ABSTRACT
Background:
Surgical lung biopsy (SLB) or video-assisted thoracic surgery (VATS) has been the traditional gold standard modality for diagnosing paediatric interstitial lung diseases. Cryobiopsy of the lung has recently been shown to be a novel technique with very good sensitivity and specificity in the diagnosis of various interstitial lung disorders in adults. Although there are a few case reports of the same in children, pediatric cryo lung biopsies are rarely performed due to the lack of the necessary equipment and the lack of expertise.
Methods:
A retrospective single-centre study was conducted with twelve consecutive children with diffuse parenchymal lung disease diagnosed both clinically and on high-resolution computed tomography (HRCT) of the chest which were included in the study between October 2020 and September 2022 to measure the diagnostic yield and safety of the procedure. The site from where cryobiopsy was to be done was chosen after a multidisciplinary meeting with the paediatric radiologist.
Results:
Twelve children (eight males and four females) were included in the study who underwent a cryobiopsy in the duration of two years. The mean age of the cases involved was 8 years and 3 months. With the youngest and oldest being 12 days and 15 years, respectively, all children underwent cryobiopsy as mentioned above. Diagnostic yield was achieved in 92% of cases.
Conclusion:
Cryobiopsy is a valuable diagnostic tool in childhood interstitial lung diseases, which offers a less invasive option for obtaining lung tissue samples with a better yield which can aid in accurate diagnosis, a good safety profile and a shorter hospital stay. Our study emphasizes that in trained centres, TBCB is a safe, effective and less invasive way to obtain tissue diagnosis in children with ChILD.
KEY WORDS: Cryobiopsy, Diagnostic yield, Open lung biopsy, Paediatric
INTRODUCTION
Childhood interstitial lung disease (ChILD) is a heterogeneous group of rare pulmonary conditions associated with significant mortality and morbidity, and most centres see only a few cases per year, with an incidence of 1.3–3.6 cases/1,00,000 children <17 years of age/year.[1] As lung biopsy specimens are not always available in children, instead of a classification based on histopathology, a classification based on aetiology was thought rationale for ChILD. Advances in genetic studies and procedural skills with the availability of paediatric-specific equipment to obtain biopsies have made the diagnosis of ChILD’s facile, thus continuously amending the classification of ChILD. There are significant anatomical and physiological differences in the adult and paediatric populations, and the biopsy techniques were modified based on these differences to ensure a safe procedure and to achieve desired outcomes. The common methods for histological diagnosis in pulmonary diseases include X-ray/ultrasound-guided percutaneous lung biopsy, SLB and transbronchial lung forceps (TBFB) and cryobiopsy (TBCB). Historically, SLB has been considered the definite means of obtaining adequate specimens, until the invention of the novel technique of cryobiopsy in 2009.[2] Several cryotherapeutic techniques are available, which work based on the Joule–Thomson effect, to freeze tissues to extremely low temperatures for destruction (cryoablation), adhesion (cryo-adhesion) or biopsy (cryobiopsy).[3]
TBFB is a standard practice for surveillance of acute cellular rejection to monitor the lung allograft after lung transplantation in the paediatric population.[4] TBCB is now being increasingly used in interventional pulmonology, more so in the diagnosis of ChILD,[5,6] though data on its safety, cost-effectiveness and yield are limited compared to adult studies which have a yield ranging from 75 to 91%.[7,8] When compared to SLB, TBCB has fewer adverse events post-procedure, with shorter hospital stays and high diagnostic yield,[9] whereas transbronchial biopsy via forceps frequently yields insufficient samples with crush artefacts. Although moderate bleeding and pneumothorax were reported[10] in adults, there are no reported complications in the paediatric population apart from minor bleeding.
Cryobiopsy seems to be a safe and efficacious tool in the hands of a skilled and experienced pulmonologist in diagnosing ChILD. This single-centre study aims to report indications, technique, safety and clinical efficacy of cryobiopsy in paediatrics.
MATERIALS AND METHODS
A retrospective review of the clinical records of 12 consecutive patients who underwent TBCB for the diagnosis of paediatric lung diseases between October 2020 and September 2022. Twelve children who underwent TBCB were suspected of having diffuse parenchymal lung disease (DPLD) on clinical and radiological examinations. Analysis of medical records, data, procedure details, complications and diagnostic results is done with the consent of the patients after counselling about TBFB, TBCB and SLB.
Procedure
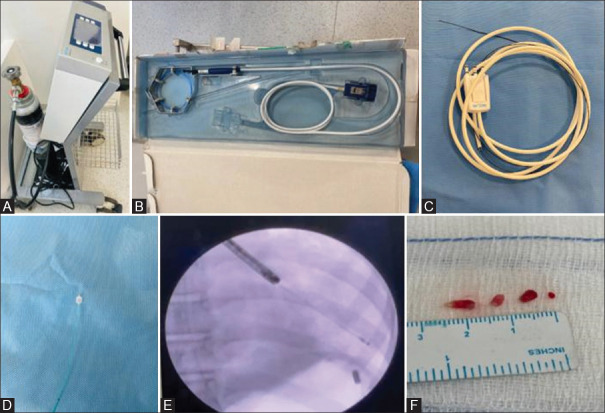
Rigid bronchoscopy (ventilating bronchoscope, size depending on child’s age, height and weight), a flexible bronchoscope (diagnostic scope (BF-P190), channel 2 mm, Olympus Corporation, Tokyo, Japan), a standard flexible cryoprobe (ERBE, Germany), 90 cm length and a diameter ranging from 1.1 to 1.9 mm and a Fogarty catheter (ranging from 3 to 5) were used in this procedure. All procedures were performed under general anaesthesia by an interventional paediatric pulmonologist. Intubation was done with a rigid bronchoscope and a flexible bronchoscope (diagnostic scope (BF-P190), channel 2 mm, Olympus Corporation, Tokyo, Japan) was introduced through the rigid scope to facilitate passage of flexible accessories. We routinely use a Fogarty catheter in anticipation of bleeding, which is usually placed proximally in the bronchus where a biopsy is planned. We used balloons ranging from 3 to 5 Fr depending on the age of the child, i.e., 3 for a neonate and 5 for an adolescent. A standard flexible cryoprobe (ERBE, Germany), 90 cm in length and diameter ranging from 1.1 to 1.9 mm, was introduced under fluoroscopic guidance via the flexible bronchoscope. In our centre, 1.1 mm, 1.7 mm (disposable), and 1.9 mm cryoprobes (ERBE, Germany) are available and the size of the probe was selected according to the age [Figure 1].
Figure 1.
(A) ERBECRYO 2 machine, (B) Flexible 1.9 mm diameter, 900 mm length cryoprobe, (C) ERBE 1.1 mm, 1.15 m length cryoprobe, (D) Fogarty catheter, 5 Fr, inflated balloon diameter of 9 mm, 40 cm length, (E) Fluoroscopic view of biopsy from the left lower lobe, (F) Tissue pieces post-biopsy
The biopsy process involved freezing the lung tissue for 3 seconds under fluoroscopic guidance approximately 10-15 millimetres (cryo-adhesion) from the pleural surface (prevents pneumothorax), followed by yanking the probe with scope amid continuous freeze. The frozen specimen obtained is then thawed in normal saline and transferred to formalin for specimen processing and sent for histopathology. During the yanking process, simultaneous coordinated inflation of the Fogarty balloon is done and maintained for the 30 s to 1 min followed by deflation of the balloon and check for any bleeding. The whole process is repeated 3 to 4 times to get the desired number of samples deemed sufficient for processing.
RESULTS
Baseline characteristics
Twelve children (eight males and four females) were included in the study who underwent cryobiopsy during the study period. The mean age of the group was 8 years and 3 months with the youngest and oldest being 12 days and 15 years, respectively. All children underwent the procedure as mentioned above.
Specimens
The total number of specimens obtained was 41. The median number of specimens obtained per procedure was 3.4 (range, 3–5). The median diameter of the specimens was 5.5 mm2 (range, 2.0–10 mm2). 58% of the cryobiopsy samples were collected from the left lower lobe, and 42% were collected from the right lower lobe. The distribution of biopsy sites is listed in Table 2.
Table 2.
Age , sex, region of biopsy and number of samples collected in each patient and their days of hospital stay
| Age | Gender | Region of biopsy | No. of Tissue samples | Hospital stay |
|---|---|---|---|---|
| 1 year | Female | Left lower lobe | 3 | 1 day |
| 4 years | Male | Left lower lobe | 5 | 1 day |
| 12 days | Male | Right lower lobe | 3 | 3 days |
| 14 years | Male | Right lower lobe | 3 | 1 day |
| 2 years | Male | Left lower lobe | 4 | 1 day |
| 8 years | Male | Left lower lobe | 4 | 1 day |
| 10 years | Male | Left lower lobe | 3 | 1 day |
| 13 years | Female | Right lower lobe | 3 | 1 day |
| 10 years | Female | Right lower lobe | 4 | 1 day |
| 15 years | Male | Left lower lobe | 3 | 1 day |
| 12 years | Female | Left lower lobe | 3 | 1 day |
| 9 years | Male | Right lower lobe | 3 | 1 day |
Table 1.
Complications during cryobiopsy
| Complications | No. |
|---|---|
| 1. Haemorrhage | |
| Total | 9 |
| Mild superficial bleeding | 9 |
| Moderate bleeding requiring vasoactive agents | 0 |
| Severe bleeding requiring blood transfusion | 0 |
| 2. Pneumothorax | |
| Total | 1 |
| Need for chest tube drainage (Detected by fluoroscopy) | 1 |
| 3. Airway perforation | 0 |
| 4. Mortality | 0 |
Diagnosis
The diagnosis was made on histopathological examination in all the cases making the diagnostic yield 92%. One adolescent had clinic radiological incongruence, treated as ChILD for years, for which, underwent a lung biopsy which was unyielding. That child was finally diagnosed to have William-Campbell syndrome. The final diagnosis of all the patients is listed in Table 3. The most common histopathological diagnosis was pulmonary alveolar proteinosis (PAP) (five patients), followed by hypersensitivity pneumonitis (two patients) followed by idiopathic pulmonary hemosiderosis, sarcoidosis, non-specific interstitial pneumonia with follicular bronchitis and cryptogenic organising pneumonia in each, respectively. Though TBCB was performed in the child with Williams–Campbell syndrome, the diagnosis was made by the characteristic findings on computed tomography. In our study, we noted clinic-radiological and histopathological congruence in 58% of the patients with an incongruence among them noted to be as high as 42%, emphasising the importance of biopsy sampling [Table 3]. The radiological and histological descriptions of a few are shown in Figure 2.
Table 3.
Comparison of clinic-radiological and histopathological diagnosis
| Clinico-Radiological diagnosis | Histopathological diagnosis | Final diagnosis |
|---|---|---|
| Interstitial lung disease? genetic | Pulmonary hemosiderosis | Idiopathic pulmonary hemosiderosis |
| Hypersensitivity pneumonitis | Hypersensitivity pneumonitis | Hypersensitivity pneumonitis |
| Pulmonary alveolar proteinosis | Pulmonary alveolar proteinosis | Pulmonary alveolar proteinosis |
| Miliary tuberculosis | Sarcoidosis | Sarcoidosis |
| Pulmonary alveolar proteinosis | Pulmonary alveolar proteinosis | Pulmonary alveolar proteinosis—SPC deficiency |
| Pulmonary alveolar proteinosis | Pulmonary alveolar proteinosis | Pulmonary alveolar proteinosis—MARS mutation (novel) |
| Pulmonary alveolar proteinosis | Pulmonary alveolar proteinosis | Pulmonary alveolar proteinosis—ABCA 3 deficiency |
| Pulmonary alveolar proteinosis | Pulmonary alveolar proteinosis | Pulmonary alveolar proteinosis—SPC deficiency |
| Nonspecific interstitial pneumonia | Nonspecific interstitial pneumonia | Anti-synthetase syndrome |
| Hypersensitivity pneumonitis | Hypersensitivity pneumonitis | Pigeon breeder’s lung |
| Pulmonary alveolar proteinosis | Cryptogenic organising pneumonia | Cryptogenic organising pneumonia |
| Interstitial lung disease | Deficiency of cartilage | William–Campbell syndrome |
Figure 2.
(A) Crazy-paving pattern, suggesting pulmonary alveolar proteinosis, (A2) Accumulation of amorphous, periodic acid-Schiff (PAS)—positive lipoproteinaceous material within alveolar spaces suggesting PAP, (B) Ground glass opacities with mild interstitial thickening—suggesting ILD, (B2) Perl’s Prussian blue stain by extracellular fibrin suggesting pulmonary hemosiderosis, (C) Diffuse interstitial thickening with traction bronchiectasis—likely fibrotic ILD, (C2) Subepithelial fibrous plugs with spindled fibroblasts within the airspaces suggesting cryptogenic organising pneumonia
Complications
Complications mainly encountered during TBCB were bleeding and pneumothorax. Bleeding was characterized as mild (mucosal bleeding needing no intervention), moderate (requiring cold saline, epinephrine apart from Fogarty balloon) or severe (requiring more than the above-mentioned interventions) depending on the modality used to control it. In our series, 9 children had mild bleeding that settled with cold saline instillation. One neonate developed pneumothorax that required pleural drainage.
DISCUSSION
To our best knowledge, this is the largest case series evaluating, utility, safety, and diagnostic yield of TBCB in the pediatric population in India.[6] Because of the relative sparsity of data on pediatric TBCB, there is no procedural consensus for the same. The Indian Association for Bronchology for performing TBCB suggests 1) securing the airway with a rigid bronchoscope or an endotracheal tube, 2) using a 1.9 mm over a 2.4mm cryoprobe 3) usage of fluoroscopic guidance and balloon occlusion 4) biopsy site 10 mm away from the pleura 5) freezing time of 3-6 seconds and 6) to obtain 3-4 samples from two lobes or different segments of the lower lobe.[11] We performed all our biopsies according to standard operating procedures and our biopsy samples are obtained approximately 10 to 15 mm from the visceral pleura to minimize the complications of pneumothorax.
Our study demonstrated a diagnostic yield of 92%. Moslehi[5] et al demonstrated a similar yield (92.8%) across 28 children, whereas studies in adults population have had a diagnostic yield of about 82%.[7,9,12] Cryoprobes used, comprised of both disposable and non-disposable sizes ranging from 1.1 mm to 1.9 mm. In our initial cohort of adolescents and older children, a 1.9 mm cryoprobe was used and with the advent of a 1.1 mm probe, sampling with smaller sizes bronchoscopes became feasible. In cases 1 and 5, a 4.5 size rigid scope was used and in case 3, a 3.5 size rigid scope was used respectively [Table 2]. In all the 3 cases, a 3 mm flexible bronchoscope (diagnostic scope (BF-P190), Olympus Corporation, Tokyo, Japan) with 1.7 mm working channel was used. The use of smaller-sized cryoprobes allows for better manoeuvrability and reduces the risk of complications such as bleeding and pneumothorax.[13-16] In previous reports, the average diameter of the specimen obtained for better diagnostic yield was reported to be 6.5 between 15.7 mm2.[11] Though the median diameter of specimen in our subjects was 5 mm2 (range, 3.0 mm2 – 10.0 mm2), this did not impact the diagnostic yield.
In our study, PAP was diagnosed in 5 children (42%), of which 3 were comprehensively diagnosed as interstitial PAP and the rest were alveolar type of PAP. In the adult population, a combination of CT chest with bronchoalveolar lavage (BAL) analysis is sufficient for the diagnosis, but in children, the histopathologic examination by lung biopsy is still considered the gold standard.[17] In our series, only one child (case number 6) had significant proteinaceous material in the effluent whereas the rest had none to minimal [Table 2]. Due to the aforementioned reasons a biopsy was done in all to minimize both risk of recurrent sedation as well as for a conclusive diagnosis. Among the PAPs, a noteworthy mention is a 12-day-old neonate. To the best of our knowledge, this is the youngest age in the literature to have undergone a TBCB successfully.
Case 12 was diagnosed and treated as ChILD from a very young age. He was treated for the same with multiple courses of steroids both intravenous and oral and referred in view of poor response to treatment. As there was clinic-radiological incongruence with CT of the chest showing minimal ground glass opacities, a TBCB was done to conclusively rule to a ChILD. This helped us in comprehensively ruling out one and also look for other rare causes such as William-Campbell Syndrome which are very uncommon [Table 2].
Case 4 was diagnosed and treated as tuberculosis with no response. Due to the progressive nature of illness with multisystem involvement, a need for microbiological and histological diagnosis was of paramount importance, hence a TBCB (Table 2). Häntschel et al,[18] in a prospective study, showed that TBCB was superior in diagnosing sarcoidosis compared to TBFB.
With TBCB resulting in a good quality specimen and a higher diagnostic yield, we were able to recognize and treat various uncommon chILDs like HP, Non-specific interstitial pneumonia with follicular bronchitis and cryptogenic organizing pneumonia.
The main advantages of TBCB over SLB in the diagnosis of ILD are a) shorter hospital stay and b) reduced occurrence of complications.[12] None of the children in our series had any major complications. In a recent meta-analysis of 13 studies, among adults undergoing TBCB, the incidence of grade 2 bleeds was 4.9% and that of pneumothorax was 9.5%.[12] In our study, although there was mild bleeding in 9 children (75%), no additional interventions were required to arrest bleeding. A prospective multi-centre study among children undergoing any sort of cryotherapy observed pneumothorax in 6.7% and mild bleeding in 26.7%.[19] Only a single patient (8.3%) had pneumothorax requiring chest tube drainage. Patients undergoing TBCB also have a shorter hospitalisation (2.6 vs 6.1 days) and a lower mortality (0.7% vs 1,8%) as compared to SLB.[12] There was no 30–60-day mortality observed among the children in our series.
The study also emphasises the fact that in experienced hands, the procedure can be performed even in neonates to not only achieve a desirable sample for diagnosis but also reduce morbidity and mortality which might be quite significant with SLB. The limitations of our study were that it is a retrospective study conducted in a single centre with a small sample size. A multicentre randomised control trial comparing the efficacy of TBCB with SLB would be ideal.
CONCLUSION
TBCB is a promising technique which substantially improved the histological diagnosis of chILD. Our study emphasizes that in trained centres, TBCB is a safe, effective and less invasive way to obtain tissue diagnosis in children with ChILD.
Abbreviations
ChILD—Childhood interstitial lung disease
DPLD—Diffuse parenchymal lung disease
HRCT—High-resolution computed tomography
SLB—Surgical lung biopsy
PAP—Pulmonary alveolar proteinosis
TBFB—Transbronchial lung forceps
TBCB—Transbronchial cryobiopsy
VATS—Video-assisted thoracic surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kitazawa H, Kure S. Interstitial lung disease in childhood: Clinical and genetic aspects: Supplementary issue: Current developments in interstitial lung disease. Clin Med Insights Circ Respir Pulm Med. 2015;9:CCRPM–S23282. doi: 10.4137/CCRPM.S23282. doi:10.4137/CCRPM. S23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kania A, Misiaszek M, Vašáková M, Szlubowski A, Bugalho A, Pankowski J, et al. Cryobiopsy in the diagnosis of idiopathic pulmonary hemosiderosis: A case report. J Thorac Dis. 2019;11:3195–3201. doi: 10.21037/jtd.2019.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant CSVA. Cryotherapy. Diag-nostic and Interventional Bronchoscopy in Children. In: Goldfarb S, Piccione J, editors. Respiratory Medicine. Cham: Springer International Publishing; 2021. [Google Scholar]
- 4.Faro A, Visner G. The use of multiple transbronchial biopsies as the standard approach to evaluate lung allograft rejection. Pediatr Transplant. 2004;8:322–8. doi: 10.1111/j.1399-3046.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 5.Moslehi MA. Transbronchial lung cryobiopsy in children. Expert Rev Respir Med. 2022;16:333–9. doi: 10.1080/17476348.2021.1987884. [DOI] [PubMed] [Google Scholar]
- 6.Dhochak N, Mittal S, Mohan A, Jain D, Jat KR, Jana M, et al. Transbronchial lung cryobiopsy for diffuse lung diseases in children: A case series. Pediatr Pulm. 2022;57:2851–4. doi: 10.1002/ppul.26074. [DOI] [PubMed] [Google Scholar]
- 7.Bango-Álvarez A, Ariza-Prota M, Torres-Rivas H, Fernández-Fernández L, Prieto A, Sánchez I, et al. Transbronchial cryobiopsy in interstitial lung disease: Experience in 106 cases–how to do it. ERJ Open Res. 2017;3 doi: 10.1183/23120541.00148-2016. doi:10.1183/23120541.00148-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharp C, McCabe M, Adamali H, Medford AR. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease—A systematic review and cost analysis. QJM. 2017;110:207–14. doi: 10.1093/qjmed/hcw142. [DOI] [PubMed] [Google Scholar]
- 9.Ravaglia C, Bonifazi M, Wells AU, Tomassetti S, Gurioli C, Piciucchi S, et al. Safety, and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: A comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration. 2016;91:215–27. doi: 10.1159/000444089. [DOI] [PubMed] [Google Scholar]
- 10.Smith D, Raices M, Bequis MA, Las Heras M, Wainstein E, Castro R, et al. Complications following transbronchial biopsy: The role of cryobiopsy in the incidence of postoperative pneumothorax. Rev Fac Cien Méd Univ Nac Cordoba. 2021;78:29–32. doi: 10.31053/1853.0605.v78.n1.28798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhooria S, Agarwal R, Sehgal IS, Aggarwal AN, Goyal R, Guleria R, et al. Vol. 36. Lung India: Official Organ of Indian Chest Society; 2019. Bronchoscopic lung cryobiopsy: An Indian association for bronchology position statement; p. 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iftikhar IH, Alghothani L, Sardi A, Berkowitz D, Musani AI. Transbronchial lung cryobiopsy and video-assisted thoracoscopic lung biopsy in the diagnosis of diffuse parenchymal lung disease. A meta-analysis of diagnostic test accuracy. Annals of the American Thoracic Society. 2017;14:1197–211. doi: 10.1513/AnnalsATS.201701-086SR. [DOI] [PubMed] [Google Scholar]
- 13.Kho SS, Chai CS, Nyanti LE, Ismail AM, Tie ST. Combination of 1.1 mm flexible cryoprobe with conventional guide sheath and therapeutic bronchoscope in biopsy of apical upper lobe solitary pulmonary nodule. BMC pulmonary medicine. 2020;20:1–6. doi: 10.1186/s12890-020-01199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke KJ, Linzenbold W, Nuessle D, Enderle M, Boesmueller H, Nilius G, et al. A new tool for transbronchial cryobiopsies in the lung:an experimental feasibility ex vivo study. Respiration. 2016;91:228–34. doi: 10.1159/000443990. [DOI] [PubMed] [Google Scholar]
- 15.Yarmus LB, Semaan RW, Arias SA, Feller-Kopman D, Ortiz R, Bösmüller H, Illei PB, Frimpong BO, Oakjones-Burgess K, Lee HJ. A randomized controlled trial of a novel sheath cryoprobe for bronchoscopic lung biopsy in a porcine model. Chest. 2016;150:329–36. doi: 10.1016/j.chest.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado F, Danoff SK, Wells AU, Colby TV, Ryu JH, Liberman M, et al. Transbronchial cryobiopsy for the diagnosis of interstitial lung diseases: CHEST guideline and expert panel report. Chest. 2020;157:1030–42. doi: 10.1016/j.chest.2019.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Cheng SL, Chang HT, Lau HP, Lee LN, Yang PC. Pulmonary alveolar proteinosis:treatment by bronchofiberscopic lobar lavage. Chest. 2002;122:480–5. doi: 10.1378/chest.122.4.1480. [DOI] [PubMed] [Google Scholar]
- 18.Yarmus LB, Semaan RW, Arias SA, Feller-Kopman D, Ortiz R, Bösmüller H, et al. A randomized controlled trial of a novel sheath cryoprobe for bronchoscopic lung biopsy in a porcine model. Chest. 2016;150:329–36. doi: 10.1016/j.chest.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Maldonado F, Danoff SK, Wells AU, Colby TV, Ryu JH, Liberman M, et al. Transbronchial cryobiopsy for the diagnosis of interstitial lung diseases: CHEST guideline and expert panel report. Chest. 2020;157:1030–42. doi: 10.1016/j.chest.2019.10.048. [DOI] [PubMed] [Google Scholar]