Abstract
We compared the biological activity of a new group of keto-C-glycosides to that of a narrow spectrum of unsaturated ketonucleosides in a panel of non-small-cell lung cancer (NSCLC) cells with various levels of intrinsic resistance to standard chemotherapy drugs. Unlike cisplatin, etoposide, adriamycin, or taxol, for which a significant difference in the cytotoxic effect was observed between sensitive cell lines (H460, H125, and MGH4) and drug-resistant cell lines (H661, MGH7, and FADU), nucleoside analogs were equally cytotoxic in NSCLC cell lines, with compound 92 being 10-fold more active than compound 43, 44, 81, or 161, while compound 3 was the least active. Apoptotic measurements with flow cytometric analysis of terminal uridine deoxynucleotide nick end-labeled cells revealed that the cytotoxic activity of these nucleosides correlated with their potency to induce apoptosis. Compound 92 triggered death in cells with wild-type p53, mutated p53, or p53 gene deletion. Our findings suggest that keto-C-glycosides may be promising alternative anticancer agents which merit further studies in in vivo cancer models refractory to standard chemotherapy drugs.
Therapeutic management of solid tumors such as non-small-cell lung cancer (NSCLC) is frequently impeded by resistance to chemotherapy drugs, even without previous drug treatment. This phenomenon, often referred to as intrinsic drug resistance, constitutes a limitation for the successful management of NSCLC. A variety of molecular markers associated with drug resistance in NSCLC have been reported, including altered expression of tyrosine kinase receptors of the erbB family, e.g., overexpression of erbB1 (EGFR) and/or erbB2 receptors, expression of neuroendocrine markers, p53 mutations, and altered cell cycle checkpoints (19–27; reviewed in reference 5). Some of these markers have been used as potential targets in developing novel anticancer agents.
A variety of alternative therapeutic approaches have been under investigation in attempts to improve the efficacy of chemotherapy in NSCLC. Promising results have been reported for pyrimidine nucleoside analogs such as gemcitabine (2,2-difluorodeoxycytidine), both in experimental and clinical studies (12). However, clinical response to this drug given alone or in combination showed only limited improvement in overall survival, and its use has been hampered by the presence of dose-limiting toxicities, including myelosuppression (1).
In an effort to design drugs that can overcome common mechanisms of resistance and are suitable for clinical development, we have investigated the design and synthesis of novel nucleoside analogs distinct from gemcitabine or its parent compound, 1-β-d-arabinofuranosylcytosine. We have previously reported the chemical synthesis (4, 6, 14, 15) and biological activity of unsaturated ketonucleosides in various cellular models (3, 13). We have shown that these compounds interact with the sulfhydryl (SH) group of cellular proteins and enzymes (13). These findings with ketonucleosides led to the design and synthesis of a new generation of unsaturated keto-C-glycosides from 6-hydroxy 2- and 4-keto-unsaturated D-C-glycosides (16, 17). Simple C-glycosides have very low potency compared to keto-glycosides. Furthermore, conjugates of keto-C-glycosides bound to arachidonic acid are more potent antiproliferative agents than simple keto-C-glycosides (16), suggesting that lipid conjugates may have enhanced delivery to cell compartments (18).
In this study, we report the evidence of antiproliferative and apoptotic activity of keto-C-glycoside compound 92 in a panel of human NSCLC cell lines expressing various drug resistance markers. Results are compared to a series of structurally related ketonucleosides and C-glycosides referred to as compounds 3, 43, 44, 81, and 161.
MATERIALS AND METHODS
Chemicals.
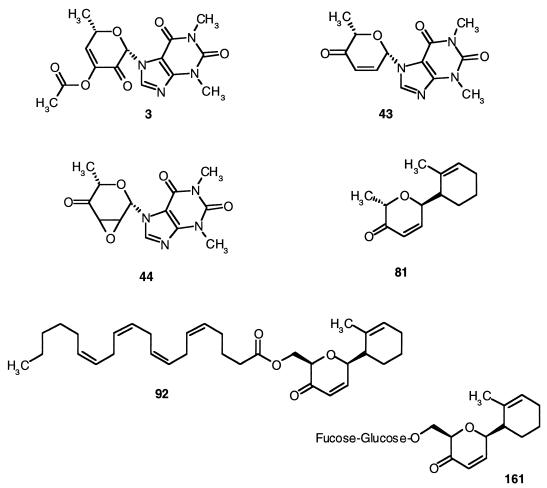
The chemical synthesis and physical properties of ketonucleosides and keto-C-glycosides were reported earlier (7, 13–17, 31). Chemical structures are described in Figure 1. Cisplatin (David Bull Laboratories, Horner, Canada), etoposide (Bristol-Myers GMBH, Troisdorf, Germany), adriamycin (Adria Laboratories, Columbus, Ohio), taxol (Rhone-Poulenc RORER Canada, Inc), and gemcitabine (Eli Lilly & Co., Indianapolis, Ind.) were obtained from the Department of Oncology at the Jewish General Hospital (Montreal, Canada). All drugs were freshly prepared in sterile water (cisplatin and adriamycin) or dimethyl sulfoximide (DMSO) (ketonucleosides and keto-C-glycosides and etoposide, taxol, and gemcitabine). The final concentration of DMSO was 0.05% of cell culture medium. All drugs were protected against light.
FIG. 1.
Structure of ketonucleosides and keto-C-glycosides.
Cell lines and cell culture.
The NSCLC cell lines H125 (adenosquamous carcinoma), H460 and H661 (large-cell carcinoma), and FADU (squamous cell carcinoma) were obtained from the American Tissue Culture Collection (ATCC) (5a). MGH4 (adenocarcinoma) and MGH7 (epidermoid) were provided by M. Tsao (20). Saos-2 clones were established in this laboratory by transfection of the parental p53-deficient cell line, Saos-2 (obtained from ATCC), with the type p53 tumor suppressor gene (Saos-2-p53). Cells were grown in either RPMI 1640 (Mediatech Inc., Herndon, Va.) (H661, H460, H522, and H125 cell lines), α-MEM (Mediatech Inc.) (FADU and Saos-2 cell lines), or ACL4 serum-free media (MGH4 and MGH7 cell lines). ACL4 was prepared as a 1:1 mixture of RPMI 1640 and Dulbecco’s modification of Eagle’s medium (Mediatech Inc.) supplemented with various growth factors and supplements as described by the ATCC (5a). RPMI 1640 and α-MEM media were supplemented with 10% fetal bovine serum (GIBCO). Media were supplemented with 100 U of penicillin per ml and 100 μg of streptomycin per ml. All cell lines were maintained in culture at 37°C in an atmosphere of 5% CO2.
Cytotoxicity.
Exponentially growing cells (2 × 103 to 3 × 103 cells/100 μl) were seeded in 96-well plates and incubated for 16 h. Cells were then treated continuously with the nucleoside analogs. After 72 h, cell survival was evaluated by replacing the culture media with 150 μl of fresh medium containing 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (pH 7.4), and 50 μl of 2.5 mg of 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT) per ml in phosphate-buffered saline (PBS) (pH 7.4) was then added. After 3 to 4 h of incubation at 37°C, the medium and the MTT were removed and 200 μl of DMSO was added to dissolve the precipitate of reduced MTT, followed by the addition of 25 μl of glycine buffer (0.1 M glycine plus 0.1 M NaCl [pH 10.5]). The formazan crystals were then dissolved, and the absorbance was determined at 570 nm with a microplate reader (model 450; Bio-Rad). The MTT assay distinguishes between viable and nonviable cells on the basis of the requirement of physiologically active mitochondria to metabolize the MTT only in viable cells. The IC50 was calculated as the concentration of drug causing a 50% inhibition in absorbance compared to that of cells treated with solvent alone.
Apoptosis assay.
Cells (106 per 75 cm2 tissue culture flask) were seeded and then left to attach overnight. The cells were then continuously exposed to drug for 72 h. Cells were then collected and washed twice with PBS and then diluted to 106 cells/100 μl of PBS and plated in a 96-well plate. Fixation was performed with 200 μl of 70% ethanol with gentle shaking at 4°C for 30 min. Cells were then washed once with PBS and permeabilized with 1% Triton X-100 in 0.1% sodium citrate on ice for 2 min. Cells were washed twice with PBS and then labeled with TUNEL (terminal uridine deoxynucleotide nick end labeling) reaction mixture (50 μl/well; Boehringer Mannheim, Laval, Quebec, Canada) with the in situ cell death detection kit at 37°C in darkness for 1 h. Cells were then washed three times with 1% bovine serum albumin diluted in PBS and resuspended in 500 μl of PBS for analysis by flow cytometry with an Epics-Profile II flow cytometer. The cell death TUNEL assay estimates the extent of DNA fragmentation. The fragmented DNA is labeled at the free 3′ OH group with terminal deoxynucleotide transferase. Fluorescein labels are incorporated into nucleotide polymers that are attached to the DNA fragments. The labeling is specific to fragmented DNA, and not to degraded DNA, due to the required presence of the 3′ OH group. Thus, the level of fluorescence as measured by a flow cytometer is correlated to the level of DNA fragmentation and hence to the number of apoptotic cells.
RESULTS
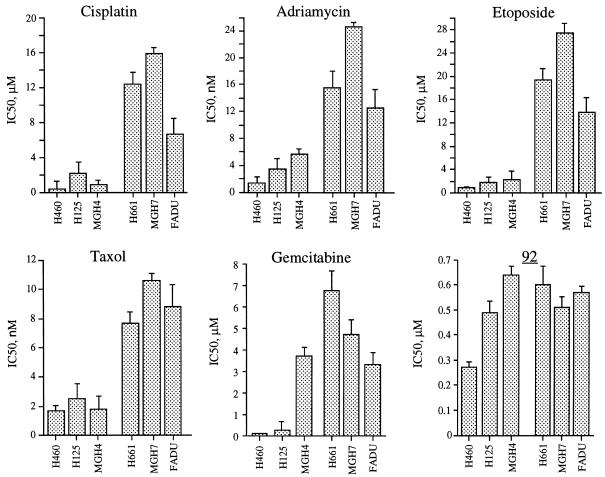
The sensitivity of various NSCLC cell lines to the unsaturated keto-nucleosides 3, 43, and 44 and to keto-C-glycosides 81, 92, and 161 (Fig. 1) was examined by continuous exposure of cells to a range of drug concentrations, and cell survival was monitored after 72 h by the MTT assay. Figure 2 shows the survival curves of the cell lines treated with these drugs, and Table 1 summarizes the average concentrations required to inhibit 50% of cell growth, pooled from independent experiments. The keto-C-glycoside 92 (IC50s range from 0.17 to 0.64 μM) was at least 10-fold more potent than 43, 44, 81, or 161 (IC50s range from 3.89 to 14.22 μM). The ketonucleoside 3 (IC50 ≥ 135 μM) was the least active, in agreement with our previous studies (2, 3). Compared to the NSCLC cell lines H460, MGH4, and H125, the cell lines H661, MGH7, and FADU were more resistant to a variety of drugs, including cisplatin, adriamycin, taxol, and gemcitabine (Fig. 3). Interestingly, keto-C-glycoside 92 as well as most of the other analogs were equally active in all these cell lines regardless of their resistance to the standard chemotherapy drugs.
FIG. 2.
Dose-response antiproliferative activity of ketonucleosides and keto-C-glycosides. NSCLC cell lines H125, H661, H460, FADU, MGH4, and MGH7 were treated continuously with unsaturated ketonucleosides 3, 43, or 44 or keto-C-glycosides 81, 92, or 161, at various concentrations. Seventy-two hours later, cytotoxicity was determined by the MTT assay as described in Materials and Methods. The average drug concentrations required to inhibit 50% of cell growth, obtained from pooled independent experiments, are reported in Table 1.
TABLE 1.
IC50s (μM) of compounds 3, 43, 44, 81, 92, and 161a
| Cell line | IC50 (μM)b
|
|||||
|---|---|---|---|---|---|---|
| 3 | 43 | 44 | 81 | 92 | 161 | |
| H460 | 135 ± 12 | 7.75 ± 0.6 | 4.39 ± 0.3 | 10.15 ± 3.6 | 0.27 ± 0.03 | 8.35 ± 1.6 |
| H125 | 175 ± 27 | 6.76 ± 0.2 | 4.97 ± 0.7 | NDb | 0.49 ± 0.05 | 14.22 ± 3.1 |
| MGH4 | 155 ± 17 | 6.25 ± 1.1 | 3.89 ± 0.6 | 5.86 ± 1.1 | 0.64 ± 0.04 | ND |
| H661 | >200 | 7.09 ± 1.0 | 5.54 ± 0.8 | 7.73 ± 0.9 | 0.60 ± 0.08 | 10.35 ± 3.5 |
| MGH7 | >200 | 4.62 ± 0.5 | 5.60 ± 0.3 | 4.16 ± 0.7 | 0.51 ± 0.05 | 7.7 ± 1.7 |
| FADU | 137 ± 29 | 7.79 ± 0.6 | 4.1 ± 0.3 | 9.55 ± 1.3 | 0.57 ± 0.03 | ND |
| Saos-2 | 179 ± 23 | ND | ND | ND | 0.22 ± 0.03 | ND |
| Saos-2/p53 | >200 | ND | ND | ND | 0.17 ± 0.02 | ND |
NSCLC cell lines H460, H125, H661, MGH4, MGH7, and FADU and parental osteosarcoma Saos-2 and Saos-2 cells transfected with wild-type p53 gene (Saos-2/p53) were treated continuously with the keto-C-glycoside 81, 92, or 161 or the unsaturated ketonucleosides 3, 43, or 44 for 72 h. Cytotoxicity was determined by the MTT assay as described in Materials and Methods.
IC50 was calculated from survival curves of at least two independent experiments. Values correspond to averages ± standard deviations. ND, not determined.
FIG. 3.
Cross-resistance profile of NSCLC cell lines expressing low (H460, H125, and MGH4) or high levels (H661, MGH7, and FADU) of erbB2 tyrosine kinase receptor. Cells were treated continuously with cisplatin, adriamycin, taxol, gemcitabine, or keto-C-glycoside 92. Seventy-two hours later, cytotoxicity was determined by the MTT assay as described in Materials and Methods. The average drug concentration required to inhibit 50% of cell growth was calculated from independent survival curves and expressed as average ± standard deviation.
To investigate the possibility that the antiproliferative potency of unsaturated ketonucleosides and C-glycosides was mediated by the induction of apoptosis (programmed cell death), cells were seeded at high density and treated with various concentrations of these drugs and the percentage of apoptotic cells was determined after 72 h by the TUNEL assay coupled with flow cytometry. Compounds 3 and 44 were selected to compare the structure-activity relationship, and cisplatin was used as a positive control. As indicated in Table 2, the antiproliferative activity of compound 92 correlated with its potency to induce apoptosis. Compound 44 was 10-fold less active than compound 92, while compound 3 was approximately 3- to 5-fold less active than compound 44. This relationship correlated with the antiproliferative activity of these compounds (Fig. 2 and Table 2). With the exception of H460, which has a wild-type p53, all the other cell lines have p53 mutations, suggesting that keto-C-glycoside-induced apoptosis was independent of p53 status. To confirm this hypothesis, apoptosis induction by compound 92 was examined by using an osteosarcoma Saos-2 cell line with the p53 gene deleted and the same cell line transfected with a plasmid containing wild-type human p53 gene (pC53-SN3, kindly provided by B. Vogelstein). Compound 92 induced apoptosis in both Saos 2 and Saos2-p53 cell lines, suggesting that this compound triggered apoptosis through a p53-independent pathway(s). The ketonucleoside 44 also induced apoptosis in these cells but at concentrations approximately 10-fold higher than keto-C-glycoside 92.
TABLE 2.
Induction of apoptosis following exposure of NSCLC cells to compounds 3, 44, and 92 and cisplatina
| Cell line | % Apoptotic cells at indicated dose (μM)
|
||||||
|---|---|---|---|---|---|---|---|
| 3
|
44
|
92
|
25 μM Cisplatin | ||||
| 25 | 50 | 25 | 50 | 2.5 | 5.0 | ||
| H460 | 5 ± 1 | 13 ± 2 | 22 ± 7 | 32 ± 12 | 23 ± 4 | 78 ± 15 | 65 ± 17 |
| H125 | 10 ± 3 | 11 ± 3 | 21 ± 3 | 41 ± 7 | 31 ± 7 | 65 ± 8 | 48 ± 15 |
| MGH4 | 7 ± 3 | 13 ± 2 | 15 ± 9 | 37 ± 5 | 17 ± 2 | 66 ± 12 | 70 ± 13 |
| H661 | 3 ± 1 | 17 ± 5 | 17 ± 4 | 41 ± 11 | 29 ± 10 | 57 ± 11 | 31 ± 12 |
| MGH7 | 2 ± 1 | 11 ± 4 | 27 ± 2 | 46 ± 15 | 19 ± 3 | 49 ± 11 | 23 ± 8 |
| FADU | 6 ± 0.5 | 9 ± 2 | 30 ± 7 | 49 ± 5 | 33 ± 11 | 63 ± 19 | 38 ± 6 |
| Saos-2 | NDb | ND | 12 ± 2 | 45 ± 7 | 26 ± 9 | 32 ± 7 | ND |
| Saos-2/p53 | ND | ND | 19 ± 1 | 33 ± 11 | 33 ± 12 | 57 ± 5 | ND |
NSCLC cell lines H460, H125, H661, MGH4, MGH7, and FADU and osteosarcoma cell line Saos-2 transfected with wild-type p53 gene (Saos-2/p53) were treated continuously with the keto-C-glycoside 81, 92, or 161 or with the unsaturated ketonucleosides 3, 43, or 44 for 72 h. The percentage of apoptotic cells was determined by the TUNEL assay as described in Materials and Methods. Each value represents the average of three independent determinations, each in duplicate, ± standard deviation.
ND, not determined.
DISCUSSION
Chemotherapy management of NSCLC is widely used as a primary or adjuvant treatment. However, treatments with conventional drugs have limited success, and in the majority of patients with advanced NSCLC, median survival is less than 5 years. Drug regimens for metastatic NSCLC include a combination of drugs such as cisplatin, etoposide, adriamycin, and/or gemcitabine. These agents exhibit a steep linear-log dose-response curve. However, only a limited number of patients respond to these drugs, presumably because of “intrinsic” resistance. Among the most common drug resistance markers found in NSCLC is the overexpression of the erbB tyrosine kinase receptors such as EGFR and erbB2 (5, 9, 10) and p53 mutations (8, 11, 23, 24). Cells overexpressing erbB receptors have been reported to acquire resistance to most standard chemotherapy drugs used in NSCLC patients, including cisplatin, etoposide, adriamycin, and taxol (28–30). This cross-resistance between unrelated drugs may limit the benefit of drug combination.
In this study, we have examined the cytotoxic and apoptotic potency of a series of nucleoside analogs, using a panel of NSCLC cell lines representative of human lung cancer, including adenocarcinoma and squamous and large-cell carcinomas. As reported earlier in human leukemia and rodent transformed cell lines, the same structure-activity relationship of the first spectrum of ketonucleosides was observed in NSCLC cell lines. The presence of the O=C−C=C in compound 43 or that of O=C−C−C in compound 44 augments the cytotoxicity of \/ O ketonucleosides, whereas the presence of O-acetyl at position 3′ of the sugar moiety of compound 3 reduces cytotoxicity (3). Compared to compounds 3, 43, 44, 81, and 161, keto-C-glycoside 92 is the most potent. The structures of compounds 43 and 81 differ by the substitution of a theophylline group for a methyl cyclohexene group (Fig. 1). The cytotoxicity values obtained with 43 and 81 (IC50s = 6.50 μM for 43 versus 6.58 μM for 81) suggest that these substitutions have no significant effect on the potency of these nucleosides to induce apoptosis. Compounds 81 and 92 are structurally similar; however, compound 92 has an arachidonic acid side group in place of the methyl group in compound 81. We have previously reported that coupling keto-C-glycosides with unsaturated fatty acids such as arachidonates enhances antiproliferative potency. This differential activity may be related to the high interaction of arachidonic acid side chain with lipid membrane, perhaps enhancing the delivery of these molecules to intracellular compartments. Halmos et al. (13) demonstrated that ketonucleoside analogs such as compounds 3 and 43 interact strongly with the SH groups of cell membranes. However, interaction of the keto-C-glycosides with SH groups and its relationship to apoptosis is still unknown.
Results obtained with the keto-C-glycosides, and particularly with compound 92, indicate that this class of chemicals is not a substrate for drug resistance mechanisms operating in NSCLC cells, since no cross-resistance was observed with other standard chemotherapy drugs. Furthermore, no cross-resistance between adriamycin and keto-C-glycoside 92 was observed in a human breast adenocarcinoma MCF7 cell line or a rat mammary carcinoma cell line (MatB) selected for resistance to adriamycin and overexpressing the MDR1 gene, indicating that compound 92 is not a substrate for the P-glycoprotein encoded by the MDR1 gene (data not shown). The apoptotic activity of compound 92 was observed in p53-mutated cell lines as well as in an osteosarcoma cell line that is lacking p53, suggesting that keto-C-glycoside-induced apoptosis is mediated by a p53-independent mechanism. This finding could have important implications in a clinical setting, since over 50% of NSCLC tumors have p53 alterations and mutated p53 has been shown to be associated with a poor response to chemotherapy (25).
In summary, the potency of C-glycoside molecules such as 92 to inhibit cell proliferation and induce apoptosis in NSCLC cells highlights the importance of further in vivo studies in experimental tumor models to examine their potential applications in therapy.
ACKNOWLEDGMENTS
This work was supported by grant no. MT-12732 from the Medical Research Council and grant no. 008491 from the National Cancer Institute, Canada (M.A.A.-J.) and by the Centre National de la Recherche Scientifique and Association Pour la Recherche sur le Cancer-ARC, France (J.H. and K.A.).
REFERENCES
- 1.Abbruzzese J L, Grunewald R, Weeks E A, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W, Raber M N. Phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9:491–498. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- 2.Alaoui-Jamali M A, Lasne C, Antonakis K, Chouroulinkov I. Absence of genotoxic effects in cells exposed to four ketonucleoside derivatives. Mutagenesis. 1986;1:411–417. doi: 10.1093/mutage/1.6.411. [DOI] [PubMed] [Google Scholar]
- 3.Alaoui-Jamali M A, Arvor-Ergon M J, Bessodes M, Antonakis K, Chouroulinkov I. Relationship between the structure and cytotoxic activity of new unsaturated ketonucleosides tested on eight cell lines. Eur J Med Chem. 1987;22:305–310. [Google Scholar]
- 4.Alaoui-Jamali M A, Tapiero H, Antonakis K, Chouroulinkov I. Structure activity relationship of ketonucleosides. Anticancer Res. 1987;7:501–504. [PubMed] [Google Scholar]
- 5.Alaoui-Jamali M A, Paterson J, Al-Mostapha A E, Yen L. The role of erbB2 tyrosine kinase receptor in cellular intrinsic chemoresistance: mechanisms and implications. Biochem Cell Biol. 1997;75:315–325. [PubMed] [Google Scholar]
- 5a.American Type Culture Collection. Catalogue of cell lines & hybridomas. 7th ed. Rockville, Md: American Type Culture Collection; 1992. [Google Scholar]
- 6.Antonakis K. Synthesis of nucleoside analogs. Chimia. 1975;29:59. [Google Scholar]
- 7.Antonakis K. Synthesis of ketonucleosides with biological activity. Carbohydr Chem Biochem. 1984;42:227. [Google Scholar]
- 8.Bates S, Vousden K H. p53 in signaling checkpoint arrest or apoptosis. Curr Opin Genet Dev. 1996;6:12–18. doi: 10.1016/s0959-437x(96)90004-0. [DOI] [PubMed] [Google Scholar]
- 9.Dougall C W, Qian X, Peterson C N, Miller J M, Samanta A, Greene I M. The neu-oncogene: signal transduction pathways, transformation mechanisms and evolving therapies. Oncogene. 1994;9:2109–2123. [PubMed] [Google Scholar]
- 10.Drebin A J, Link C V, Stern F D, Weinberg A R, Greene I M. Down modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985;41:695–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 11.Fan S, el-Deiry W S, Bae I, Freeman J, Jondle D, Bhatia K, Fornace A J, Jr, Magrath I, Kohn K W, O’Connor P M. p53 gene mutations are associated with decreased sensitivity of human lymphoma cells to DNA damaging agents. Cancer Res. 1994;54:5824–5830. [PubMed] [Google Scholar]
- 12.Goss G D, Dahrouge S, Lochrin C A. Recent advances in the treatment of non-small cell lung cancer. Anti-Cancer drugs. 1996;7:3633–3685. doi: 10.1097/00001813-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Halmos T, Cardon A, Antonakis K. Interactions of cytostatic unsaturated ketonucleosides with sulfhydryl containing cell constituents. Chem Biol Interact. 1983;46:11–29. doi: 10.1016/0009-2797(83)90003-0. [DOI] [PubMed] [Google Scholar]
- 14.Herscovici J, Argoullon J M, Egron M J, Antonakis K. Nucleophilic additions on a β-unsaturated ketonucleoside. Preparation of amino- and thio-ketonucleosides derived from 2′,3′,6′-trideoxy sugars. Carbohydr Res. 1983;112:301–306. [Google Scholar]
- 15.Herscovici J, Egron M J, Antonakis K. Synthesis of spiroepoxynucleosides. J Chem Soc Perkin Trans I. 1986;2:1297. [Google Scholar]
- 16.Herscovici J, Bennani-Baiti I, Frayssinet C, Antonakis K. Keto-C-glycosides. A new class of antitumor compounds. Biomed Chem Lett. 1991;1:395–398. [Google Scholar]
- 17.Herscovici J, Bennani-Baiti I, Montserret R, Frayssinet C, Antonakis K. Design, synthesis and cytotoxic evaluation of keto-C-glycoside fatty acid conjugates. Biomed Chem Lett. 1991;1:721–724. [Google Scholar]
- 18.Herscovici J, Uriel C, Uriel J, Antonakis K. Unsaturated epoxy-C-glycosides. A new class of antitumor compounds with DNA cleavage properties. Biomed Chem Lett. 1994;4:421–426. [Google Scholar]
- 19.Kern J A, Schwartz D A, Nordberg J E, Weiner D B, Greene M I, Torney L, Robinson R A. p185neu expression in human lung adenocarcinomas predicts shortened survival. Cancer Res. 1990;50:5184–5191. [PubMed] [Google Scholar]
- 20.Liu C, Tsao M-S. Proto-oncogene and growth factor/receptor expression in the establishment of primary human non-small cell lung carcinoma cell lines. Am J Pathol. 1993;142:413–423. [PMC free article] [PubMed] [Google Scholar]
- 21.Marchetti A, Buttitta F, Merlo G, Diella F, Pellegrini S, Pepe S, Macchiarini P, Chella A, Angeletti C A, Callahan R, et al. p53 alterations in non-small cell lung cancers correlate with metastatic involvement of hilar and mediastinal lymph nodes. Cancer Res. 1993;53:2846. [PubMed] [Google Scholar]
- 22.Miller P, DiOrio C, Moyer M, Schnur C R, Bruskin A, Cullen W, Moyer D J. Depletion of the erbB-2 gene product p185 by benzoquinoid ansamycins. Cancer Res. 1994;54:2724–2730. [PubMed] [Google Scholar]
- 23.Mitsudomi T, Oyama T, Kusano T, Osaki T, Nakanishi R, Shirakusa T. Mutations of the p53 gene as a predictor of poor prognosis in patients with non-small cell lung cancer. J Natl Cancer Inst. 1993;84:2018–2023. doi: 10.1093/jnci/85.24.2018. [DOI] [PubMed] [Google Scholar]
- 24.Mitsudomi T, Steinberg S M, Nau M M, Carbone D, D’Amico D, Bodner S, Oie H K, Linnoila R I, Mulshine J L, Minna J D, et al. p53 gene mutations in non-small-cell lung cancer cell lines and their correlation with the presence of ras mutations and clinical features. Oncogene. 1992;7:171–180. [PubMed] [Google Scholar]
- 25.Mulshine, J. L., B. E. Johnson, A. F. Gazdar, G. L. Shaw, B. S. Kramer, T. Mitsudomi, J. D. Minna, H. Pass, R. Phelps, B. Ghosh, et al. 1994. Integrated clinical and basic studies related to circumventing non-small cell lung cancer drug resistance. Lung Cancer 10(Suppl. 1):S73–S81. [DOI] [PubMed]
- 26.Shi D, He G, Cao S, Pan W, Zhang H-Z, Yu D, Hung M-C. Overexpression of the c-erbB2/neu-encoded p185 protein in primary lung cancer. Mol Carcinog. 1992;5:213–218. doi: 10.1002/mc.2940050308. [DOI] [PubMed] [Google Scholar]
- 27.Slamon D J, Godolphin W, Jones L A, Wong S G, Keith D E, Levin W J, Stuart S G, Udove J, Ullrich A, Press M F. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 28.Tsai C-M, Chang K-T, Perng R-P, Mitsudomi T, Chen M-H, Kadoyama C, Gazdar A F. Correlation of intrinsic chemoresistance of non-small-cell lung cancer cell lines with HER-2/neu gene expression but not with ras gene mutations. J Natl Cancer Inst. 1993;85:897–901. doi: 10.1093/jnci/85.11.897. [DOI] [PubMed] [Google Scholar]
- 29.Tsai C M, Yu D, Chang K T, Wu L H, Perng R P, Ibrahim N K, Hung M C. Enhanced chemoresistance by elevation of p185neu levels in HER-2/neu-transfected human lung cancer cells. J Natl Cancer Inst. 1995;87:682–684. doi: 10.1093/jnci/87.9.682. [DOI] [PubMed] [Google Scholar]
- 30.Tsai C-M, Chang K-T, Chen J-Y, Chen Y-M, Chen M-H, Perng R-P. Cytotoxic effects of gemcitabine-containing regimens against human non-small cell lung cancer cell lines which express different levels of p185neu. Cancer Res. 1996;56:794–801. [PubMed] [Google Scholar]
- 31.Uriel C. Conception, synthèse et étude structurale. Application au ciblage de drogues par préparation de conjugués lipidiques et glycosidiques. Ph.D. thesis. Paris, France: Université de Paris VI; 1995. C-glycosides antitumoraux. [Google Scholar]