of the WorldProfessional Association for Transgender Health (WPATH)drericaanderson@gmail.comJamison GreenIndependent Legal Scholar & Past President and Co-Chair of the Ethics Committee, World Professional Association for Transgender Health (WPATH)jgreenphd1@gmail.comLin FraserPrivate Practice-Counseling & Psychotherapyand Past President and Co-Chair of theEthics Committee, World ProfessionalAssociation for Transgender Health (WPATH)linfraser@gmail.comLiza JohannessonAnnette C. and Harold C. Simmons TransplantInstitute, Baylor University Medical Center,Dallas, TX, USA & Department of Obstetrics and Gynecology, Baylor University Medical Center,Dallas, TX, USALiza.Johannesson@bswhealth.orgGiuliano TestaAnnette C. and Harold C. Simmons Transplant Institute, Baylor University Medical Center, Dallas, TX, USA & Department of Obstetrics and Gynecology, Baylor University Medical Center, Dallas, TX, USAGiuliano.Testa@bswhealth.orgLoren S. SchechterDivision of Plastic & Reconstructive Surgery Rush University Medical Center, Chicago, IL, USA &The Center for Gender Confirmation SurgeryWeiss Memorial Hospital, The University of Illinoisat Chicago, IL, USAlss@univplastics.com
References
- Alghrani, A. (2016). Uterus transplantation: Does procreative liberty encompass a right to gestate? Journal of Law and the Biosciences, 3(3), 636–641. 10.1093/jlb/lsw048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghrani, A. (2018). Uterus transplantation in and beyond cisgender women: Revisiting procreative liberty in light of emerging reproductive technologies. Journal of Law and the Biosciences, 5(2), 301–328. 10.1093/jlb/lsy012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Api, M., Boza, A., & Ceyhan, M. (2017). Could the female-to-male transgender population be donor candidates for uterus transplantation? Turkish Journal of Obstetrics and Gynecology, 14(4), 233–237. 10.4274/tjod.55453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arian, S. E., Flyckt, R. L., Farrell, R. M., Falcone, T., & Tzakis, A. G. (2017). Characterizing women with interest in uterine transplant clinical trials in the United States: Who seeks information on this experimental treatment? American Journal of Obstetrics and Gynecology, 216(2), 190–191. 10.1016/j.ajog.2016.11.1028 [DOI] [PubMed] [Google Scholar]
- Baker, K. E. (2017). The future of transgender coverage. The New England Journal of Medicine, 376(19), 1801–1804. 10.1056/NEJMp1702427 [DOI] [PubMed] [Google Scholar]
- Balayla, J., Pounds, P., Lasry, A., Volodarsky-Perel, A., & Gil, Y. (2021). The Montreal Criteria and uterine transplants in transgender women. Bioethics. 10.1111/bioe.12832 [DOI] [PubMed] [Google Scholar]
- Beauchamp, T. L., & Childress, J. F. (2009). Principles of biomedical ethics. Oxford University Press. [Google Scholar]
- Berli, J. U., Knudson, G., Fraser, L., Tangpricha, V., Ettner, R., Ettner, F. M., Safer, J. D., Graham, J., Monstrey, S., & Schechter, L. (2017). What surgeons need to know about gender confirmation surgery when providing care for transgender individuals: A review. JAMA Surgery, 152(4), 394–400. 10.1001/jamasurg.2016.5549 [DOI] [PubMed] [Google Scholar]
- Brännström, M., Dahm-Kähler, P., Ekberg, J., Akouri, R., Groth, K., Enskog, A., Broecker, V., Mölne, J., Ayoubi, J. M., & Kvarnström, N. (2020). Outcome of recipient surgery and 6-month follow-up of the Swedish live donor robotic uterus transplantation trial. Journal of Clinical Medicine, 9(8), 2338. 10.3390/jcm9082338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brännström, M., Johannesson, L., Bokström, H., Kvarnström, N., Mölne, J., Dahm-Kähler, P., Enskog, A., Milenkovic, M., Ekberg, J., Diaz-Garcia, C., Gäbel, M., Hanafy, A., Hagberg, H., Olausson, M., & Nilsson, L. (2015). Livebirth after uterus transplantation. Lancet (London, England), 385(9968), 607–616. 10.1016/S0140-6736(14)61728-1 [DOI] [PubMed] [Google Scholar]
- Brännström, M., Johannesson, L., Dahm-Kähler, P., Enskog, A., Mölne, J., Kvarnström, N., Diaz-Garcia, C., Hanafy, A., Lundmark, C., Marcickiewicz, J., Gäbel, M., Groth, K., Akouri, R., Eklind, S., Holgersson, J., Tzakis, A., & Olausson, M. (2014). First clinical uterus transplantation trial: A six-month report. Fertility and Sterility, 101(5), 1228–1236. 10.1016/j.fertnstert.2014.02.024 [DOI] [PubMed] [Google Scholar]
- Bretschneider, C. E., Sheyn, D., Pollard, R., & Ferrando, C. A. (2018). Complication rates and outcomes after hysterectomy in transgender men. Obstetrics and Gynecology, 132(5), 1265–1273. 10.1097/AOG.0000000000002936 [DOI] [PubMed] [Google Scholar]
- Brucker, S. Y., Strowitzki, T., Taran, F.-A., Rall, K., Schöller, D., Hoopmann, M., Henes, M., Guthoff, M., Heyne, N., Zipfel, S., Schäffeler, N., Bösmüller, H., Fend, F., Rosenberger, P., Heim, E., Wiesing, U., Nikolaou, K., Fleischer, S., Bakchoul, T., … Königsrainer, A. (2020). Living-donor uterus transplantation: Pre-, intra-, and postoperative parameters relevant to surgical success, pregnancy, and obstetrics with live births. Journal of Clinical Medicine, 9(8), 2485. 10.3390/jcm9082485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D., Simons, L., Johnson, E. K., Lockart, B. A., & Finlayson, C. (2017). Fertility preservation for transgender adolescents. The Journal of Adolescent Health, 61(1), 120–123. 10.1016/j.jadohealth.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, E., Bockting, W., Botzer, M., Cohen-Kettenis, P., DeCuypere, G., Feldman, J., Fraser, L., Green, J., Knudson, G., Meyer, W. J., Monstrey, S., Adler, R. K., Brown, G. R., Devor, A. H., Ehrbar, R., Ettner, R., Eyler, E., Garofalo, R., Karasic, D. H., … Zucker, K. (2012). Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. International Journal of Transgenderism, 13(4), 165–232. 10.1080/15532739.2011.700873 [DOI] [Google Scholar]
- Dahm-Kähler, P., Diaz-Garcia, C., & Brännström, M. (2016). Human uterus transplantation in focus. British Medical Bulletin, 117(1), 69–78. 10.1093/bmb/ldw002 [DOI] [PubMed] [Google Scholar]
- De Sutter, P., Verschoor, A., Hotimsky, A., & Kira, K. (2002). The desire to have children and the preservation of fertility in transsexual women: A survey. International Journal of Transgenderism, 6(3), 215–221. [Google Scholar]
- Defreyne, J., van Schuylenbergh, J., Motmans, J., Tilleman, K., & T’Sjoen, G. (2020). Parental desire and fertility preservation in assigned male at birth transgender people living in Belgium. International Journal of Transgender Health, 21(1), 45–57. 10.1080/15532739.2019.1692750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejzenberg, D., Andraus, W., Baratelli Carelli Mendes, L. R., Ducatti, L., Song, A., Tanigawa, R., Rocha-Santos, V., Macedo Arantes, R., Soares, J. M., Jr., Serafini, P. C., Bertocco de Paiva Haddad, L., Pulcinelli Francisco, R., Carneiro D’Albuquerque, L. A., & Chada Baracat, E. (2019). Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. The Lancet (London, England), 392(10165), 2697–2704. 10.1016/S0140-6736(18)31766-5 [DOI] [PubMed] [Google Scholar]
- Fageeh, W., Raffa, H., Jabbad, H., & Marzouki, A. (2002). Transplantation of the human uterus. International Journal of Gynecology & Obstetrics, 76(3), 245–251. 10.1016/S0020-7292(01)00597-5 [DOI] [PubMed] [Google Scholar]
- Flores, A. R., Herman, J. L., Gates, G. J., Brown, T. N. (2020). How many adults identify as transgender in the United States? Retrieved February 25, 2021, from https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Flyckt, R., Falcone, T., Quintini, C., Perni, U., Eghtesad, B., Richards, E. G., Farrell, R. M., Hashimoto, K., Miller, C., Ricci, S., Ferrando, C. A., D’Amico, G., Maikhor, S., Priebe, D., Chiesa-Vottero, A., Heerema-McKenney, A., Mawhorter, S., Feldman, M. K., & Tzakis, A. (2020). First birth from a deceased donor uterus in the United States: From severe graft rejection to successful cesarean delivery. American Journal of Obstetrics and Gynecology, 223(2), 143–151. 10.1016/j.ajog.2020.03.001 [DOI] [PubMed] [Google Scholar]
- Fronek, J., Janousek, L., Kristek, J., Chlupac, J., Pluta, M., Novotny, R., Maluskova, J., & Olausson, M. (2020). Live birth following uterine transplantation from a nulliparous deceased donor. Transplantation. 10.1097/TP.0000000000003346 [DOI] [PubMed] [Google Scholar]
- Fronek, J., Kristek, J., Chlupac, J., Janousek, L., & Olausson, M. (2021). Human uterus transplantation from living and deceased donors: The interim results of the first 10 cases of the czech trial. Journal of Clinical Medicine, 10(4), 586. 10.3390/jcm10040586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, C. R., & Siemionow, M. (2009). Requirements for the development of a hand transplantation program. Annals of Plastic Surgery, 63(3), 262–273. 10.1097/SAP.0b013e31818d45e9 [DOI] [PubMed] [Google Scholar]
- Herlin, M. K., Petersen, M. B., & Brännström, M. (2020). Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome: A comprehensive update. Orphanet Journal of Rare Diseases, 15(1), 214. 10.1186/s13023-020-01491-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson, L., Kvarnström, N., Mölne, J., Dahm-Kähler, P., Enskog, A., Diaz-Garcia, C., Olausson, M., & Brännström, M. (2015). Uterus transplantation trial: 1-year outcome. Fertility and Sterility, 103(1), 199–204. 10.1016/j.fertnstert.2014.09.024 [DOI] [PubMed] [Google Scholar]
- Johannesson, L., Testa, G., Putman, J. M., McKenna, G. J., Koon, E. C., York, J. R., Bayer, J., Zhang, L., Rubeo, Z. S., Gunby, R. T., & Gregg, A. R. (2021). Twelve live births after uterus transplantation in the Dallas UtErus Transplant Study. Obstetrics and Gynecology, 137(2), 241–249. 10.1097/AOG.0000000000004244 [DOI] [PubMed] [Google Scholar]
- Johannesson, L., Wall, A., Tzakis, A., Quintini, C., Richards, E. G., O’Neill, K., Porrett, P. M., & Testa, G. (2020). Life underneath the VCA umbrella: Perspectives from the US uterus transplant consortium. American Journal of Transplantation. 10.1111/ajt.16445 [DOI] [PubMed] [Google Scholar]
- Johannesson, L., Wallis, K., Koon, E. C., McKenna, G. J., Anthony, T., Leffingwell, S. G., Klintmalm, G. B., Gunby, R. T., Jr., & Testa, G. (2018). Living uterus donation and transplantation: Experience of interest and screening in a single center in the United States. American Journal of Obstetrics and Gynecology, 218(3), 331.e1–331–e7. 10.1016/j.ajog.2017.11.594 [DOI] [PubMed] [Google Scholar]
- Jones, B. P., Rajamanoharan, A., Vali, S., Williams, N. J., Saso, S., Thum, M. Y., Ghaem-Maghami, S., Quiroga, I., Diaz-Garcia, C., Thomas, P., Wilkinson, S., Yazbek, J., & Smith, J. R. (2021). Perceptions and motivations for uterus transplant in transgender women. JAMA Network Open, 4(1), e2034561. 10.1001/jamanetworkopen.2020.34561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, B. P., Saso, S., Bracewell-Milnes, T., Thum, M. Y., Nicopoullos, J., Diaz-Garcia, C., Friend, P., Ghaem-Maghami, S., Testa, G., Johannesson, L., Quiroga, I., Yazbek, J., & Smith, J. R. (2019). Human uterine transplantation: A review of outcomes from the first 45 cases. BJOG, 126(11), 1310–1319. 10.1111/1471-0528.15863 [DOI] [PubMed] [Google Scholar]
- Jones, B. P., Williams, N. J., Saso, S., Thum, M. Y., Quiroga, I., Yazbek, J., Wilkinson, S., Ghaem-Maghami, S., Thomas, P., & Smith, J. R. (2019). Uterine transplantation in transgender women. BJOG, 126(2), 152–156. 10.1111/1471-0528.15438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kailas, M., Lu, H., Rothman, E. F., & Safer, J. D. (2017). Prevalence and types of gender-affirming surgery among a sample of transgender endocrinology patients prior to state expansion of insurance coverage. Endocrine Practice, 23(7), 780–786. 10.4158/EP161727.OR [DOI] [PubMed] [Google Scholar]
- Lefkowitz, A., Edwards, M., & Balayla, J. (2013). Ethical considerations in the era of the uterine transplant: An update of the Montreal Criteria for the Ethical Feasibility of Uterine Transplantation. Fertility and Sterility, 100(4), 924–926. 10.1016/j.fertnstert.2013.05.026 [DOI] [PubMed] [Google Scholar]
- Light, A. D., Obedin-Maliver, J., Sevelius, J. M., & Kerns, J. L. (2014). Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstetrics and Gynecology, 124(6), 1120–1127. 10.1097/AOG.0000000000000540 [DOI] [PubMed] [Google Scholar]
- Meerwijk, E. L., & Sevelius, J. M. (2017). Transgender population size in the United States: A meta-regression of population-based probability samples. American Journal of Public Health, 107(2), e1–e8. 10.2105/AJPH.2016.303578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Marcotty, M., Rennekampff, H., Haerle, M., Knobloch, K., & Vogt, P. (2010). Composite tissue allotransplantation in Europe. Logistics and infrastructure of a centre. Annals of Transplantation, 15(3), 87–92. [PubMed] [Google Scholar]
- Mookerjee, V. G., & Kwan, D. (2020). Uterus transplantation as a fertility option in transgender healthcare. International Journal of Transgender Health, 21(2), 122–124. 10.1080/15532739.2019.1599764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, F. D. (2000). Ethical problems special to surgery: Surgical teaching, surgical innovation, and the surgeon in managed care. Archives of Surgery, 135(1), 14–16. 10.1001/archsurg.135.1.14 [DOI] [PubMed] [Google Scholar]
- Murphy, T. F. (2015). Assisted gestation and transgender women. Bioethics, 29(6), 389–397. 10.1111/bioe.12132 [DOI] [PubMed] [Google Scholar]
- Nahata, L., Tishelman, A. C., Caltabellotta, N. M., & Quinn, G. P. (2017). Low fertility preservation utilization among transgender youth. The Journal of Adolescent Health, 61(1), 40–44. 10.1016/j.jadohealth.2016.12.012 [DOI] [PubMed] [Google Scholar]
- Nolan, I. T., Kuhner, C. J., & Dy, G. W. (2019). Demographic and temporal trends in transgender identities and gender confirming surgery. Translational Andrology and Urology, 8(3), 184–190. 10.21037/tau.2019.04.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomahac, B. (2012). Establishing a composite tissue allotransplantation program. Journal of Reconstructive Microsurgery, 28(1), 3–6. 10.1055/s-0031-1285823 [DOI] [PubMed] [Google Scholar]
- Puntambekar, S., Telang, M., Kulkarni, P., Puntambekar, S., Jadhav, S., Panse, M., Sathe, R., Agarkhedkar, N., Warty, N., Kade, S., Manchekar, M., Parekh, H., Parikh, K., Desai, R., Mehta, M., Chitale, M., Kinholkar, B., Jana, J. S., Pare, A., Sadre, A., … Phadke, U. (2018). Laparoscopic-assisted uterus retrieval from live organ donors for uterine transplant: Our experience of two patients. Journal of Minimally Invasive Gynecology, 25(4), 622–631. 10.1016/j.jmig.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Robertson, J. A. (2017). Is there a right to gestate? Journal of Law and the Biosciences, 4(3), 630–636. 10.1093/jlb/lsx010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa, B., Lin, W. C., Salim, A. M., Deschamps-Braly, J. C., & Poh, M. M. (2019). Current concepts in masculinizing gender surgery. Plastic and Reconstructive Surgery, 143(4), 857e–871e. 10.1097/PRS.0000000000005453 [DOI] [PubMed] [Google Scholar]
- Siemionow, M. Z., & Gordon, C. R. (2010a). Institutional review board-based recommendations for medical institutions pursuing protocol approval for facial transplantation. Plastic and Reconstructive Surgery, 126(4), 1232–1239. 10.1097/PRS.0b013e3181ee482d [DOI] [PubMed] [Google Scholar]
- Siemionow, M., & Gordon, C. R. (2010b). Overview of guidelines for establishing a face transplant program: A work in progress. American Journal of Transplantation, 10(5), 1290–1296. 10.1111/j.1600-6143.2010.03096.x [DOI] [PubMed] [Google Scholar]
- Sineath, R. C., Woodyatt, C., Sanchez, T., Giammattei, S., Gillespie, T., Hunkeler, E., Owen-Smith, A., Quinn, V. P., Roblin, D., Stephenson, R., Sullivan, P. S., Tangpricha, V., & Goodman, M. (2016). Determinants of and barriers to hormonal and surgical treatment receipt among transgender people. Transgender Health, 1(1), 129–136. 10.1089/trgh.2016.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taran, F. A., Schöller, D., Rall, K., Nadalin, S., Königsrainer, A., Henes, M., Bösmüller, H., Fend, F., Nikolaou, K., Notohamiprodjo, M., Grasshoff, C., Heim, E., Zipfel, S., Schäffeler, N., Bakchoul, T., Heyne, N., Guthoff, M., Krämer, B., Reisenauer, C., Hoopmann, M., … Brucker, S. Y. (2019). Screening and evaluation of potential recipients and donors for living donor uterus transplantation: Results from a single-center observational study. Fertility and Sterility, 111(1), 186–193. 10.1016/j.fertnstert.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Testa, G., McKenna, G. J., Bayer, J., Wall, A., Fernandez, H., Martinez, E., Gupta, A., Ruiz, R., Onaca, N., Gunby, R. T., Gregg, A. R., Olausson, M., Koon, E. C., & Johannesson, L. (2020). The evolution of transplantation from saving lives to fertility treatment: DUETS (Dallas UtErus Transplant Study). Annals of Surgery, 272(3), 411–417. 10.1097/SLA.0000000000004199 [DOI] [PubMed] [Google Scholar]
- Testa, G., McKenna, G. J., Gunby, R. T., Jr., Anthony, T., Koon, E. C., Warren, A. M., Putman, J. M., Zhang, L., dePrisco, G., Mitchell, J. M., Wallis, K., Klintmalm, G. B., Olausson, M., & Johannesson, L. (2018). First live birth after uterus transplantation in the United States. American Journal of Transplantation, 18(5), 1270–1274. 10.1111/ajt.14737 [DOI] [PubMed] [Google Scholar]
- Wei, L., Xue, T., Tao, K. S., Zhang, G., Zhao, G. Y., Yu, S. Q., Cheng, L., Yang, Z. X., Zheng, M. J., Li, F., Wang, Q., Han, Y., Shi, Y. Q., Dong, H. L., Lu, Z. H., Wang, Y., Yang, H., Ma, X. D., Liu, S. J., Liu, H. X., … Chen, B. L. (2017). Modified human uterus transplantation using ovarian veins for venous drainage: The first report of surgically successful robotic-assisted uterus procurement and follow-up for 12 months. Fertility and Sterility, 108(2), 346–356.e1. 10.1016/j.fertnstert.2017.05.039 [DOI] [PubMed] [Google Scholar]
- Wiegmann, A. L., Young, E. I., Baker, K. E., Khalid, S. I., Seu, M., Shenaq, D. S., Dorafshar, A. H., & Schechter, L. S. (2021). The affordable care act and its impact on plastic and gender-affirmation surgery. Plastic and Reconstructive Surgery, 147(1), 135e–153e. 10.1097/PRS.0000000000007499 [DOI] [PubMed] [Google Scholar]
- Wierckx, K., Van Caenegem, E., Elaut, E., Dedecker, D., Van de Peer, F., Toye, K., Weyers, S., Hoebeke, P., Monstrey, S., De Cuypere, G., & T’Sjoen, G. (2011). Quality of life and sexual health after sex reassignment surgery in transsexual men. The Journal of Sexual Medicine, 8(12), 3379–3388. 10.1111/j.1743-6109.2011.02348.x [DOI] [PubMed] [Google Scholar]
- Worthen, M. (2019, April 13). Lili Elbe. Retrieved February 25, 2021, from https://www.biography.com/artist/lili-elbe