Abstract
Background
There is increasing evidence that physical activity supports healthy ageing. Exercise is helpful for cardiovascular, respiratory and musculoskeletal systems, among others. Aerobic activity, in particular, improves cardiovascular fitness and, based on recently reported findings, may also have beneficial effects on cognition among older people.
Objectives
To assess the effect of aerobic physical activity, aimed at improving cardiorespiratory fitness, on cognitive function in older people without known cognitive impairment.
Search methods
We searched ALOIS ‐ the Cochrane Dementia and Cognitive Improvement Group's Specialized Register, the Cochrane Controlled Trials Register (CENTRAL) (all years to Issue 2 of 4, 2013), MEDLINE (Ovid SP 1946 to August 2013), EMBASE (Ovid SP 1974 to August 2013), PEDro, SPORTDiscus, Web of Science, PsycINFO (Ovid SP 1806 to August 2013), CINAHL (all dates to August 2013), LILACS (all dates to August 2013), World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch), ClinicalTrials.gov (https://clinicaltrials.gov) and Dissertation Abstracts International (DAI) up to 24 August 2013, with no language restrictions.
Selection criteria
We included all published randomised controlled trials (RCTs) comparing the effect on cognitive function of aerobic physical activity programmes with any other active intervention, or no intervention, in cognitively healthy participants aged over 55 years.
Data collection and analysis
Two review authors independently extracted the data from included trials. We grouped cognitive outcome measures into eleven categories covering attention, memory, perception, executive functions, cognitive inhibition, cognitive speed and motor function. We used the mean difference (or standardised mean difference) between groups as the measure of the treatment effect and synthesised data using a random‐effects model. We conducted separate analyses to compare aerobic exercise interventions with no intervention and with other exercise, social or cognitive interventions. Also, we performed analyses including only trials in which an increase in the cardiovascular fitness of participants had been demonstrated.
Main results
Twelve trials including 754 participants met our inclusion criteria. Trials were from eight to 26 weeks in duration.
We judged all trials to be at moderate or high risk of bias in at least some domains. Reporting of some risk of bias domains was poor.
Our analyses comparing aerobic exercise to any active intervention showed no evidence of benefit from aerobic exercise in any cognitive domain. This was also true of our analyses comparing aerobic exercise to no intervention. Analysing only the subgroup of trials in which cardiorespiratory fitness improved in the aerobic exercise group showed that this improvement did not coincide with improvements in any cognitive domains assessed. Our subgroup analyses of aerobic exercise versus flexibility or balance interventions also showed no benefit of aerobic exercise in any cognitive domain.
Dropout rates did not differ between aerobic exercise and control groups. No trial reported on adverse effects.
Overall none of our analyses showed a cognitive benefit from aerobic exercise even when the intervention was shown to lead to improved cardiorespiratory fitness.
Authors' conclusions
We found no evidence in the available data from RCTs that aerobic physical activities, including those which successfully improve cardiorespiratory fitness, have any cognitive benefit in cognitively healthy older adults. Larger studies examining possible moderators are needed to confirm whether or not aerobic training improves cognition.
Plain language summary
Aerobic exercise to improve cognitive function in older people without known cognitive impairment
Aerobic exercise is beneficial for healthy ageing. It has been suggested that the increased fitness brought about by aerobic exercise may help to maintain good cognitive function in older age. We looked for randomised controlled trials of aerobic exercise programmes for people over the age of 55 years, without pre‐existing cognitive problems, which measured effects on both fitness and cognition. The aerobic exercise programmes could be compared with no intervention (e.g. being on a waiting list for the exercise group) or with other kinds of activity (including non‐aerobic exercises such as strength or balance exercises, or social activities).
In this Cochrane Review, 12 trials including 754 participants met our inclusion criteria. Eight of the 12 trials reported that the aerobic exercise interventions resulted in increased fitness of the trained group. However, when we combined results across the trials, we did not find any significant benefits of aerobic exercise or increased fitness on any aspect of cognition. Many included trials had problems with their methods or reporting which reduced our confidence in the findings.
We did not find evidence that aerobic exercise or increased fitness improves cognitive function in older people. However, it remains possible that it may be helpful for particular subgroups of people, or that more intense exercise programmes could be beneficial. Therefore further research in this area is necessary.
Background
Description of the condition
In 2005, there were over 925 million people worldwide aged 55 years or older according to the population database of the United Nations (WPP 2006). It is predicted that in 10 years this will increase to over 1.4 billion people. Subjective complaints about cognitive capacities increase with (older) age (Martin 2003; Newson 2006) and an objective decline in cognitive performance accelerates around the age of 50 (Salthouse 2003; Verhaeghen 1997), with the exception of cognitive skills with a large crystallised intelligence component. Research has shown that a regular exercise programme can slow down or prevent functional decline associated with ageing and improve health in this age group. The physical health benefits for older people who regularly participate in endurance, balance and resistance training programmes are well established. Such health benefits include improved muscle mass, arterial compliance, energy metabolism, cardiovascular fitness, muscle strength and overall functional capacity (Lemura 2000). It is suspected that physical activity may also enhance cognitive function (Colcombe 2003).
Description of the intervention
In this Cochrane Review we included the interventions of exercise programmes for older people which aimed to improve cardiorespiratory fitness, the ability of the circulatory and respiratory to supply oxygen to muscles during sustained physical activity, through for example walking, running or cycling. We compared their effects with a variety of control interventions: either no intervention or exercise interventions which would not be expected to enhance cardiorespiratory fitness, such as strength or balance programmes, or social or mental activities. Cardiorespiratory fitness may be assessed in a variety of ways. A common method is to measure VO2 max, which is the maximal oxygen uptake measured during exercise on a treadmill or cycle, although other physiological measures or walk times may also be used.
How the intervention might work
Research using animal models has provided insight into the possible cellular and molecular mechanisms that could underlie an effect of physical activity on cognitive function. Increased aerobic fitness increases oxygen extraction, glucose utilisation and cerebral blood flow (Churchill 2002). Cerebral blood flow meets metabolic needs of the brain and removes waste (Lojovich 2010). Increased aerobic fitness also increases Brain‐Derived Neurotrophic Factor (BDNF) and other growth factors which mediate structural changes (Cotman 2002; Cotman 2007). For example, BDNF is implicated in neurogenesis, synaptogenesis, dendritic branching and neuroprotection (Lojovich 2010). A preliminary survey of the literature on human research points towards the same possible physiological mechanisms that could explain the association between physical activity and cognitive vitality (Aleman 2000; Brown 2008; Colcombe 2006; Davenport 2012; Erickson 2009; McAuley 2004; Prins 2002). Hence it is hypothesised that improvements in cardiovascular (aerobic) fitness mediate the benefits of physical activity on cognitive capacity (Etnier 2007; McAuley 2004). Therefore this cardiovascular fitness hypothesis implies that changes in cognitive function are preceded by changes in aerobic fitness. The evidence for this hypothetical link between physical activity, cardiovascular fitness and cognitive function in older people comes from several longitudinal studies (Abbott 2004; Barnes 2003; Etgen 2010; Laurin 2001; Middleton 2011; Richards 2003; Sturman 2005; van Gelder 2004). However, results from training studies by Hill 1993 and Blumenthal 1991 failed to correlate changes in aerobic power (VO2 max) with changes in cognitive measures. At the same time, trials seldom report combinations of activity, fitness and cognition in a single trial.
Why it is important to do this review
Previous meta‐analyses have reported a robust effect of physical activity on cognitive function in older adults (Colcombe 2003; Etnier 1997b; Heyn 2004;Smith 2010), but it remains unclear whether improvement in cardiovascular fitness (as reflected by cardiovascular parameters such as VO2 max) accounts for the effects of physical activity on cognitive capacity. Physiological or psychological mechanisms other than aerobic fitness might still account for the effects found in these meta‐analyses. This Cochrane Review intends to investigate a hypothesised link between physical activity specifically aimed at the improvement of cardiorespiratory fitness and cognitive function. Such information will be useful in the quest to identify interventions that may be helpful for healthy ageing and protective against the development of neurodegenerative disorders such as Alzheimer's disease.
Objectives
To assess the effectiveness of physical activity, aimed at improving cardiorespiratory fitness, on cognitive function in older people without known cognitive impairment.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomised controlled clinical trials (RCTs). Blinding of outcome assessors was not required for inclusion in this review. We did not apply any language restrictions but trials must have been published in peer‐reviewed journals.
Types of participants
Participants were aged 55 or older and not objectively cognitively impaired in any way greater than that expected from age alone. Hence, we excluded patients with mild cognitive impairment (MCI) or any form of dementia and patients with other conditions likely to be associated with cognitive impairment, such as stroke and depression. However, we included trials of participants with age‐related illnesses (e.g. osteoporosis, arthrosis) or specific disorders (e.g. chronic obstructive pulmonary disease (COPD), heart failure).
Types of interventions
We included the physical activity interventions of any programme of exercise of any intensity, duration or frequency which was aimed at improving cardiorespiratory fitness. Therefore, trials must have reported at least one objective measure of cardiorespiratory fitness. Acceptable comparator interventions were: no treatment; a strength or balance programme; or a programme of social activities or mental activities. Trials which had both an active comparator group and a no treatment group could contribute data to the 'aerobic exercise vs. any active intervention' meta‐analyses and to the 'aerobic exercise vs. no intervention' meta‐analyses.
Types of outcome measures
Trials had to report an objective measure of cardiorespiratory fitness. Acceptable measures included, but were not limited to: VO2 max, Graded Exercise Test (GXT) rate‐pressure product, heart rate and blood pressure during modified step test, the Six‐Minute Walk Test (6MWT), 400‐metre walk time, and ¼ mile walk time. Where trials measured more than one fitness parameter, we preferred the measure that we considered to be the purest measure of cardiorespiratory fitness, or was previously show to be correlated with VO2 max, or both.
Primary outcomes
The primary outcome measurement was cognitive function, tested with a neuropsychological test (sensitive to changes in cognitive function in adults) or test battery (a combination of several neuropsychological tests).
Secondary outcomes
Other outcome measures were drop‐out, as a measure of acceptability, and adverse events.
Search methods for identification of studies
Electronic searches
We searched ALOIS ‐ the Cochrane Dementia and Cognitive Improvement Group's Specialized Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1946 to August 2013), EMBASE (Ovid SP 1974 to August 2013), PEDro, SPORTDiscus, Web of Science (Web of Science platform), PsycINFO (Ovid SP 1806 to August 2013), CINAHL (EBSCOhost), LILACS (BIREME), World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch), ClinicalTrials.gov (https://clinicaltrials.gov) and Dissertation Abstracts International (DAI) up to 24 August 2013 with no language restrictions.
We used a combination of MeSH and free text terms to find records of physical activity, including: exercise*, motor activit*, leisure activit*, physical fitness, physical endurance, exercise tolerance, exercise test, aerobic, aerobic capacity, physical activity, physical capacity, physical performance, training. We have listed the search strategy details in Appendix 1.
We performed a further search update up to November 2014. We have inserted the search results into the Studies awaiting classification section and will fully incorporate these trials in the next review update.
Searching other resources
We checked reference lists of the included trials and in reviews of the literature screened for relevant trials. Also we contacted experts in this area and relevant associations.
Data collection and analysis
Selection of studies
The Cochrane Trials Search Coordinator (ANS) assessed the titles and available abstracts of all trials identified by the initial search and excluded irrelevant trials. Two review authors (JY and NT; or MA and GA previously) independently assessed full paper copies of reports of potentially relevant trials. We resolved any disagreements on inclusion by discussion and through arbitration by a third review author (JR). Details of the study selection process can be found in Figure 1.
1.

Study flow diagram for the August 2013 update search
Data extraction and management
Two review authors (JY and NT) independently extracted data from the published reports and JY entered them into RevMan 2014, with full agreement of the second review author. The summary statistics required for each trial and each outcome for continuous data were the mean (or mean change from baseline), the standard deviation (SD) and the number of participants for each treatment group at each assessment. For cognitive data in which a higher score denotes worse performance (e.g. reaction times, digit vigilance, trail making part A, trail making part B, Stroop interference data and error rates), we entered the mean as a negative variable. If only the standard error of the mean was reported, we calculated the SD using SD = SE x sqrt(N). For dichotomous data, we extracted the number of participants with each outcome in each group.
The included articles measured cognitive function using various rating scales. We grouped neuropsychological tests measuring approximately the same construct in a total of eleven categories (see Table 1; Kessels 2000; Lezak 2004). For each trial, only a single test was admitted to each category. Where a trial used more than one test within a category, then first we chose the one which was used most frequently in the included trials; if not, then the one that had been found to load onto the category in previous factor analysis (Salthouse 1996) or which we considered closer to the core construct of the category. We chose all included tests prior to extraction of results.
1. Grouping of cognitive tests and studies over cognitive functions.
| Cognitive function | Cognitive tests | Trial |
| Cognitive speed | Simple RT | Panton 1990, Oken 2006 |
| Choice RT | Hassmén 1997, Whitehurst 1991 | |
| Trailmaking part A | Emery 1998, Legault 2011, Langlois 2012 | |
| Digit symbol substitution | Blumenthal 1989, Kramer 2001, Emery 1990a | |
| Verbal memory functions (immediate) | Randt memory test story recall | Blumenthal 1989 |
| 16 words immediate recall | Hassmén 1997 | |
| Ross Information Processing Assessment memory immediate recall | Moul 1995 | |
| Wechsler Adult Intelligence Scales logical memory immediate recall | Fabre 2002 | |
| Rey auditory verbal learning test trail I‐V | Kramer 2001, Langlois 2012 | |
| Hopkins Verbal Learning Test | Legault 2011 | |
| Visual memory functions (immediate) | Benton visual retention | Blumenthal 1989 |
| Wechsler Memory Scales visual reproduction immediate recall | Fabre 2002 | |
| Working memory | Digit span backward | Blumenthal 1989, Kramer 2001, Langlois 2012 |
| 2‐Back | Legault 2011 | |
| Memory function (delayed) | 16 words delayed recall | Hassmén 1997 |
| Rey auditory verbal learning test delayed recall trail | Kramer 2001, Langlois 2012 | |
| 10 words delayed recall | Oken 2006 | |
| Hopkins Verbal Learning Test ‐ 12 words | Legault 2011 | |
| Executive functions | Trailmaking part B | Blumenthal 1989, Legault 2011, Langlois 2012 |
| Ross Information Processing Assessment problem solving and abstract reasoning | Moul 1995 | |
| Wechsler Memory Scales mental control | Fabre 2002 | |
| Task switching paradigm | Kramer 2001 | |
| Verbal fluency | Emery 1990a | |
| Letter number sequencing | Oken 2006 | |
| Perception | Face recognition | Hassmén 1997, Kramer 2001 |
| Ross Information Processing Assessment auditory processing | Moul 1995 | |
| Wechsler Adult Intelligence Scales visual reproduction | Fabre 2002 | |
| Cognitive inhibition | Stroop colour word test | Blumenthal 1989, Oken 2006, Langlois 2012, Predovan 2012 |
| Stopping task | Kramer 2001 | |
| Flanker Task | Legault 2011 | |
| Visual attention | Digit vigilance | Emery 1990a |
| Tracking | Bakken 2001 | |
| 2&7 test | Blumenthal 1989 | |
| Visual search | Kramer 2001 | |
| Covert orienting of visuospatial attention | Oken 2006 | |
| Auditory attention | Digit span forward | Blumenthal 1989, Emery 1990a, Fabre 2002, Hassmén 1997, Kramer 2001 |
| Motor function | Finger tapping | Bakken 2001, Blumenthal 1989, Emery 1998 |
| Pursuit rotor task | Kramer 2001 |
One trial (Blumenthal 1989) reported results for men and women separately in the same paper. In this case, we calculated pooled means and SDs by combining results for both genders.
Assessment of risk of bias in included studies
Two review authors (JY, NT) independently evaluated the methodological quality of the selected articles using two different methods. We used the criteria list for quality assessment of non‐pharmaceutical trials (CLEAR NPT) developed using consensus (Boutron 2005). This checklist includes information on sampling method, measurement, intervention and reporting of biases and limitations (see Table 2). We performed a small pilot exercise to clarify the method with some articles that we already excluded from the review process. We calculated Cohen's kappa (K) as a measure of inter‐observer agreement, and we relied on Landis 1977's benchmarks for assessing the relative strength of agreement. We resolved any discordance in assessment through a single round of discussion and arbitration by a third review author (JR).
We also used the recommended approach for assessing risk of bias in trials included in Cochrane Reviews, which is based on the evaluation of six specific methodological domains (namely, sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues). For each trial the six domains are analysed, described as reported in the trial and a final judgment on the likelihood of bias is provided. This is achieved by answering a pre‐specified question about the adequacy of the trial in relation to each domain, such that a judgement of "yes" indicates low risk of bias, "no" indicates high risk of bias, and "unclear" indicates unclear or unknown risk of bias. To make these judgments we used the criteria indicated by the Cochrane Handbook for Systematic Reviews of Interventions (see Higgins 2011 for a detailed description) and their applicability on the addiction field. We assessed the included trials using the criteria and the method indicated in Higgins 2011.
Measures of treatment effect
For continuous outcome data, we used the weighted mean difference (WMD) if trials used the same cognitive tests and if the outcome measurements were on the same scale. We calculated the standardised mean difference (SMD) in all other cases. For dichotomous data, such as drop‐out, we used the odds ratio (OR).
Dealing with missing data
To allow an intention‐to‐treat (ITT) analysis, we sought data on every participant randomised irrespective of compliance, whether or not the participant was subsequently deemed ineligible, or otherwise excluded from treatment or follow‐up. If ITT data were unavailable in the publications, we sought "on‐treatment" data or the data of those who completed the trial, where indicated.
Data synthesis
For each cognitive outcome category, we synthesised the data using a random‐effects model. We analysed the possible effects of aerobic exercise versus any active comparator (strength programme, flexibility or balance programme, social or mental programme) and versus no intervention (usual care or waiting list).
Subgroup analysis and investigation of heterogeneity
Heterogeneity was low across all domains in all meta‐analyses, therefore we did not subgroup analyses to explore heterogeneity.
In order to explore further the potential effects the different forms of exercise, we conducted subgroup analyses which compared aerobic exercise with (a) flexibility or balance interventions and (b) strength training. We further explored our hypothesis by performing analyses of only those trials in which an increase in fitness was demonstrated.
As an extension to subgroup analyses, a meta‐regression would allow the effect of cardiovascular fitness (VO2 max or any other measure of the degree of aerobic fitness) on cognitive outcomes to be investigated. However, we did not consider meta‐regression in this Cochrane Review due to the small number of included trials (< eight trials) in all meta‐analyses.
Results
Description of studies
Results of the search
The August 2013 search identified 352 promising abstracts (see PRIMSA flow diagram). We identified seven potentially relevant theses but these had no associated peer‐reviewed publications. We asked the authors of the theses to provide information on published data, but none were provided. We examined the full texts of 82 articles. We identified 2 new trials for inclusion bringing the total number of trials included to 12 trials involving 754 participants.
Included studies
We have listed the details of the methods, participants, interventions and outcomes for each included trial in the Characteristics of included studies table. Also, we have summarized the intervention types in each trial in Table 2.
2. Types of interventions in each trial.
| Trial | Aerobic exercise | Strength | Flexibility/balance | Social | Cognitive | Education | Miscellaneous | No intervention |
| Bakken 2001 | x | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | x |
| Blumenthal 1989 | x | ‐ | x | ‐ | ‐ | ‐ | ‐ | x |
| Emery 1990a | x | ‐ | ‐ | x | ‐ | ‐ | ‐ | x |
| Fabre 2002 | x | ‐ | ‐ | x | x | ‐ | ‐ | ‐ |
| Kramer 2001 | x | ‐ | x | ‐ | ‐ | ‐ | ‐ | ‐ |
| Langlois 2012 | x | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | x |
| Legault 2011 | x | ‐ | ‐ | ‐ | x | x | ‐ | ‐ |
| Madden 1989 | x | ‐ | x | ‐ | ‐ | ‐ | ‐ | x |
| Moul 1995 | x | x | x | ‐ | ‐ | ‐ | ‐ | ‐ |
| Oken 2006 | x | ‐ | x | ‐ | ‐ | ‐ | ‐ | x |
| Panton 1990 | x | x | ‐ | ‐ | ‐ | ‐ | ‐ | x |
| Whitehurst 1991 | x | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | x |
Bakken 2001 conducted a small RCT (N = 15) comparing an aerobic exercise group to a waiting list control group for eight weeks. Both groups showed slight improvement in a measure of aerobic fitness over the course of the trial. The only cognitive outcome parameter was the accuracy index ‐ a test of visual attention.
Blumenthal 1989 randomised 101 participants to aerobic exercise training, a yoga/flexibility programme or a waiting list control group over 16 weeks. Participants in the aerobic training group only experienced a significant increase in their VO2 max. Outcomes included tests of cognitive speed, verbal, visual and working memory, executive functions, cognitive inhibition, visual and auditory attention and motor function.
Madden 1989 reported different cognitive outcomes for a subset of the participants from Blumenthal 1989. We did not included any of the data from this paper in the analyses because Blumenthal 1989 reported data for the same outcome categories.
Emery 1990a assigned 48 participants to an exercise programme, a social activity group or a waiting list control group for 12 weeks. No effect of the exercise programme on cardiovascular function was demonstrated. As attrition from the social group was comparable to that of the control group, and attendance for the social group was poor overall (ranged from 10% to 94%), the trial authors pooled data from the social activity and waiting list control groups (we included this pooled group in the 'exercise versus any intervention' analyses). This trial included tests for cognitive speed and auditory attention.
Fabre 2002 presented data from 32 participants randomly assigned to an aerobic exercise programme, a mental training programme, a combined aerobic/mental programme or a social activity group. We did not use data from the combined aerobic exercise/mental training group in this review. There was a significant increase in VO2 max in the aerobic training group but no change in the other two groups. The trial included tests for verbal and visual memory, perception and executive functions.
Kramer 2001 recruited a total of 174 participants and randomly assigned participants to an aerobic walking group or a stretching and toning group. The aerobic walking group improved their VO2 max measures while the stretching and toning group decreased their VO2 max measures. The trial authors assessed cognitive speed, verbal and visual memory, perception, executive and motor functions as well as cognitive inhibition, visual and auditory attention with various cognitive tests. Mean results of the subtests of the pursuit rotor task, Rey's auditory verbal learning test, spatial attention and visual search task were summed and divided by the number of tasks. SD values of these subtests were pooled.
Langlois 2012 randomly assigned 83 participants, ensuring gender ratio equivalence, to a 12‐week exercise training group or a control group that maintained their previous activity levels. Participants in the exercise training group improved in physical fitness, as measured by the 6MWT, significantly more than controls. Outcomes included tests of cognitive speed, verbal and working memory, executive functions and inhibition.
Legault 2011 published a pilot RCT of 73 participants randomly assigned to a physical activity training group, a cognitive training group, a combined intervention group or a 'healthy aging' control group, which we considered an active intervention. We did not use data from the combined intervention group in this review. The physical activity training group improved in a fitness measure while the cognitive training and control group did not. Cognitive speed, verbal memory, working memory, executive function and cognitive inhibition were tested in the participants.
Moul 1995 recruited 30 participants and randomly assigned them to a walking condition, weight training or control condition, which we considered to be a flexibility intervention, for 16 weeks. VO2 max significantly increased in the walking group but not in the weight training or control conditions. The Ross Information Processing Assessment was used to evaluate changes in cognitive function.
Oken 2006 randomised 135 participants into an aerobic group, a yoga group or a waiting list control group for six months. There were no significant differences between the groups in their fitness measure. Cognitive speed, delayed memory functions, executive functions, visual attention and cognitive inhibition were assessed in order to test for effects on cognition.
Panton 1990 included data on 49 participants randomly assigned to a walk/jog group, a strength group or a no intervention control condition for 26 weeks. VO2 max significantly improved for the walk/jog group while there was no significant change for strength as well as the control groups. Tests for cognitive speed were performed to analyse cognitive function.
Whitehurst 1991 recruited 14 participants and randomly assigned them to an exercise programme or a no intervention control condition for eight weeks. Participants in the exercise group significantly increased their VO2 max scores, whereas participants in the control group did not. Choice reaction times were tested for evaluation of cognitive function.
Excluded studies
We have listed details of excluded trials in the Characteristics of excluded studies table. We excluded trials because they were not RCTs (19), did not use a cognitively normal older population (11), did not meet other inclusion criteria (1: Kharti 2001 included depressed participants), did not have objective aerobic fitness parameters (16), did not have objective cognitive outcomes (5), assessed cognition during exercise (3), did not have pre‐ to post‐ intervention data (4), did not have a non‐aerobic control group (2), had not been published (7), the data was published in an already included trial (2), or for other reasons: objective cognitive measures were not analysed by group (Emery 1990b) or the control group was exercising but not given a formal program (Etnier 2001).
Risk of bias in included studies
We have presented the results of the quality assessment of non‐pharmaceutical trials (CLEAR NPT) (Boutron 2005) in Table 3. The overall methodological quality score of the included trials ranged from 24 to 39 (minimum possible score of 14 points, maximum possible score of 48 points; lower scores denote a better methodological quality). For most trials, the blinding treatment providers and participants was scored "no, because blinding is not feasible". Two review authors (JY, NT) calculated Cohen's kappa (K) as a measure of inter‐observer reliability after the initial screening and reached 0.84, almost perfect according to Landis 1977.
3. Methodological quality of included trials (CLEAR NPT score).
| Study ID | Number | |||||||||
| 1 / 2 | 3 | 4 | 5 | 6 / 6.1.1 / 6.1.2 | 7 / 7.1.1 / 7.1.2 | 8 / 8.1.1 | 9 | 10 | Total | |
| Bakken 2001 | 3 / 3 | 1 | 3 | 1 | 2 / 3 / 2 | 2 / 3 / 2 | 1 / 0 | 1 | 2 | 28 |
| Blumenthal 1989 | 3 / 3 | 1 | 3 | 1 | 2 / 2 / 2 | 2 / 2 / 2 | 4 / 3 | 1 | 3 | 34 |
| Emery 1990a | 3 / 3 | 1 | 3 | 1 | 2 / 2 / 2 | 2 / 2 / 2 | 4 / 3 | 1 | 2 | 33 |
| Fabre 2002 | 3 / 3 | 1 | 3 | 2 | 2 / 1 / 1 | 2 / 1 / 1 | 4 / 3 | 1 | 1 | 29 |
| Kramer 2001 | 3 / 3 | 1 | 3 | 1 | 2 / 3 / 1 | 2 / 3 / 1 | 4 / 3 | 1 | 2 | 33 |
| Langlois 2012 | 3 / 3 | 1 | 3 | 3 | 2 / 3 / 2 | 2 / 3 / 2 | 4 / 3 | 1 | 2 | 37 |
| Legault 2011 | 3 / 3 | 1 | 3 | 1 | 2 / 3 / 1 | 2 / 3 / 1 | 4 / 3 | 1 | 1 | 31 |
| Madden 1989 | 3 / 3 | 1 | 3 | 1 | 2 / 3 / 2 | 2 / 3 / 2 | 4 / 3 | 1 | 2 | 34 |
| Moul 1995 | 3 / 3 | 1 | 3 | 3 | 2 / 3 / 1 | 2 / 3 / 1 | 4 / 3 | 1 | 1 | 34 |
| Oken 2006 | 1 / 1 | 1 | 3 | 1 | 2 / 2 /1 | 2 / 2 / 1 | 1 / 3 | 1 | 2 | 24 |
| Panton 1990 | 3 / 3 | 1 | 3 | 2 | 2 / 3 / 3 | 2 / 3 / 3 | 4 / 3 | 1 | 2 | 38 |
| Whitehurst 1991 | 3 / 3 | 1 | 3 | 2 | 2 / 3 / 3 | 2 / 3 / 3 | 4 / 3 | 1 | 3 | 39 |
See Table 4 for CLEAR NPT items.
4. CLEAR NPT checklist items.
| Number | Checklist item |
| 1 | Was the generation of allocation sequences adequate? |
| 2 | Was the treatment allocation concealed? |
| 3 | Were the details of the intervention administered to each group made available?a |
| 4 | Were care providers' experience or skillb in each arm appropriate?c |
| 5 | Was participant (i.e. patients) adherence assessed quantitatively?d |
| 6 | Were participants adequately blinded? |
| 6.1.1 | If participants were not adequately blinded, were all other treatments and care (cointerventions) the same in each randomised group? |
| 6.1.2 | If participants were not adequately blinded, were withdrawals and lost to follow‐up the same in each randomised group? |
| 7 | Were care providers or persons caring for the participants adequately blinded? |
| 7.1.1 | If care providers were not adequately blinded, were all other treatments and care (cointerventions) the same in each randomised group? |
| 7.1.2 | If care providers were not adequately blinded, were withdrawals and losses to follow‐up the same in each randomised group? |
| 8 | Were outcome assessors adequately blinded to assess the primary outcomes? |
| 8.1.1 | If outcome assessors were not adequately blinded, were specific methods used to avoid ascertainment bias?e |
| 9 | Was the follow‐up schedule the same in each group?f |
| 10 | Were the main outcomes analysed according to the ITT principle? |
| a | The answer should be "Yes" if these data are either described in the report or made available for each arm (reference to preliminary report, online addendum, etc.). |
| b | Care provider experience or skill will be assessed only for therapist‐dependent interventions (where the success of the intervention is directly linked to the providers' technical skill. For other treatment this item is not relevant and should be answered "Unclear". |
| c | Appropriate experience or skill should be determined according to published data, preliminary studies, guidelines, run‐in period, or a group of experts and should be specified in the protocol for each study arm before the beginning of the survey. |
| d | Treatment adherence will be assessed only for the treatments necessitating iterative interventions (physiotherapy that supposes several sessions, in contrast to a one‐shot treatment such as surgery). For one‐shot treatments, this item is not relevant and should be answered "Unclear". |
| e | The answer is "0" if the answer to 8 is "Yes". The answer should be "Yes" if the main outcome is objective or hard, or if outcomes were assessed by a blinded or at least an independent endpoint review committee, or if outcomes were assessed by an independent outcome assessor trained to perform the measurements in a standardised manner, or if the outcome assessor was blinded to the study purpose and hypothesis. |
| f | This item is not relevant if follow‐up is part of the question. For example, this item is not relevant for a trial assessing frequent versus less frequent follow‐up for cancer recurrence. In these situations, this item should be answered "Unclear". |
| For items 6, 7 and 8 a score of 1 was given for a "Yes", a score of 2 for "No, because blinding is not feasible", a score of 3 for "No, although blinding is feasible" and a score of 4 for "Unclear". The other items of the checklist (1 to 5, 6.1.1, 6.1.2, 7.1.1, 7.1.2, 8.1.1, 9 and 10) were given a score of 1 for "Yes", 2 for "No" and 3 for "Unclear". | |
We have presented the results of our 'Risk of bias' assessment in the Characteristics of included studies tables and in Figure 2. We only considered one trial to be at low risk of bias for sequence generation (Oken 2006). We judged the remaining 11 trials to be at unclear risk of bias for sequence generation. Procedures to ensure allocation concealment were not described in the included papers; all 12 papers were judged to be at unclear risk of bias in this domain. In all 12 included trials blinding of participants and trainers was not feasible. This was unlikely to introduce bias in trainers, so we considered all 12 trials to be at low risk of bias for blinding trainers. This may have introduced bias in participants, so all 12 trials were judged to be at high risk of blinding of the participants. We judged five trials (Bakken 2001; Legault 2011; Oken 2006; Panton 1990; Whitehurst 1991) to be at low risk of bias for blinding of the assessors for the cognitive outcomes because assessment of cognition was by means of computerised tests. We considered the other seven trials to be at unclear risk of bias for this item. Four trials (Fabre 2002; Legault 2011; Moul 1995; Whitehurst 1991) were judged to be at low risk of bias for addressing incomplete data. Besides Legault 2011, in all cases this was due to the fact that there were no drop‐outs from these trials. Legault 2011 reported drop‐outs per group and analysed using ITT principles. All other eight trials were judged being at high risk of bias for this item since they reported drop‐outs but either lacked information on the group assignment of these drop‐outs (Panton 1990) or lacked ITT analysis, or both. We judged all trials, except Blumenthal 1989, to be at unclear risk of bias for selective reporting since there was insufficient information to permit a judgment. Blumenthal 1989 was judged being at high risk for this item since data on one pre‐specified primary cognitive outcome was missing. We considered all trials to be at low risk of bias for other potential threats to validity. However, we could not rule out risk of contamination bias, where the control group, on finding out the purpose of a trial, could have increased their levels of aerobic exercise as well.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
Aerobic exercise versus any active intervention
Eight trials including 506 participants contributed data on at least one cognitive domain. Duration of the intervention in these trials ranged from eight weeks to 26.07 weeks. In six trials, trial authors showed an increase in aerobic fitness in the active intervention but not the comparison group. We were able to conduct meta‐analyses for all 11 of our pre‐specified cognitive domains (Analysis 1.1 to Analysis 1.11; Figure 3; Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; Figure 9; Figure 10; Figure 11; Figure 12; Figure 13). There was no evidence of benefit of the aerobic exercise intervention in any cognitive domain.
1.1. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 1 Cognitive speed.
1.11. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 11 Motor function.
3.

Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.1 Cognitive speed.
4.

Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.2 Verbal memory functions (immediate).
5.

Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.3 Visual memory functions (immediate).
6.
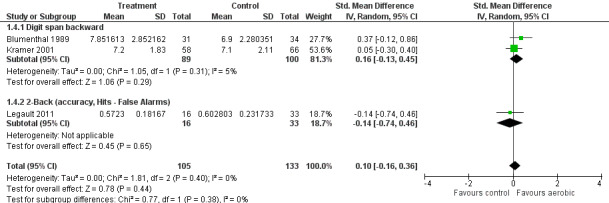
Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.4 Working memory.
7.

Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.5 Memory functions (delayed).
8.

Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.6 Executive functions.
9.

Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.7 Perception.
10.

Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.8 Cognitive inhibition.
11.

Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.9 Visual attention.
12.

Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.10 Auditory attention.
13.

Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.11 Motor function.
There was no difference in dropout rates between the aerobic exercise intervention and comparison groups (OR 0.96, 95% CI 0.44 to 2.10; seven trials, 469 participants; Analysis 1.12; Figure 14).
1.12. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 12 Drop‐out.
14.

Forest plot of comparison: 1 Aerobic exercise versus any active intervention, outcome: 1.12 Drop‐out.
Aerobic exercise versus no intervention
Six trials including 296 participants contributed data on at least one cognitive domain. The duration of the intervention in these trials ranged from eight to 26.07 weeks. In four trials, trial authors showed an increase in aerobic fitness in the active intervention but not the comparison group. We were able to conduct meta‐analyses for 10 of our 11 pre‐specified cognitive domains, besides perception (Analysis 2.1 to Analysis 2.10; Figure 15; Figure 16; Figure 17; Figure 18; Figure 19; Figure 20; Figure 21; Figure 22; Figure 23; Figure 24). There was no evidence of benefit of the aerobic exercise intervention in any cognitive domain.
2.1. Analysis.

Comparison 2 Aerobic exercise versus no intervention, Outcome 1 Cognitive speed.
2.10. Analysis.

Comparison 2 Aerobic exercise versus no intervention, Outcome 10 Motor function.
15.

Forest plot of comparison: 2 Aerobic exercise versus no intervention, outcome: 2.1 Cognitive speed.
16.

Forest plot of comparison: 2 Aerobic exercise versus no intervention, outcome: 2.2 Verbal memory functions (immediate).
17.

Forest plot of comparison: 2 Aerobic exercise versus no intervention, outcome: 2.3 Visual memory functions (immediate).
18.

Forest plot of comparison: 2 Aerobic exercise versus no intervention, outcome: 2.4 Working memory.
19.

Forest plot of comparison: 2 Aerobic exercise versus no intervention, outcome: 2.5 Memory functions (delayed).
20.

Forest plot of comparison: 2 Aerobic exercise versus no intervention, outcome: 2.6 Executive functions.
21.

Forest plot of comparison: 2 Aerobic exercise versus no intervention, outcome: 2.7 Cognitive inhibition.
22.

Forest plot of comparison: 2 Aerobic exercise versus no intervention, outcome: 2.8 Visual attention.
23.

Forest plot of comparison: 2 Aerobic exercise versus no intervention, outcome: 2.9 Auditory attention.
24.

Forest plot of comparison: 2 Aerobic exercise versus no intervention, outcome: 2.10 Motor function.
There was no difference in dropout rates between the aerobic exercise intervention and comparison groups (OR 1.84, 95% CI 0.79 to 4.29; five trials, 267 participants; Analysis 2.11; Figure 25).
2.11. Analysis.

Comparison 2 Aerobic exercise versus no intervention, Outcome 11 Drop‐out.
25.

Forest plot of comparison: 2 Aerobic exercise versus no intervention, outcome: 2.11 Drop‐out.
Aerobic exercise versus flexibility/balance intervention
Analysing only the subgroup of trials in which the aerobic exercise intervention was compared to flexibility or balance control groups, four trials (351 participants) contributed data on at least one cognitive domain (Blumenthal 1989; Kramer 2001; Moul 1995; Oken 2006). Intervention duration in these trials ranged from 16 to 26.07 weeks. We were able to conduct meta‐analyses on all 11 of our pre‐specified cognitive domains (Analysis 3.1 to Analysis 3.11). There was no evidence of benefit of the aerobic exercise intervention in any cognitive domain.
3.1. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 1 Cognitive speed.
3.11. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 11 Motor function.
There was no difference in dropout rates between the aerobic exercise intervention and comparison groups (OR 0.99, 95% CI 0.58 to 1.72; four trials, 351 participants; Analysis 3.12).
3.12. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 12 Drop‐out.
Aerobic exercise versus strength training intervention
Subgroup analyses of aerobic exercise intervention compared to strength training controls was not possible since we could only include one trial in these analyses.
Fitness improved: aerobic exercise versus any active intervention
Analysing only the subgroup of trials in which the aerobic exercise intervention was shown to enhance fitness relative to any active intervention control groups, six trials including 367 participants contributed data on at least one cognitive domain (Blumenthal 1989; Fabre 2002; Kramer 2001; Legault 2011; Moul 1995; Panton 1990). The duration of the intervention in these trials ranged from eight to 26.07 weeks. We were able to conduct meta‐analyses for all 11 of our pre‐specified cognitive domains (Analysis 5.1 to Analysis 5.11). There was no evidence of benefit of the aerobic exercise intervention in any cognitive domain.
5.1. Analysis.
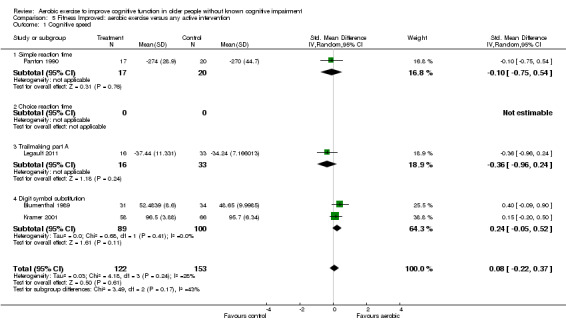
Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 1 Cognitive speed.
5.11. Analysis.

Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 11 Motor function.
There was no difference in dropout rates between the aerobic exercise intervention and comparison groups (OR 1.22, 95% CI 0.66 to 2.25; five trials, 330 participants; Analysis 5.12).
5.12. Analysis.

Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 12 Drop‐out.
Fitness improved: aerobic exercise versus no intervention
Analysing only the subgroup of trials in which the aerobic exercise intervention was shown to significantly improve fitness relative to no intervention control groups, four trials involving 183 participants contributed data on at least one cognitive domain. Intervention duration in these trials ranged from eight to 26 weeks (Blumenthal 1989; Langlois 2012; Panton 1990; Whitehurst 1991). We were able to conduct meta‐analyses for 10 of our 11 pre‐specified cognitive domains, besides perception (Analysis 6.1 to Analysis 6.10). There was no evidence of benefit of the aerobic exercise intervention in any cognitive domain.
6.1. Analysis.

Comparison 6 Fitness improved: aerobic exercise versus no intervention, Outcome 1 Cognitive speed.
6.10. Analysis.

Comparison 6 Fitness improved: aerobic exercise versus no intervention, Outcome 10 Motor function.
There was no difference in dropout rates between the aerobic exercise intervention and comparison groups (OR 1.50, 95% CI 0.50 to 4.50; three trials, Analysis 6.11).
6.11. Analysis.

Comparison 6 Fitness improved: aerobic exercise versus no intervention, Outcome 11 Drop‐out.
All analyses showed no difference on cognitive test scores between aerobic exercise groups and either active comparator or no treatment groups (controls or waiting list groups). In terms of dropout (without Panton 1990, which did not include dropouts by group), there were no differences between aerobic exercise and any of our other intervention groups. Also, no trial included adverse events as an outcome and none of the trial reports made any mention of adverse events.
Discussion
Summary of main results
This Cochrane Review examined the effect of physical activity aimed at improving cardiorespiratory fitness on cognitive function in healthy older people without known cognitive impairment. The hypothesis being tested is that physical activity brings about improvements in cognition which are mediated by increased cardiovascular (aerobic) fitness (Colcombe 2004; Kramer 1999; McAuley 2004). If true, this would imply that a physically active lifestyle resulting in enhanced fitness could positively affect people's cognitive abilities as they age and may even prevent, or at least delay, the onset of neurodegenerative disorders such as Alzheimer's disease.
Nine of the 12 included trials reported that aerobic exercise interventions resulted in increased cardiorespiratory fitness of the intervention group. This is not unexpected as significant evidence already points to exercise having a beneficial effect on cardiorespiratory fitness. However, this was not accompanied by any impact on cognitive function. Several issues need further consideration. Firstly, the quality of the included trials could have also affected our results. Reporting of methods in the included papers was generally quite poor. For all but one trial, the randomisation methods were unclear. It was not feasible to blind participants and trainers, but for most trials it was also unclear if outcome assessors were blinded, raising the risk of detection bias. Attrition was poorly reported. No trials had published protocols so it was not possible to tell if there was selective reporting of results. Of note, no included trials assessed for contamination bias which could have worked against finding group differences. Secondly, with healthy older populations, it is possible that "ceiling effects" prevented detection of cognitive improvement. The risk of this will depend on the task used and what is being measured. In the included papers, no trial author discussed any potential impact of a ceiling effect on the variables measured. However, there was much variation in each measure included in our analyses which makes ceiling effects unlikely.
Agreements and disagreements with other studies or reviews
Five meta‐analytic studies and one systematic review published data based on very similar hypotheses yet failed to find comparable results:
Etnier 1997b included 134 articles in their review. Their aim was to give a comprehensive overview of all literature available with sufficient information to calculate effect sizes. Therefore, apart from RCTs, the review included several cross‐sectional studies. It reported data on the acute effects of exercise and data on strength and flexibility regimens as well as results for younger age groups and cognitively impaired individuals. The authors concluded that exercise has a small positive effect on cognition and with the effect size depending on the exercise paradigm, the quality of the trial, the participants and the cognitive tests used as outcome measures.
van Uffelen 2008 set out to systematically review the effect of exercise on cognitive performance in older adults with and without dementia. They found 23 papers that met their inclusion criteria. They included strength exercise interventions, trials which did not assess any fitness parameters and a trial where both groups received aerobic training, while this review did not. Their review observed exercise programmes in healthy older adults improved memory, information processing abilities and executive function.
Smith 2010 meta‐analytic review assessed the effects of aerobic exercise on cognitive performance. Their criteria differed from this Cochrane Review in including participants with MCI, younger participants and trials which did not assess cardiorespiratory fitness. They also included some unpublished trials. The authors concluded that aerobic exercise is significantly and positively related to modest improvements in attention and processing speed, executive function and memory.
The meta‐analysis presented by Colcombe 2003 included 18 studies. Their aim ("to examine the hypothesis that aerobic fitness training enhances the cognitive vitality of healthy but sedentary older adults") and exclusion criteria (cross‐sectional design, no random assignment, unsupervised exercise programme, training lacking in fitness component and an average age below 55) were similar to ours. The reviews differed in that we excluded trials in which allocation was clearly quasi‐randomised or did not present any fitness parameter. We also excluded interventions that were not purely exercise and which included participants who were cognitively impaired or suffered from depression. Colcombe 2003 concluded that physical activity is beneficial for all analysed cognitive functions.
Etnier 2006 published a meta‐analytic review on the relationship between aerobic fitness and cognitive performance. Their primary goal was "to provide a statistically powerful test of the viability of the cardiovascular fitness hypothesis by examining the dose‐response relationship between aerobic fitness and cognition". Their search identified 30 studies which reported data on cross‐sectional comparisons, pre‐post comparisons and RCTs. Etnier 2006 included only those studies which assessed aerobic fitness by maximal, submaximal or a composite measure of fitness which included VO2 max, whereas we included all measures of aerobic fitness. We imposed a lower age limit and did not include trials on depressed participants whereas Etnier 2006 included all ages and at least one trial on depressed subjects. Etnier 2006 included unpublished master theses and doctoral dissertations, whereas we only included data published in peer reviewed journals. Post‐test comparisons showed no significant relationships between aerobic fitness and cognitive performance. For the exercise groups, increased fitness was associated with worse cognitive function. Age interacted with fitness and was a significant negative predictor of cognitive performance for older adults.
Although we did not identify any relationship between physical activity or cardiorespiratory fitness and cognitive function, it is possible that certain subgroups of the population, such as those starting from a lower baseline of fitness, could react differently to aerobic training. Other factors which might influence the relationship include: age, frequency of cognitive activities (Christensen 1993; Hultsch 1993; Hultsch 1999; Lachman 2010; Marquine 2012; Wilson 1999; Wilson 2005), social network (Crooks 2008; Seeman 2001), and adherence to a Mediterranean diet (Panagiotakos 2007; Tangney 2011). The search for possible subgroups has provided some promising results (examples in Etnier 2007; Podewils 2005; Schuit 2001).
It is possible that the intensity of physical activities is important (Angevaren 2007; Brown 2012; Tierney 2010; van Gelder 2004) which may have implications for the effectiveness of some of the training programmes in the included RCTs. However, Smith 2010 did not find any relationship between intensity of physical activity and change in cognitive function.
Authors' conclusions
Implications for practice.
We found no evidence that improving cardiorespiratory fitness necessarily results in improvements in cognitive performance in healthy older adults without known cognitive impairment.
Implications for research.
We consider that larger studies with robust methodology exploring possible moderators are still required to confirm whether or not aerobic training improves cognition in this population.
We wish to emphasise two important points:
Our review includes results from as many as 40 different cognitive tests. This is already a smaller sample of tests than the absolute total reported in the included trials (tests were lost from analyses in order to avoid double representation of trials within cognitive categories). A broad battery of tests can give insight into the specificity of physical activity effects. At the same time, too great a number of cognitive tests can be confusing and obscure overall effects. We would recommend that researchers in the field seek agreement on a smaller battery of cognitive tests to use in order to increase comparability between trials. This smaller core‐set of cognitive tests should incorporate measures of key cognitive domains which are important both scientifically and clinically.
Any intervention that is to be effective against age‐related cognitive decline should be assessed over a significant period of time. A limitation of the included RCTs is the lack of long‐term follow‐up (with an average duration of 15.62 weeks). Longer‐term intervention trials would be very valuable in the future.
What's new
| Date | Event | Description |
|---|---|---|
| 14 April 2015 | New search has been performed | We performed a literature search update in November 2014. We have put the search results into the Studies awaiting classification section of this review. We will fully incorporate them into the next review update. |
| 14 April 2015 | New citation required but conclusions have not changed | We performed a literature search update in November 2014. We have put the search results into the Studies awaiting classification section of this review. We will fully incorporate them into the next review update. The conclusions are unchanged. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 24 August 2013 | New search has been performed | A new update search was performed for this review on 24 August 2013 |
| 17 December 2008 | New citation required but conclusions have not changed | The update rendered one study (Oken 2006) which met the inclusion criteria. The results of the review have slightly changed. |
| 17 December 2008 | Amended | Incorporation of the risk of bias tables for all included studies |
| 15 July 2008 | New search has been performed | A new update search was performed for this review on 15 July 2008 |
| 10 April 2008 | New search has been performed | The delayed memory functions data have been corrected |
| 10 April 2008 | New citation required and conclusions have changed | Errors in the data entry for the outcome delayed memory function have been corrected. The effect of physical exercise on this outcome are not statistically significant |
Acknowledgements
We thank Jenny McCleery, Co‐ordinating Editor of the Cochrane Dementia and Cognitive Improvement Group (CDCIG), for assistance. We are grateful to Anna Noel‐Storr, Trials Search Coordinator, for her initial assessment of trials identified by searches in this iteration. We thank Sue Marcus, Managing Editor of CDCIG, for assistance. Also, we thank Geert Aufdemkampe, HJJ Verhaar, A Aleman and Luc Vanhees for their help with a previous version of this manuscript.
Appendices
Appendix 1. Search strategies: August 2013
| Source | Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) ‐ all dates to August 2013 | Keyword search: "physical activity" OR exercise | 8 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946 to August 2013 (Ovid SP) | 1. exercise.mp. or Exercise/ 2. exercis*.mp. 3. motor activit*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 4. leisure activit*.mp. 5. physical fitness.mp. 6. physical endurance.mp. or Physical Endurance/ 7. exercise tolerance.mp. or Exercise Tolerance/ 8. aerobic.mp. 9. physical activity.mp. 10. Motor Activity/ 11. physical capacity.mp. 12. physical performance training.mp. 13. cognit*.mp. 14. Mental Processes/ or mental process*.mp. 15. maximal VO2.mp. 16. METS.mp. 17. Watts.mp. 18. treadmill speed.mp. 19. inclination.mp. 20. Adult/ or adult*.mp. 21. middle aged.mp. or Middle Aged/ 22. aged.mp. or Aged/ 23. elderly.mp. 24. old*.mp. 25. geriatric.mp. or Geriatrics/ 26. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 27. 13 or 14 or 15 or 16 or 17 or 18 or 19 28. 20 or 21 or 22 or 23 or 24 or 25 29. 26 and 27 and 28 30. randomised controlled trial.pt. 31. controlled clinical trial.pt. 32. randomized.ab. 33. placebo.ab. 34. drug therapy.fs. 35. randomly.ab. 36. trial.ab. 37. groups.ab. 38. or/30‐37 39. 29 and 38 40. (2012* or 2013*).ed. 41. 39 and 40 |
650 |
| 3. EMBASE 1974 to 2013 week 27 (Ovid SP) |
1. exercise.mp. or Exercise/ 2. exercis*.mp. 3. motor activit*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 4. leisure activit*.mp. 5. physical fitness.mp. 6. physical endurance.mp. or Physical Endurance/ 7. exercise tolerance.mp. or Exercise Tolerance/ 8. aerobic.mp. 9. physical activity.mp. 10. Motor Activity/ 11. physical capacity.mp. 12. physical performance training.mp. 13. cognit*.mp. 14. Mental Processes/ or mental process*.mp. 15. maximal VO2.mp. 16. METS.mp. 17. Watts.mp. 18. treadmill speed.mp. 19. inclination.mp. 20. Adult/ or adult*.mp. 21. middle aged.mp. or Middle Aged/ 22. aged.mp. or Aged/ 23. elderly.mp. 24. old*.mp. 25. geriatric.mp. or Geriatrics/ 26. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 27. 13 or 14 or 15 or 16 or 17 or 18 or 19 28. 20 or 21 or 22 or 23 or 24 or 25 29. 26 and 27 and 28 30. "randomi?ed controlled trial".mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 31. "controlled clinical trial".mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 32. random*.mp. 33. randomised controlled trial/ 34. clinical trial.mp. 35. or/30‐34 36. 29 and 35 37. (2012* or 2013*).em. 38. 36 and 37 |
750 |
| 4. PSYCINFO 1806 to August week 5 2011 (Ovid SP) |
1. exercise.mp. or Exercise/ 2. exercis*.mp. 3. motor activit*.mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] 4. leisure activit*.mp. 5. physical fitness.mp. 6. physical endurance.mp. or Physical Endurance/ 7. exercise tolerance.mp. or Exercise Tolerance/ 8. aerobic.mp. 9. physical activity.mp. 10. Motor Activity/ 11. physical capacity.mp. 12. physical performance training.mp. 13. cognit*.mp. 14. Mental Processes/ or mental process*.mp. 15. maximal VO2.mp. 16. METS.mp. 17. Watts.mp. 18. treadmill speed.mp. 19. inclination.mp. 20. Adult/ or adult*.mp. 21. middle aged.mp. or Middle Aged/ 22. aged.mp. or Aged/ 23. elderly.mp. 24. old*.mp. 25. geriatric.mp. or Geriatrics/ 26. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 27. 13 or 14 or 15 or 16 or 17 or 18 or 19 28. 20 or 21 or 22 or 23 or 24 or 25 29. 26 and 27 and 28 30. "randomi?ed controlled trial".mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] 31. "controlled clinical trial".mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] 32. random*.mp. 33. randomised controlled trial/ 34. clinical trial.mp. 35. 30 or 31 or 32 or 33 or 34 36. 29 and 35 37. (2012* or 2013*).up. 38. 36 and 37 |
92 |
| 5. CINAHL (EBSCOhost) to August 2013 | S1 TX exercis* S2 TX "physical activit*" S3 TX cycling S4 TX swim* S5 TX gym* S6 TX walk* OR treadmill S7 TX danc* S8 TX yoga* S9 TX "tai chi" S10 (MH "Exercise+") S11 (MH "Clinical Trials") S12 TX trial S13 TX RCT OR CCT S14 TX placebo* S15 TX "double‐blind*" OR "single‐blind*" S16 TX groups OR "control group" S17 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 S18 S11 OR S12 OR S13 OR S14 OR S15 OR S16 S19 TX elderly S20 (MH "Aged") S21 TX geriatric S22 S19 OR S20 OR S21 S23 TX cognition S24 cognition S25 (MH "Cognition") S26 TX cognitive S27 AB brain OR mental OR memory OR "executive function*" S28 S23 OR S24 OR S25 OR S26 OR S27 S29 S17 AND S18 AND S22 AND S28 S30 EM 2012 S31 EM 2013 S32 S30 OR S31 S33 S29 AND S32 |
213 |
| 6. Web of Science (1945 to August 2013) (ISI Web of Knowledge) | Topic=("physical activity" OR "physical exercise" OR cycling OR yoga OR swim* OR danc* OR aerobic*) AND Topic=(cogni* OR elderly OR memory OR geriatric) AND Topic=(randomly OR trial OR RCT) Timespan=2012‐2013. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, CCR‐EXPANDED, IC. |
869 |
| 7. LILACS (BIREME) All dates to August 2013 | "exercício físico" OR "physical exercise" OR aerobic$ OR aeróbico OR aerobio OR yoga OR "physical activit$" OR "actividad física" OR "atividade física" [Words] and randomised OR randomized OR trial OR randomly OR groups [Words] and elderly OR idoso OR anciano [Words] | 165 |
| 8. CENTRAL (the Cochrane Library; Issue 2 of 4, 2013) | #1 "cognit* impair*" #2 MeSH descriptor: [Cognition Disorders] explode all trees #3 MCI #4 ACMI #5 ARCD #6 SMC #7 CIND #8 BSF #9 AAMI #10 LCD #11 QD or "questionable dementia" #12 AACD #13 MNCD #14 MCD #15 "N‐MCI" or "A‐MCI" or "M‐MCI" #16 (cognit* or memory or cerebr* or mental*) near/3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*) #17 "preclinical AD" #18 "pre‐clinical AD" #19 "preclinical alzheimer*" or "pre‐clinical alzheimer*" #20 aMCI or MCIa #21 "CDR 0.5" or "clinical dementia rating scale 0.5" #22 "GDS 3" or "stage 3 GDS" #23 "global deterioration scale" and "stage 3" #24 "Benign senescent forgetfulness" #25 "mild neurocognit* disorder*" #26 (prodrom* near/2 dement*) #27 episodic* near/2 memory #28 "preclinical dementia" or "pre‐clinical dementia" #29 episodic near/2 memory #30 "pre‐clinical dementia" or "preclinical dementia" #31 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 #32 "Physical therap*" #33 "physical activit*" #34 fitness #35 exercis* #36 aerobic #37 "physical* fit*" #38 "physical capacity" #39 "physical training" #40 Cycling #41 swim* #42 gym* #43 danc* #44 yoga #45 "tai chi" #46 walk* #47 flexibility #48 motor* #49 "leisure activit*" #50 "physical endurance" #51 MeSH descriptor: [Exercise Therapy] explode all trees #52 #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 in Trials #53 #31 and #52 from 2009 to 2011, in Trials #54 #52 and (brain or MMSE or cognition or cognitive or memory) from 2012 to 2013, in Trials |
191 |
| 9. ClinicalTrials.gov (www.clinicaltrials.gov) All dates to August 2013 | Interventional Studies | cognition OR cognitive OR memory OR MMSE OR brain | "Physical therapy" OR "physical activity" OR "physical exercise" OR cycling OR yoga OR swim OR swimming OR dance OR aerobic | Adult, Senior | received from 01/01/2012 to 08/03/2013 | 273 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] All dates to August 2013 | Interventional Studies | cognition OR cognitive | "Physical therapy" OR "physical activity" OR "physical exercise" OR cycling OR yoga OR swim OR swimming OR dance OR aerobic | Adult, Senior | received from 01/01/2012 to 08/03/2013 | 187 |
| TOTAL before removal of duplicates and first assessment | 3398 | |
| TOTAL after removal of duplicates and first assessment | 352 | |
Data and analyses
Comparison 1. Aerobic exercise versus any active intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive speed | 6 | 389 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.08, 0.33] |
| 1.1 Simple reaction time | 2 | 113 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.28, 0.46] |
| 1.2 Choice reaction time | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Trailmaking part A | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.96, 0.24] |
| 1.4 Digit symbol substitution | 3 | 227 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.03, 0.50] |
| 2 Verbal memory functions (immediate) | 5 | 292 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.38, 0.55] |
| 2.1 16 words immediate recall | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Randt Memory test story recall | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.15, 0.83] |
| 2.3 Ross Information Processing Assessment immediate memory | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.18, 1.37] |
| 2.4 Wechsler Adult Intelligence Scales logical memory immediate recall | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐2.36, ‐0.45] |
| 2.5 Rey auditory verbal learning trial I‐V | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.25, 0.45] |
| 2.6 Hopkins Verbal Learning Test (immediate) | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.27, 0.94] |
| 3 Visual memory functions (immediate) | 2 | 89 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.97, 0.44] |
| 3.1 Benton visual retention (#error) | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.47, 0.50] |
| 3.2 Wechsler Memory Scales visual reproduction | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.61, 0.15] |
| 4 Working memory | 3 | 238 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.16, 0.36] |
| 4.1 Digit span backward | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.13, 0.45] |
| 4.2 2‐Back (accuracy, Hits ‐ False Alarms) | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.74, 0.46] |
| 5 Memory functions (delayed) | 3 | 249 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.16, 0.35] |
| 5.1 16 words delayed recall | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Rey auditory verbal learning delayed recall trial | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.17, 0.54] |
| 5.3 10 words delayed recall | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.55, 0.35] |
| 5.4 Hopkins Verbal Learning Test ‐ 12 words (delayed) | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.42, 0.78] |
| 6 Executive functions | 6 | 367 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.14, 0.90] |
| 6.1 Trailmaking part B | 2 | 113 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.11, 0.65] |
| 6.2 Ross Information Processing Assessment problem solving and abstract reasoning | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 2.75 [1.69, 3.82] |
| 6.3 Wechsler Memory Scales mental control | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.16, 0.55] |
| 6.4 Task switching paradigm (accuracy) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.32, 0.38] |
| 6.5 Verbal fluency | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Letter number sequencing | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.38, 0.52] |
| 7 Perception | 3 | 178 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.50, 0.48] |
| 7.1 Face recognition (delayed recall) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.53] |
| 7.2 Ross Information Processing Assessment auditory processing | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.55, 0.97] |
| 7.3 Wechsler Adult Intelligence Scales visual reproduction | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.61, 0.15] |
| 8 Cognitive inhibition | 4 | 314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.28, 0.17] |
| 8.1 Stroop colour word (interference) | 2 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.46, 0.20] |
| 8.2 Stopping task (accuracy choice RT) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.35, 0.36] |
| 8.3 Flanker Task (Incongruent RT) | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.59, 0.60] |
| 9 Visual attention | 3 | 265 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.03, 0.46] |
| 9.1 Digit vigilance | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Tracking (accuracy index) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 2&7 test | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.19, 0.79] |
| 9.4 Visual search (accuracy) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.10, 0.60] |
| 9.5 Covert orienting of visuospatial attention | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.36, 0.54] |
| 10 Auditory attention | 4 | 251 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.38, 0.69] |
| 10.1 Digit span forward | 4 | 251 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.38, 0.69] |
| 11 Motor function | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.20, 0.37] |
| 11.1 Finger tapping | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.30, 0.68] |
| 11.2 Pursuit rotor task (tracking error) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.33, 0.38] |
| 12 Drop‐out | 7 | 469 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.44, 2.10] |
1.2. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 2 Verbal memory functions (immediate).
1.3. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 3 Visual memory functions (immediate).
1.4. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 4 Working memory.
1.5. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 5 Memory functions (delayed).
1.6. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 6 Executive functions.
1.7. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 7 Perception.
1.8. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 8 Cognitive inhibition.
1.9. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 9 Visual attention.
1.10. Analysis.

Comparison 1 Aerobic exercise versus any active intervention, Outcome 10 Auditory attention.
Comparison 2. Aerobic exercise versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive speed | 5 | 260 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.16, 0.41] |
| 1.1 Simple reaction time | 2 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.47, 0.29] |
| 1.2 Choice reaction time | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.60, 0.54] |
| 1.3 Trailmaking part A | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.15, 0.78] |
| 1.4 Digit symbol substitution | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.05, 0.94] |
| 2 Verbal memory functions (immediate) | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.24, 0.43] |
| 2.1 Randt Memory test story recall | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.53, 0.45] |
| 2.2 16 words immediate recall | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Ross Information Processing Assessment immediate memory | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Wechsler Adult Intelligence Scales logical memory immediate recall | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Rey auditory verbal learning trial I‐V | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.25, 0.67] |
| 2.6 Hopkins Verbal Learning Test (immediate) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Visual memory functions (immediate) | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.57, 0.40] |
| 3.1 Benton visual retention (#error) | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.57, 0.40] |
| 3.2 Wechsler Memory Scales visual reproduction | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Working memory | 2 | 137 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.54, 1.15] |
| 4.1 Digit span backward | 2 | 137 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.54, 1.15] |
| 4.2 2‐Back (accuracy, Hits ‐ False Alarms) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Memory functions (delayed) | 2 | 152 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.23, 0.41] |
| 5.1 16 words delayed recall | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Rey auditory verbal learning delayed recall trial | 1 | 72 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.21, 0.72] |
| 5.3 10 words delayed recall | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.49, 0.38] |
| 5.4 Hopkins Verbal Learning Test ‐ 12 words (delayed) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Executive functions | 3 | 217 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.16, 0.53] |
| 6.1 Trailmaking part B | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.16, 0.76] |
| 6.2 Ross Information Processing Assessment problem solving and abstract reasoning | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Wechsler Memory Scales mental control | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Task switching paradigm (accuracy) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 Verbal fluency | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Letter number sequencing | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.47, 0.41] |
| 7 Cognitive inhibition | 3 | 217 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.06, 0.47] |
| 7.1 Stroop colour word (interference) | 3 | 217 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.06, 0.47] |
| 7.2 Stopping task (accuracy choice RT) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Flanker Task (Incongruent RT) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Visual attention | 3 | 155 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.26, 0.37] |
| 8.1 Digit vigilance | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Tracking (accuracy index) | 1 | 10 | Std. Mean Difference (IV, Random, 95% CI) | 0.76 [‐0.55, 2.07] |
| 8.3 2&7 test | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.44, 0.53] |
| 8.4 Visual search (accuracy) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 Covert orienting of visuospatial attention | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.45, 0.42] |
| 9 Auditory attention | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐1.01, 1.33] |
| 9.1 Digit span forward | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐1.01, 1.33] |
| 10 Motor function | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐7.87, 8.08] |
| 10.1 Finger tapping | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐7.87, 8.08] |
| 10.2 Pursuit rotor task (tracking error) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Drop‐out | 5 | 267 | Odds Ratio (IV, Random, 95% CI) | 1.84 [0.79, 4.29] |
2.2. Analysis.

Comparison 2 Aerobic exercise versus no intervention, Outcome 2 Verbal memory functions (immediate).
2.3. Analysis.

Comparison 2 Aerobic exercise versus no intervention, Outcome 3 Visual memory functions (immediate).
2.4. Analysis.

Comparison 2 Aerobic exercise versus no intervention, Outcome 4 Working memory.
2.5. Analysis.

Comparison 2 Aerobic exercise versus no intervention, Outcome 5 Memory functions (delayed).
2.6. Analysis.

Comparison 2 Aerobic exercise versus no intervention, Outcome 6 Executive functions.
2.7. Analysis.

Comparison 2 Aerobic exercise versus no intervention, Outcome 7 Cognitive inhibition.
2.8. Analysis.

Comparison 2 Aerobic exercise versus no intervention, Outcome 8 Visual attention.
2.9. Analysis.

Comparison 2 Aerobic exercise versus no intervention, Outcome 9 Auditory attention.
Comparison 3. Aerobic exercise versus flexibility/balance programme.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive speed | 3 | 265 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.01, 0.47] |
| 1.1 Simple reaction time | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.27, 0.63] |
| 1.2 Choice reaction time | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Trailmaking part A | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Digit symbol substitution | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.04, 0.54] |
| 2 Verbal memory functions (immediate) | 3 | 209 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.09, 0.80] |
| 2.1 Randt Memory test story recall | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.15, 0.83] |
| 2.2 16 words immediate recall | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Ross Information Processing Assessment immediate memory | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 1.14 [0.18, 2.10] |
| 2.4 Wechsler Adult Intelligence Scales logical memory immediate recall | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Rey auditory verbal learning trial I‐V | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.25, 0.45] |
| 3 Visual memory functions (immediate) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.65, 1.76] |
| 3.1 Benton visual retention (#error) | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.65, 1.76] |
| 3.2 Wechsler Memory Scales visual reproduction | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Working memory | 2 | 189 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.41, 1.12] |
| 4.1 Digit span backward | 2 | 189 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.41, 1.12] |
| 5 Memory functions (delayed) | 2 | 200 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.20, 0.36] |
| 5.1 16 words delayed recall | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Rey auditory verbal learning delayed recall trial | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.17, 0.54] |
| 5.3 10 words delayed recall | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.55, 0.35] |
| 6 Executive functions | 4 | 285 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.09, 0.55] |
| 6.1 Trailmaking part B | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.13, 0.85] |
| 6.2 Ross Information Processing Assessment problem solving and abstract reasoning | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 1.08 [0.13, 2.03] |
| 6.3 Wechsler Memory Scales mental control | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Task switching paradigm (accuracy) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.32, 0.38] |
| 6.5 Verbal fluency | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Letter number sequencing | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.38, 0.52] |
| 7 Perception | 2 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.11, 0.54] |
| 7.1 Face recognition (delayed recall) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.53] |
| 7.2 Ross Information Processing Assessment auditory processing | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.41, 1.38] |
| 7.3 Wechsler Adult Intelligence Scales visual reproduction | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Cognitive inhibition | 3 | 265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.31, 0.18] |
| 8.1 Stroop colour word (interference) | 2 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.46, 0.20] |
| 8.2 Stopping task (accuracy choice RT) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.35, 0.36] |
| 9 Visual attention | 3 | 265 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.03, 0.46] |
| 9.1 Digit vigilance | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Tracking (accuracy index) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 2&7 test | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.19, 0.79] |
| 9.4 Visual search (accuracy) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.10, 0.60] |
| 9.5 Covert orienting of visuospatial attention | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.36, 0.54] |
| 10 Auditory attention | 2 | 189 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.83, 0.49] |
| 10.1 Digit span forward | 2 | 189 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.83, 0.49] |
| 11 Motor function | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.20, 0.37] |
| 11.1 Finger tapping | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.30, 0.68] |
| 11.2 Pursuit rotor task (tracking error) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.33, 0.38] |
| 12 Drop‐out | 4 | 351 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.58, 1.72] |
3.2. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 2 Verbal memory functions (immediate).
3.3. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 3 Visual memory functions (immediate).
3.4. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 4 Working memory.
3.5. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 5 Memory functions (delayed).
3.6. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 6 Executive functions.
3.7. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 7 Perception.
3.8. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 8 Cognitive inhibition.
3.9. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 9 Visual attention.
3.10. Analysis.

Comparison 3 Aerobic exercise versus flexibility/balance programme, Outcome 10 Auditory attention.
Comparison 4. Aerobic exercise versus strength programme.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Verbal memory functions (immediate) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐4.17, 4.77] |
| 1.1 Randt Memory test story recall | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 16 words immediate recall | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Ross Information Processing Assessment immediate memory | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐4.17, 4.77] |
| 1.4 Wechsler Adult Intelligence Scales logical memory immediate recall | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Rey auditory verbal learning trial I‐V | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Executive functions | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐4.49, ‐0.11] |
| 2.1 Trailmaking part B | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Ross Information Processing Assessment problem solving and abstract reasoning | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐4.49, ‐0.11] |
| 2.3 Wechsler Memory Scales mental control | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Word comparison (#error) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Task switching paradigm (accuracy) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Verbal fluency | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Perception | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.93, 1.93] |
| 3.1 Face recognition (delayed recall) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Ross Information Processing Assessment auditory processing | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.93, 1.93] |
| 3.3 Wechsler Adult Intelligence Scales visual reproduction | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cognitive speed | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐27.93, 19.93] |
| 4.1 Simple reaction time | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐27.93, 19.93] |
4.1. Analysis.

Comparison 4 Aerobic exercise versus strength programme, Outcome 1 Verbal memory functions (immediate).
4.2. Analysis.

Comparison 4 Aerobic exercise versus strength programme, Outcome 2 Executive functions.
4.3. Analysis.

Comparison 4 Aerobic exercise versus strength programme, Outcome 3 Perception.
4.4. Analysis.

Comparison 4 Aerobic exercise versus strength programme, Outcome 4 Cognitive speed.
Comparison 5. Fitness Improved: aerobic exercise versus any active intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive speed | 4 | 275 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.22, 0.37] |
| 1.1 Simple reaction time | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.75, 0.54] |
| 1.2 Choice reaction time | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Trailmaking part A | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.96, 0.24] |
| 1.4 Digit symbol substitution | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.05, 0.52] |
| 2 Verbal memory functions (immediate) | 5 | 292 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.38, 0.55] |
| 2.1 16 words immediate recall | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Randt Memory test story recall | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.15, 0.83] |
| 2.3 Ross Information Processing Assessment immediate memory | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.18, 1.37] |
| 2.4 Wechsler Adult Intelligence Scales logical memory immediate recall | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐2.36, ‐0.45] |
| 2.5 Rey auditory verbal learning trial I‐V | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.25, 0.45] |
| 2.6 Hopkins Verbal Learning Test (immediate) | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.27, 0.94] |
| 3 Visual memory functions (immediate) | 2 | 89 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐2.04, 0.87] |
| 3.1 Benton visual retention (#error) | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐1.65, 1.76] |
| 3.2 Wechsler Memory Scales visual reproduction | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐3.50, 0.60] |
| 4 Working memory | 3 | 238 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.16, 0.36] |
| 4.1 Digit span backward | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.13, 0.45] |
| 4.2 2‐Back (accuracy, Hits ‐ False Alarms) | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.74, 0.46] |
| 5 Memory functions (delayed) | 2 | 173 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.29, 1.25] |
| 5.1 16 words delayed recall | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Rey auditory verbal learning delayed recall trial | 1 | 124 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.44, 1.44] |
| 5.3 10 words delayed recall | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Hopkins Verbal Learning Test ‐ 12 words (delayed) | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.94, 1.82] |
| 6 Executive functions | 5 | 291 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.18, 1.15] |
| 6.1 Trailmaking part B | 2 | 113 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.11, 0.65] |
| 6.2 Ross Information Processing Assessment problem solving and abstract reasoning | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 2.75 [1.69, 3.82] |
| 6.3 Wechsler Memory Scales mental control | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.16, 0.55] |
| 6.4 Task switching paradigm (accuracy) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.32, 0.38] |
| 6.5 Verbal fluency | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Letter number sequencing | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Perception | 3 | 178 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.50, 0.48] |
| 7.1 Face recognition (delayed recall) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.53] |
| 7.2 Ross Information Processing Assessment auditory processing | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.55, 0.97] |
| 7.3 Wechsler Adult Intelligence Scales visual reproduction | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.61, 0.15] |
| 8 Cognitive inhibition | 3 | 238 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.27, 0.24] |
| 8.1 Stroop colour word (interference) | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.55, 0.42] |
| 8.2 Stopping task (accuracy choice RT) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.35, 0.36] |
| 8.3 Flanker Task (Incongruent RT) | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.59, 0.60] |
| 9 Visual attention | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.02, 0.56] |
| 9.1 Digit vigilance | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Tracking (accuracy index) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 2&7 test | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.19, 0.79] |
| 9.4 Visual search (accuracy) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.10, 0.60] |
| 9.5 Covert orienting of visuospatial attention | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Auditory attention | 3 | 213 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.49, 0.79] |
| 10.1 Digit span forward | 3 | 213 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.49, 0.79] |
| 11 Motor function | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.20, 0.37] |
| 11.1 Finger tapping | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.30, 0.68] |
| 11.2 Pursuit rotor task (tracking error) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.33, 0.38] |
| 12 Drop‐out | 5 | 330 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.66, 2.25] |
5.2. Analysis.

Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 2 Verbal memory functions (immediate).
5.3. Analysis.

Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 3 Visual memory functions (immediate).
5.4. Analysis.

Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 4 Working memory.
5.5. Analysis.

Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 5 Memory functions (delayed).
5.6. Analysis.
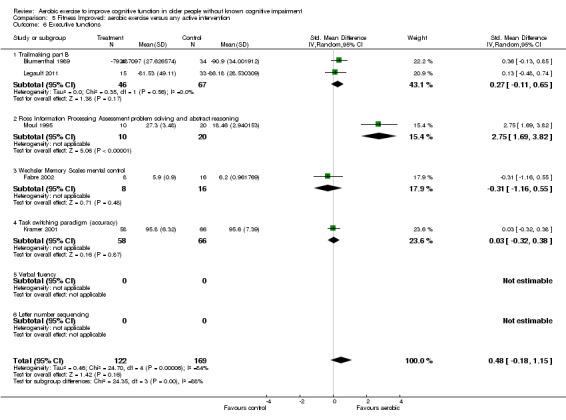
Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 6 Executive functions.
5.7. Analysis.

Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 7 Perception.
5.8. Analysis.

Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 8 Cognitive inhibition.
5.9. Analysis.

Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 9 Visual attention.
5.10. Analysis.

Comparison 5 Fitness Improved: aerobic exercise versus any active intervention, Outcome 10 Auditory attention.
Comparison 6. Fitness improved: aerobic exercise versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive speed | 4 | 180 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.05, 0.55] |
| 1.1 Simple reaction time | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.71, 0.76] |
| 1.2 Choice reaction time | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.60, 0.54] |
| 1.3 Trailmaking part A | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.15, 0.78] |
| 1.4 Digit symbol substitution | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.05, 0.94] |
| 2 Verbal memory functions (immediate) | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.24, 0.43] |
| 2.1 Randt Memory test story recall | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.53, 0.45] |
| 2.2 16 words immediate recall | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Ross Information Processing Assessment immediate memory | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Wechsler Adult Intelligence Scales logical memory immediate recall | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Rey auditory verbal learning trial I‐V | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.25, 0.67] |
| 2.6 Hopkins Verbal Learning Test (immediate) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Visual memory functions (immediate) | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐1.87, 1.30] |
| 3.1 Benton visual retention (#error) | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐1.87, 1.30] |
| 3.2 Wechsler Memory Scales visual reproduction | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Working memory | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.21, 0.46] |
| 4.1 Digit span backward | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.21, 0.46] |
| 4.2 2‐Back (accuracy, Hits ‐ False Alarms) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Memory functions (delayed) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.92 [‐0.75, 2.59] |
| 5.1 Rey auditory verbal learning delayed recall trial | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.92 [‐0.75, 2.59] |
| 6 Executive functions | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.16, 0.76] |
| 6.1 Trailmaking part B | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.16, 0.76] |
| 6.2 Ross Information Processing Assessment problem solving and abstract reasoning | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Wechsler Memory Scales mental control | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Task switching paradigm (accuracy) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 Verbal fluency | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Letter number sequencing | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Cognitive inhibition | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.04, 0.63] |
| 7.1 Stroop colour word (interference) | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.04, 0.63] |
| 7.2 Stopping task (accuracy choice RT) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Flanker Task (Incongruent RT) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Visual attention | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.44, 0.53] |
| 8.1 Digit vigilance | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Tracking (accuracy index) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 2&7 test | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.44, 0.53] |
| 8.4 Visual search (accuracy) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 Covert orienting of visuospatial attention | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Auditory attention | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐1.01, 1.33] |
| 9.1 Digit span forward | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐1.01, 1.33] |
| 10 Motor function | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐7.87, 8.08] |
| 10.1 Finger tapping | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐7.87, 8.08] |
| 10.2 Pursuit rotor task (tracking error) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Drop‐out | 3 | 164 | Odds Ratio (IV, Random, 95% CI) | 1.50 [0.50, 4.50] |
6.2. Analysis.

Comparison 6 Fitness improved: aerobic exercise versus no intervention, Outcome 2 Verbal memory functions (immediate).
6.3. Analysis.

Comparison 6 Fitness improved: aerobic exercise versus no intervention, Outcome 3 Visual memory functions (immediate).
6.4. Analysis.

Comparison 6 Fitness improved: aerobic exercise versus no intervention, Outcome 4 Working memory.
6.5. Analysis.

Comparison 6 Fitness improved: aerobic exercise versus no intervention, Outcome 5 Memory functions (delayed).
6.6. Analysis.

Comparison 6 Fitness improved: aerobic exercise versus no intervention, Outcome 6 Executive functions.
6.7. Analysis.

Comparison 6 Fitness improved: aerobic exercise versus no intervention, Outcome 7 Cognitive inhibition.
6.8. Analysis.

Comparison 6 Fitness improved: aerobic exercise versus no intervention, Outcome 8 Visual attention.
6.9. Analysis.

Comparison 6 Fitness improved: aerobic exercise versus no intervention, Outcome 9 Auditory attention.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bakken 2001.
| Methods | Parallel‐group RCT: 1 intervention group and 1 control group. At randomisation 15 enrolled; 8 in the aerobic exercise group, 7 in the control group. Follow‐up: 8 weeks | |
| Participants | 10 participants (4 males, 6 females) in the age range of 72 to 91 years from a senior housing complex in Minneapolis, Minnesota. Inclusion criteria: > 65 years of age with no history of pulmonary disease, recurring falls, orthopaedic limitations or acute arthritis in the hands. | |
| Interventions | Aerobic exercise: 1 hour sessions for 3 sessions per week for 8 consecutive weeks. 10 minutes of warming up, aerobic conditioning period that increased in duration and intensity (callisthenics, walking and cycling) systematically each week, 10 minutes of cooling down. Subjects heart rates did not exceed the upper limit of their THRR*. Control: continued their normal everyday routine, which did not include any aerobic exercise according to the subjects report. | |
| Outcomes | AI (Accuracy Index by finger movement) Resting heart rate Resting systolic BP Resting rate‐pressure product GXT test heart rate** GXT test systolic BP GXT test RPP (Rate‐Pressure product) | |
| Notes | Testing took place at the University of Minnesota. *THRR: (Karvonen) training HR = resting HR + [0.60‐0.75 (HRR)]. HRR = age‐predicted max HR ‐ resting HR **GXT: submaximal graded exercise tolerance test. Stage 1; stepping back and forth on the ground at a frequency of 20 mounts per minute for 3 minutes. Stage 2: stepping up and down a 10.16 cm high step. Stage 3: stepping up and down a 20.32 cm high step. Stage 4: stepping up and down a 30.48 cm high step. RPP; rate‐pressure product = systolic BP multiplied by heart rate. A decrease in RPP is a quantitative measure of aerobic training. Both groups showed slight increases in RPP from pre‐test to post‐test. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | Cognition was assessed with a computer and therefore adequately blinded. At the same time the researchers where unaware of the group assignment of the participants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/8 participants were lost from the exercise condition, 2/7 were lost in the control group. Main outcomes were not analysed according to the ITT principle. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk | However, we cannot rule out contamination bias. |
Blumenthal 1989.
| Methods | Parallel‐group RCT: 2 intervention groups and 1 control group. 101 participants (50 males and 51 females) were randomised either to aerobic exercise (N = 33), yoga/flexibility (N = 34) or control (N = 34). Follow‐up: 16 weeks |
|
| Participants | 101 participants aged 60 to 83 years Inclusion criteria: free from clinical manifestations of coronary disease assessed by medical history, physical examination, bicycle ergometry exercise testing. Exclusion criteria: positive ECG during exercise testing, evidence of coronary artery disease, asthma, pulmonectomy, uncontrolled hypertension, beta‐blocker therapy. | |
| Interventions | Aerobic exercise: 3 supervised sessions per week for 16 weeks. Training based on 70% of max heart rate achieved on exercise test. 10 minutes of warming up, 30 minutes of bicycle ergometry, 15 minutes of brisk walking/jogging and arm ergometry, 5 minutes of cooling down. Yoga/flexibility: 2 supervised sessions a week for 60 minutes over 16 weeks. Controls: not to change their physical activity habits and especially not to engage in any aerobic exercise for the trial period. | |
| Outcomes | Tapping (dominant/non‐dominant) Digit span (forward / backward) Benton Revised Visual Retention test (correct/error) Story Recall of the Randt Memory test (immediate) ‐ data on the delayed Story Recall of the Randt Memory test could not be traced by the authors. Selective reminding test (total/intrusions) Trail making (part B) Digit Symbol substitution 2 & 7 test (digits/letters) Stroop colour word Stroop interference Verbal fluency Non‐verbal fluency VO2 max AT | |
| Notes | Testing took place at the Duke University Medical Center. A summary combination of the scores on both 2&7 test (letters and digits) was calculated and SDs were pooled and used in analysis. Subjects in the aerobic training group experienced a significant 11.6% increase in their VO2 max (from 19.4 to 21.4 mL/kg/min), whereas the participants in the yoga/flexibility and control groups experienced a 1 to 2% decrease in VO2 max (from 18.8 to 18.7 mL/kg/min and 18.5 to 17.9 mL/kg/min, respectively). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Insufficient information is provided to judge the blinding of the cognitive outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/33 participants were lost from both the aerobic group and yoga/flexibility group and 2/34 the control group. Main outcomes were not analysed according to the ITT principle. |
| Selective reporting (reporting bias) | High risk | The methods section describes assessment of the Story Recall of the Randt Memory test after 30 minutes delay. Data on this subtest could not be traced by the authors. |
| Other bias | Low risk | Although contamination bias could not be ruled out. |
Emery 1990a.
| Methods | Parallel‐group RCT: 2 intervention groups and 1 control group. 48 subjects (8 males and 40 females) were randomly assigned to an aerobic exercise programme (N = 15), social activity group (N = 15) or a control group (N = 18). Follow‐up: 12 weeks |
|
| Participants | 48 participants aged 61 to 86 years from a metropolitan inner‐city community. Inclusion criteria not described. Exclusion criteria not described. | |
| Interventions | Exercise: 3 sessions per week for approximately 60 minutes. 10 to 15 minutes of stretching exercises followed by 20 to 25 minutes of aerobic exercise (at 70% of age‐adjusted max = 220‐age), including rapid walking as well as rhythmic muscle strengthening exercises (e.g. repeatedly standing up and sitting down). 5 minutes of cooling down with dancing and light exercises. Social activity: 3 sessions per week for 60 minutes. Participation in non‐physical activities (card games, art projects, political discussion groups, watching films). Controls: not described. | |
| Outcomes | Digit Symbol substitution Digit Span Copying Words Copying Numbers (digit/sec) Weight (kg) Resting HR Resting blood pressure (syst/diast) BP during modified step test HR during modified step test Sit‐and‐reach test | |
| Notes | Testing took place at the Duke University Medical Center. Resting heart rate, maximum heart rate and systolic/diastolic blood pressure indicated no significant differences between the groups. Both groups showed a significant time main effect decrease in diastolic blood pressure, other measures indicated no significant effects. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Insufficient information provided to assess the blinding of the cognitive outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1/15 participants was lost from the aerobic group, 4/15 from the social group and 4/18 from the control group. Main outcomes were not analysed according to the ITT principle. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Low risk | Although contamination bias could not be ruled out. |
Fabre 2002.
| Methods | Parallel‐group RCT: 3 intervention groups and 1 control group. 32 participants (5 males and 27 females) at randomisation; each group (physical training, memory training, combined physical/memory training and controls) contained 8 subjects. Follow‐up: 8 weeks |
|
| Participants | 32 participants in the age range of 60 to 76 years "from clubs". Inclusion criteria are not described. Exclusion criteria: positive ECG during exercise testing, present depression, could not breathe through the tube during exercise testing, various other reasons such as disease during training. | |
| Interventions | Physical training: two supervised 1 hour exercise sessions per week for 8 weeks: walking and running to maintain target heart rate (target heart rate corresponded to the ventilatory threshold). 5 minutes of warming up, 45 minutes of walking/running, 10 minutes of cooling down. Memory training: 90 minutes of sessions once a week for 8 weeks. 15 minutes of explaining, Israel's method in core. Combined physical training and memory training. Controls: no training whatsoever. |
|
| Outcomes | Memory quotient (= total score of al WAIS subtests) Paired associates learning Digit span forward Logical memory immediate recall Orientation General information Mental control Visual reproductions VO2 max VO2 max at Vth Max O2 pulse Max O2 pulse at Vth | |
| Notes | Testing took place at the University of Montpellier. The physical training resulted in an average significant increase in VO2 max of 12% (from 1350 to 1630 mL/min) and 11% (from 1510 to 1625 mL/min) in the aerobic training group and the combined aerobic/mental group, respectively. The VO2 max scores of the participants in the other two groups were unchanged compared to initial values (mental training group from 1060 to 999 mL/min and controls from 1256 to 1265 mL/min). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Insufficient information provided to assess the blinding of the cognitive outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Low risk | Although contamination bias could not be ruled out. |
Kramer 2001.
| Methods | Parallel‐group RCT: 1 aerobic walking intervention group and 1 stretching/toning control group.174 participants at randomisation. The trial was completed by 124 individuals; 58 (13 men) in the aerobic group and 66 (20 men) in the stretching and toning group. Follow‐up period: 6 months. |
|
| Participants | 124 participants aged 60 to 75 years of age. Inclusion criteria: aged 60 to 75 years, sedentary (no physical activity in the preceding 6 months), capable of performing exercise, physicians examination and consent to participate, successful completion of graded exercise test without evidence of cardiac abnormalities, initial depression score on the GDS below clinical level, no history of neurologic disorders, corrected (near & far) acuity of 20/40 or better, fewer than three errors on the Pfeiffer Mental Status questionnaire. Exclusion criteria: younger than 60 years, self‐reported activity on a regular basis (2 times a week) in the preceding 6 months, any physical disability that prohibits mobility, non‐consent of physician, evidence of abnormal cardiac responses during graded exercise testing, depression score on the GDS indicative of clinical depression, history of neurologic disorders, corrected (near & far) acuity greater than 20/40, more than three errors on the Pfeiffer questionnaire. | |
| Interventions | Aerobic walking exercise: 3 supervised sessions per week for 6 months. Warming up, 40 minutes of brisk walking (gradually beginning at 10 to 15 minutes up to 40 minutes), cooling down. Initial exercise was performed at 50 to 55% of VO2 max and increased to 65 to 70% of VO2 max. Stretching and toning: 3 times a week supervised sessions for 6 months. The programme emphasized stretches for all the large muscle group of the upper and lower extremities. Each stretch was held for 20 to 30 seconds and repeated 5 to 10 times. Each session was proceeded and followed by 10 minutes of warm‐up and cooling down. |
|
| Outcomes | Visual search task Response compatibility task Task switching paradigm Stopping paradigm Spatial attention task Rey auditory verbal learning test Pursuit rotor task Self‐ordered pointing task Spatial working memory Verbal working memory Face recognition task Digit‐digit and digit‐symbol tests Forward and backward digit span VO2 max (mL/kg/min) Time on treadmill (min) Rockport 1‐mile walk (min) | |
| Notes | Testing took place at the University of Illinois at Urbana‐Champaign. The physical training resulted in improvements of 5.1% on VO2 max measures (from 21.5 to 22.6 mL/kg/min). The toning group showed a 2.8% decrease in VO2 max scores (from 21.8 to 21.2 mL/kg/min). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Insufficient information provided to assess the blinding of the cognitive outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25/83 subjects from walking group and 25/91 subjects from stretching/toning group were dropped from the trial because of withdrawal from the training protocol or incomplete data. These participants did not differ in demographic characteristics from those who completed the trial. Main outcomes were not analysed according to the ITT principle. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Low risk | Although contamination bias could not be ruled out. |
Langlois 2012.
| Methods | Parallel‐group RCT: 1 one exercise training intervention group and 1 waiting list control group. 83 participants at randomisation, randomised ensuring gender ratio equivalence: 43 in the intervention group, 40 in the control group. Follow‐up: 3 months |
|
| Participants | 72 participants aged 61 to 89. Inclusion criteria: assessed via complete geriatric assessment as able to perform exercise programme at low risk. Exclusion criteria: limitations to undertaking exercise programme, MMSE < 25, or GDS > 10, or both. | |
| Interventions | Physical exercise training group: 12 weeks of 1 hour exercise 3 days/week conducted in supervised 3 to 5 participant subgroups. 10 mins stretching and balancing warm up, 10 to 30 mins aerobic workout, 10 mins strength training, 10 mins cool down. Intensity and duration of aerobic workout increased individually using modified Borg RPE reaching moderate to hard intensity. Control group: maintain level of activity during period and were offered physical training programme after trial. | |
| Outcomes | MMSE WAIS‐III Similarities WAIS‐III Digit‐Symbol Coding Trailmaking part A Trailmaking part B modified Stroop Colour‐Word Test WAIS‐III Letter‐Number Sequencing Digit Span Backwards Rey Auditory Verbal Learning Task 6MWT modified Physical Performance Test Timed Up and Go Test Gait speed (comfortable and maximum) |
|
| Notes | Testing took place at the Université du Québec à Montréal. There was a significantly larger improvement in the exercise training group in comparison to the control group in the 6MWT. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Insufficient information provided to assess the blinding of the cognitive outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/43 participants was lost from the intervention group, 4/40 from the from the control group. Main outcomes were not analysed according to the ITT principle. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Low risk | Although contamination bias could not be ruled out. |
Legault 2011.
| Methods | Parallel‐group RCT: 3 intervention groups and 1 control group. 73 participants at randomisation, 18 (10 female) were put into the physical activity training group, 18 (8 female) into the cognitive training, 19 (12 female) into the combined intervention group, and 18 (7 female) into the healthy aging control group. Follow‐up period: 4 months. |
|
| Participants | 73 participants, aged 70 to 85 years of age that were community‐dwelling. Inclusion criteria: aged 70 to 85, identified as "individuals who were appropriate candidates for physical activity and cognitive training and who appeared likely to adhere to interventions and data collection protocols" as detailed in a previous paper. Exclusion criteria: Related to Physical Activity: Severe rheumatologic or orthopedic diseases, severe pulmonary heart disease, actively participating in a formal exercise programme within the past month (> 30 min/week), severe cardiac disease, clinically significant aortic stenosis, history of cardiac arrest which required resuscitation, use of cardiac defibrillator or uncontrolled angina. Other significant co‐morbid disease that would impair ability to participate in the exercise based intervention. Receiving physical therapy for gait, balance or other lower extremity training. Serious conduction disorder, uncontrolled arrhythmia. Pulmonary embolism or deep venous thrombosis within past 6 months. Hip fracture, hip or knee replacement, or spinal surgery within past 4 months. Severe hypertension. Related to Cognition: Neurological disease, stroke that required hospitalisation, Parkinson's, multiple sclerosis, Amyotrophic Lateral Sclerosis, or MCI. Telephone interview for cognitive status ≤ 31. Current use of cognitive enhancing prescription or investigational medications. History of participation in a cognitive training programme in the last two years. 3MSE score < 88 (< 80 for ≤ 8 years education). Scores ≥ 2 SDs below normal on memory or non‐memory domain tests (speed of processing and verbal fluency). Other significant factors that may affect the ability for cognitive training, including a history of head trauma resulting in a loss of consciousness, current use of benzodiazepines, hypnotic or anticholinergic agents. Stroke within past 4 months. Baseline Geriatric Depression Scale score ≥ 8. Related to trial design or adherence: Age < 70 or > 85 years. Unwillingness to be randomized to any of the four intervention conditions. Failure to provide the name of a personal physician. Living in a nursing home. Terminal illness with life expectancy less than 8 months. Unable to communicate because of severe hearing loss or speech disorder. Severe visual impairment. Excessive alcohol use (> 14 drinks per week). Member of household is already enrolled. Lives distant from the trial site or is planning to move out of the area in the next year or leave the area for more than one month during the next year. Other temporary intervening events, such as sick spouse, bereavement or recent move. Participation in another intervention trial. Inability to commit to intervention schedule requirements. Failure to provide informed consent. |
|
| Interventions | Physical activity training: centre‐based and home‐based sessions aimed at aerobic and flexibility training targeting duration of 150 minutes/week. Two centre‐based sessions per week for four months, focus on walking (or other endurance activity if contraindicated) with explicit intent of improving cardiovascular fitness. Centre‐based sessions approximately 60 minutes ‐ 40 minutes walking, 20 minutes flexibility. Tailored home‐based walking 1 to 2 sessions per week for first month and encouraged to slowly increase duration, speed and frequency to achieve 150 min/week goal. Cognitive training: four consecutive 10 to 12 min sessions per day, administered two times per week for two months, then one time per week for two additional months at centre via computer, where participants studied a list of 30 words, then were given a recognition test consisting of the 30 studied words and 30 new words with each new word repeated once, and asked to respond "yes" to trial words or "no" to new words. Intervals between the first and second presentation of new words increased as participants reached accuracy thresholds. Combined physical activity and cognitive training: received both, cognitive was delivered prior to physical activity to avoid impact of fatigue. Controls: weekly lectures based on health education, topics such as medications, foot care, travelling and nutrition. |
|
| Outcomes | Trailmaking part A
Trailmaking part B
Hopkins Verbal Learning Test
2‐Back
Flanker Task 400‐metre walk time |
|
| Notes | Testing took place at Wake Forest University. 400‐metre walk times for the physical activity training group decreased by 5.31 seconds and were not different from the combined intervention group. Walk times for the cognitive training group and the 'healthy ageing' group did not improve. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | Trial was "single‐blinded", since it is not possible to blind participants, outcome assessors must have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses conducted according to ITT principles. 2/18 participants in the physical activity group were excluded because they did not return for the 4‐month visit. 1/18 in the physical activity group and 1/19 in the combined intervention group were excluded for not attending any of the centre‐based training sessions. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Low risk | Although contamination bias could not be ruled out. |
Madden 1989.
| Methods | Parallel‐group RCT: 2 intervention groups and 1 control group. 85 participants (44 males and 41 females) at randomisation; 28 in the aerobic group, 30 in the yoga group and 27 served as controls. Follow‐up: 16 weeks. |
|
| Participants | Participants were 60 to 83 years of age. Inclusion criteria: free of medical conditions that would preclude a programme of either aerobic exercise or yoga. Exclusion criteria: uncontrolled hypertension, diabetes, or coronary heart disease, use of beta‐blockers or psychotropic medication. | |
| Interventions | Aerobic exercise: 3 supervised sessions per week for 16 weeks. 10 minutes of warming up, 30 minutes of cycling, 15 minutes of brisk walking or jogging or both, 5 minutes of cooling down. All exercise was performed in target (training) heart range (70% of max during initial exercise test). Yoga: 2 times a week 60 minutes of supervised yoga sessions for 16 weeks. Control: no change to their physical activity habits for the length of the trial. |
|
| Outcomes | Letter search RT task (short‐term memory) Word comparison RT task (long‐term memory) VO2 max | |
| Notes | Testing took place at the Duke University Medical Center. Aerobic capacity remained constant for the yoga and control groups between pre‐ and post‐test (respectively from 18.8 to 18.6 mL/kg/min and from 19.1 to 18.6 mL/kg/min), whereas the aerobic exercise group showed a significant 11% increase in VO2 max (from 19.7 to 21.9 mL/kg/min) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Insufficient information provided to assess the blinding of the cognitive outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/28 were lost from the exercise group, 2/30 from the yoga group and 1/27 from the controls. Main outcomes were not analysed according to the ITT principle. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Low risk | Although contamination bias could not be ruled out. |
Moul 1995.
| Methods | Parallel‐group RCT: 2 intervention groups and 1 control group. 30 participants (11 males, 19 females), the walking, weight training and control group all contained 10 participants. Follow‐up: 16 weeks. |
|
| Participants | 30 participants aged 65 to 72 years. Inclusion criteria: nondiseased (no current symptoms or signs suggestive of heart disease), non active (defined as < 2 moderate to vigorous aerobic or resistance training sessions of > 20 minutes per week). | |
| Interventions | Walking: 5 sessions per week. Walking 30 minutes at 60% of HRR (as determined by treadmill testing). Walking duration was increased 2 minutes per week until they reached 40 minutes and HRR were adjusted after 8 weeks of training to 65% of HRR. Weight training: 5 sessions per week of upper and lower body exercises on alternate days of the week. Abdominal crunches and back extensions were performed in each session. Weight group employed a daily adjusted progressive resistive exercise programme (DAPRE) using weights. Controls: 5 sessions per week mild stretching exercises for 30 to 40 minutes. Minimal challenge to the cardiovascular or muscular systems. |
|
| Outcomes | Immediate Memory (Ross Information Processing Assessment) Recent Memory (RIPA) Temporal Orientation (RIPA) Problem Solving and abstract reasoning (RIPA) Organization (RIPA) Auditory Processing (RIPA) Weight (kg) Sum of seven skinfolds (mm) VO2 max (mL/kg/min) Time on treadmill (min) Ventilation (mL/min) RER Knee extension (lb) Elbow flexion (lb) | |
| Notes | Testing took place at the Human Performance Laboratory and Athletic Training Laboratory, Appalachian State University. Post‐test data revealed that the subjects in the walking condition significantly increased their VO2 max by an average of 16% (from 22.4 to 26.6 mL/kg/min), whereas there were no significant changes in VO2 max for the other two groups (weight training group from 21.4 to 20.4 mL/kg/min and controls from 20.9 to 19.3 mL/kg/min). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Insufficient information provided to assess the blinding of the cognitive outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Low risk | Although contamination bias could not be ruled out. |
Oken 2006.
| Methods | Parallel‐group RCT: 2 intervention groups and 1 control group. 135 participants were randomised in a yoga class (N = 47), exercise group (N = 44) or a wait‐list control group (N = 44). Follow‐up: 6 months. |
|
| Participants | Participants were healthy adults in the age of 65 to 85 years. Inclusion criteria: other than age not described. Exclusion criteria: subjects were screened for significant medical problems, had a physical examination and routine ECG to ensure the safety of the intervention and to exclude participants with pathology with might impair cognition. Subjects were excluded for the following reasons: insulin‐dependent diabetes, uncontrolled hypertension, evidence of liver or kidney failure, significant lung disease, alcoholism or other drug abuse, symptoms or signs of congestive heart failure, symptomatic ischemic heart disease, or significant valvular disease and significant visual impairment. Subjects also were excluded if they were actively practicing yoga or had taken a yoga or tai‐chi class in the last 6 months or if they were regularly performing aerobic exercise more than 210 minutes per week. |
|
| Interventions | Yoga was taught in one class per week along with home practice. The yoga classes were 90 minutes in duration and designed by a certified Iyengar yoga teacher, an Iyengar trained teacher and a physician. Over all weeks, eighteen poses were taught. Each class ended with a 10‐minute deep relaxation period with the participant lying supine. Daily home practice was strongly encouraged and participants were encouraged to honour their individual limits. A certified personal trainer with experience in the geriatric population directed the aerobic exercise intervention arm of the trial. The aerobic intervention consisted of 1 class per week along with home exercise. The aerobic exercise consisted of walking on an outdoor 400‐metre track for endurance training. The 1‐hour class began with walking 2 laps to warm up and then progressed to mild leg stretches. Intensity of exercise was determined by heart rate and modified Borg Rate of Perceived Exertion scale (Borg CR10 Scale). Participants wore a heart‐rate monitor, and target heart rate was initially estimated as 70% of maximum based on morning resting heart rate and age. Participants were instructed to exercise at a level of 6/7 on the Borg scale. Based on perceived exertion, the heart rate target was adjusted slightly. Participants were strongly encouraged to exercise daily at least 5 times per week in addition to the weekly class session. Participants in the wait‐list control group received no intervention. |
|
| Outcomes | Stroop colour word Covert orienting of spatial attention Simple RT (msec) Choice RT (msec) 10‐words learning task (delayed recall) Letter‐number sequencing SF‐36 Stanford sleepiness scale (SSS) Profile of mood states (POMS) Multidimensional fatigue inventory (MFI) Centre for epidemiologic studies depression scale (CESD‐10) State‐trait anxiety inventory One‐leg stand (sec) Chair sit and reach (cm) Sit and stand (sec) ¼ mile walk (sec) |
|
| Notes | Testing took place at the Oregon Health and Science University. After 6 months there were no significant differences in time at a ¼ mile walk between all three groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to treatment groups in this study with a planned modified minimization scheme". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | Outcome assessors were adequately blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/47 dropped out from yoga, 6/44 from exercise and 2/44 from the wait‐list group. ITT analysis was not performed. Quote: "No attempt was made to input missing variables". |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Low risk | Although contamination bias could not be ruled out. |
Panton 1990.
| Methods | Parallel‐group RCT: 2 intervention groups and 1 control group. 57 participants were randomised in a walk/jog group, a strength group and a control group. Analyses were performed on 17 participants in the walk/jog group, 20 participants in the strength group and 12 controls.
Participants were not blinded; it is unclear whether the outcome assessor and the caregiver were blinded. Follow‐up: 26 weeks. |
|
| Participants | Participants were retired professionals from the university community of Gainesville, FL and 70 to 79 years of age. Inclusion criteria: sedentary non‐smokers who had no contraindications to exercise testing or training. Free of any overt evidence of coronary artery disease and other conditions that would limit their participation in a vigorous exercise programme as tested with a diagnostic graded exercise test (using a modified Naughton protocol). Exclusion criteria were not described. | |
| Interventions | The walk/jog group participated in three exercise sessions per week for the duration of the trial. All training sessions were preceded by 5 to 10 minutes of stretching and warm‐up and ended with 5 min of cool‐down exercises. Initially, participants started walking/jogging for 20 minutes at 50% of their maximal heart rate reserve (HRRmax). The duration was increased by 5 min every 2 weeks until the participants walked for 40 minutes. Training intensity was gradually increased until participants could walk at 60 to 70% of their HRRmax. During the 14th week of training exercise intensity was further increased by alternating fast walk/moderate walk or fast walk/slow jog intervals. Five participants increased their training intensity by increasing the slope of the treadmill. By the 26th week of training, all participants performed at 85% of HRRmax for 35 to 45 min. Participants in the strength group participated in 30 min sessions, 3 times a week for 26 weeks. Workouts consisted of one set of 10 variable resistance Nautilus exercises (leg, arm and torso muscles). During the first 13 weeks, participants used light to moderate weights and performed 8 to 12 repetitions for each exercise. During the last 13 weeks, resistance was increased substantially and participants were encouraged to train to volitional muscular fatigue. When participants could complete 12 or more repetitions, the resistance was increased. Participants in the control group were asked not to change their lifestyle over the 6 month duration of the trial. | |
| Outcomes | Total simple Reaction time Fractionated Reaction time (PreMotorTime and MotorTime) Speed of Movement measurements Fat percentage (predicted from body density) Body density (7 skinfolds) 1RM muscle strength test VO2 max | |
| Notes | Testing took place at the University of Florida College of Medicine. Aerobic capacity significantly improved by 20.4% (from 22.5 to 27.1 mL/kg/min) for subjects in the walk/jog group; participants in both the strength as well as the control group showed no significant changes in VO2 max (from 22.5 to 23.3 and 22.2 to 22.0, respectively). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | Cognitive function was assessed by computer and therefore adequately blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/57 participants were lost to follow‐up; it is unclear from which condition these participants were lost. Main outcomes were not analysed according to the ITT principle. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Low risk | Although contamination bias could not be ruled out. |
Whitehurst 1991.
| Methods | Parallel‐group RCT: 1 intervention group and 1 control group. 14 participants at randomisation (all females): 7 in both the exercise and the control group. Follow‐up: 8 weeks. |
|
| Participants | Females in the age range of 61 to 73 years living in a rural community in North Carolina. Inclusion criteria: did not participate in aerobic exercise more than one time per week prior to the trial. Medical clearance from a physician (resting ECG and physical examination). Free of primary cardiovascular risk factors. Maintained the household. | |
| Interventions | Exercise: 3 supervised sessions per week for 8 weeks (total of 24 sessions). 5 to 10 minutes of warming up and cooling down. The participants cycled for 8 to 10 minutes the first week to provide acclimatization. Thereafter, 3 to 5 minutes was added to subsequent sessions so that by week 4 all participants were cycling for 35 to 40 minutes at their target heart rate. Control: did not engage in any form of vigorous physical activity during the course of the trial. |
|
| Outcomes | Simple reaction time Choice reaction time Estimated VO2 max | |
| Notes | Testing took place at the Human Performance Laboratory, Florida Atlantic University. The subjects in the exercise group significantly increased their VO2 max values by an average 16% (from 25.4 to 29.7 mL/kg/min), whereas the subjects in the control group increased their VO2 max by a (non significant) 2% (from 24.7 to 25.4 mL/kg/min). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) Participants | High risk | Participants were not blinded to their group assignment, but it was not feasible to do so. |
| Blinding (performance bias and detection bias) Trainers | Low risk | It is not feasible that the trainers were blinded to the condition but this non‐blinding was unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | Cognitive function was assessed by computer (quote: "a standard choice reaction‐time apparatus was used") and therefore adequately blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Other bias | Low risk | Although contamination bias could not be ruled out. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alessi 1999 | No pre to post‐intervention cognitive data. Mean MMSE scores of the participants was below the range of what is considered 'normal' cognition (mean MMSE of 13.6 ± 8.5). |
| Alves 2012 | No measure of cardiorespiratory fitness. |
| Barry 1966 | Not a RCT but a CCT. |
| Blumenthal 1988 | Participants were too young to meet the given inclusion criteria of this review. |
| Blumenthal 1991 | Data were already published in Blumenthal 1989. |
| Bream 1996 | No published data. |
| Carles 2007 | Participants were too young to meet the given inclusion criteria of this review. |
| Cassilhas 2007 | Exercise was not intended to improve aerobic fitness and no fitness parameters present. |
| Colcombe 2006 | No pre to post‐intervention cognitive data. |
| Deary 2006 | Not a RCT but a longitudinal survey. |
| Dietrich 2004 | Data could not test the cardiovascular fitness hypothesis since cognition was assessed during exercise. |
| Dorner 2007 | Participants had cognitive impairment; cognitive impairment was an exclusion criterium for our review. |
| Dustman 1984 | Not a RCT but a quasi‐randomised study (participants "alternately assigned"). |
| Emery 1990b | Perceived (subjective) measurements of cognition were analysed according to groups but the objective measures of cognition were analysed according to perceived measures of cognition. |
| Emery 1998 | Only had combined intervention groups and no pure aerobic exercise intervention group. |
| Emery 2003 | Within participants repeated measures design to evaluate the influence of music and exercise on cognition. No control group. |
| Erickson 2007 | Not a RCT but a cross‐sectional study. |
| Etnier 1997a | No exercise intervention. |
| Etnier 2001 | The control group was encouraged to continue exercising; however no formal programme was provided. |
| Fabre 1999 | No means and SDs for cognitive data. These results are described in Fabre 2002. |
| Gates 2011 | Selected participants that have early changes in memory without diagnosis and excluded people with perfect MMSE. |
| Glisky 1997 | No published data. |
| Hassmén 1992 | Not RCT but "matched controls". |
| Hassmén 1997 | Not a RCT, participants matched on cognitive performance in pairs, then randomised. |
| Hawkins 1992 | No fitness parameter present. |
| Hill 1993 | Not a RCT but a quasi‐randomised study (participants "assigned to intervention group"). |
| Ijuin 2013 | No measure of aerobic fitness and not peer‐reviewed. |
| Jacobson 2007 | No fitness parameter present and not published in a peer reviewed journal. |
| Jedrziewski 2007 | Not a RCT but a narrative review. |
| Kerschan 2002 | Both groups followed aerobic training intervention. |
| Kharti 2001 | Study participants were depressed older men and women: depression was an exclusion criterion for our review. |
| Kramer 1999 | This article provides no quantitative data on which an analysis can be based. Quantitative data of the RCT of this research group is provided in Kramer 2001, which is included in our review. |
| Kramer 2007 | Not a RCT but a narrative review. |
| Larson 2006 | Not a RCT but a prospective cohort study. |
| Lautenschlager 2008 | No assessment of fitness parameters. |
| Leinonen 2007 | No pre‐ to post‐intervention data present, only selected baseline results. |
| Littbrand 2006 | No pre‐ to post‐intervention cognitive data. Applicability study for the evaluation of attendance and adverse events of an exercise programme. |
| Liu‐Ambrose 2010 | No measure of cardiorespiratory fitness. |
| Masley 2009 | RCT included younger, middle‐aged and older adults. |
| McAuley 2006 | No pre‐ to post‐intervention cognitive parameters. |
| McKenzie 2000 | No published data. |
| Molloy 1988 | Exercise was not intended to improve aerobic fitness. |
| Mortimer 2012 | No aerobic fitness measure. |
| Munguía‐Izquierdo 2007 | Participants were too young to meet the given inclusion criteria of this review. No fitness parameters present. |
| Netz 2007 | Data could not test the cardiovascular fitness hypothesis since cognition was assessed during exercise. |
| O'Dwyer 2007 | Not a RCT but description of a study protocol. |
| Oken 2004 | Participants were too young to meet the given inclusion criteria of this review. |
| Okumiya 1996 | No fitness parameter present. |
| Palleschi 1996 | Participants were elderly patients with senile dementia of the Alzheimer type: this was an exclusion criterion for our review. |
| Palmer 1995 | No published data. |
| Perri 1984 | Not a true RCT but a clinical trial. |
| Pierce 1993 | Participants were too young to meet the inclusion criteria of this review. |
| Plati 2006 | Not a RCT but "matched controls". |
| Powell 1975 | Narrative review; this article provides no quantitative data. |
| Powers 2007 | No published data. |
| Predovan 2012 | Not a RCT, group selection was based on order of recruitment and willingness to participate in an exercise programme. |
| Prohaska 2007 | Not a RCT but a narrative article. |
| Querry 1998 | No published data. |
| Rikli 1991 | Balance, sit and reach flexibility, shoulder flexibility, and grip strength were given as fitness parameters. We excluded this RCT since neither of the two fitness parameters reflect aerobic fitness. |
| Rosendahl 2006 | No pre‐ to post‐intervention cognitive data. Mean MMSE scores of the participants was below the range of what is considered 'normal' cognition (mean MMSE of 17.8 ± 5.1). |
| Russell 1984 | No published data. |
| Sato 2007 | No cognition or fitness parameters, or both. |
| Shatil 2013 | No objective measure of aerobic fitness in all groups. |
| Sibley 2007 | Not a RCT and data could not test the cardiovascular fitness hypothesis since cognition was assessed during exercise. |
| Small 2006 | No fitness parameters present. |
| Smiley‐Oyen 2008 | Not a RCT but a quasi‐randomised study ("Group allocation alternated between CARDIO and FLEX‐TONE"). |
| Stevenson 1990 | Both intervention groups received aerobic training (different levels of intensity). |
| van Uffelen 2007 | Participants had mild cognitive impairment; cognitive impairment was an exclusion criterion for our review. |
| Verghese 2006 | Not a RCT but a prospective cohort study. |
| Wallman 2004 | Participants were too young to meet the inclusion criteria of this review. |
| Wilbur 2005 | No objective measures of cognitive parameters (symptom impact inventory). |
| Williams 1997 | No objective measures of fitness, only subjective measures (Perceived General Fitness). |
| Williamson 2009 | No assessment of fitness parameters. What could have been used to assess fitness (400 m walk) was taken here as part of an assessment of functionality (specifically normal gait speed) and because of how this measure was implemented (walked at usual pace, allowed to rest, allowed to not complete), it could not be used for fitness assessment. |
| Winter 2007 | Data could not test the cardiovascular fitness hypothesis since cognition was assessed during exercise. |
| Zlomanczuk 2006 | No fitness parameters present. |
Characteristics of studies awaiting assessment [ordered by study ID]
Chapman 2013.
| Methods | Parallel‐group, randomised, controlled trial: 1 intervention group, 1 wait‐list control group. 37 participants at randomisation; 18 (13 female) were put into the physical training group, 19 (14 female) were put into the control group. Follow‐up: 12 weeks |
| Participants | Participants were 57 to 75 years of age. Inclusion criteria: "no prior history of neurological or psychiatric conditions, average IQ range, native English speaker, and minimum of a high school diploma" Exclusion criteria: "MR scanning contraindications, cognitive impairment (TICS‐M < 28 and MoCA < 26), elevated depressive symptoms (BDI >14), left‐handedness, increased body mass BMI > 40, abnormal electrocardiographic response, significant hypertensive blood pressure response to exercise, or inability to reach 85% of maximum predicted heart rate for age... if they reported regular aerobic activity of more than twice a week for 20 min or more.They could not have regularly exercised for at least 3 months prior to enrolling in the study." |
| Interventions | Physical Training: "The training regimen consisted of three 60 min sessions of aerobic exercise training per week for a period of 12 weeks. The participants’ aerobic exercise alternated each session between exercise bike and treadmill. The exercise bike routine included: 5 min warm up at 43 watts, cycling for 50 min at a speed that increased their heart rate to 50–75% of their maximum achieved heart rate on VO2 max testing, and a 5 min cool down at 43 watts. The treadmill workout included: 5 min warmup at 2 miles per h (mph), walking on treadmill for 50 min at a speed that increased their heart rate to 50–75% of their maximum achieved heart rate on VO2 max testing, and a 5 min cool down at 2mph." Control: Wait‐list |
| Outcomes | Wechsler Abbreviated Scale of Intelligence (WASI) BDI MoCA Tics‐M Trails B ‐ Trails A CVLT‐II WMS‐IV immediate/delayed memory Delis‐Kaplan Executive Function System‐Color Word Interference subtest (DKEFS‐ color word) Backward Digit Span BMI Structural MRI absolute Cerebral Blood Flow (aCBF) RPE VO2 max |
| Notes | Testing took place at the The University of Texas at Dallas, The University of Texas Southwestern Medical Center, and The Cooper Institute. VO2 max increased significantly to a greater extent for the Physical Training group than the Control group at the mid‐point (p = .03), however at the endpoint change in VO2 max did not differ between the groups. |
Linde 2014.
| Methods | Parallel‐group, randomised, controlled trial: 3 intervention groups, 1 wait‐list control group. 70 participants were randomised: 19 (11 female) to a physical intervention group, 18 (9 female) to a cognitive intervention group, 17 (11 female) to a combined physical and cognitive intervention group, and 16 (10 female) to a wait‐list control group. Follow‐ups: 16‐weeks and 3 months after conclusion of intervention |
| Participants | Participants were aged 60‐75 from a "medium‐sized German city" Inclusion criteria: "age of 60–75 years" Exclusion criteria: "dementia, depression, and possible medical conditions (e.g., coronary heart diseases, hypertension, stroke, pulmonary diseases) that would not allow for participation in a regular exercise program." |
| Interventions | "The interventions took place in groups of 8–10 participants and were hosted at the facilities of the Faculty of Sport Sciences and its campus." Physical Activity Intervention: "Participants trained two times per week, each session lasting 60 min, for a period of 16 weeks." Sessions consisted of 20 mins progressive strength training of each major muscle group and 40 mins aerobic endurance training ‐ 5 minute warm‐up, 30 minute walking or running, 5 minute cool down. "Each individual was asked to exercise at an intensity of 40–50% heart rate reserve (moderate intensity) during the beginner’s stage; the intensity of activity was then incrementally increased to 60–70% (moderate to vigorous intensity) by the end of the developmental stage." Cognitive Activity Intervention: "Cognitive training took place once a week for approximately 30 min... The primary element of the cognitive intervention consisted of the individual editing of worksheets. In addition, some partner and group exercises were carried out. During the first 5 min, warm‐up exercises were performed as a group (e.g., training of short‐term memory) or homework was discussed. Some small amount of information was then given relating to one of the following topics: information processing speed, attention, introduction to the memory model, sensory memory, short‐term memory, mnemonics, long‐term memory, and memory aids. Following the distribution of information, the following cognitive abilities were practiced for 25 min: short‐term memory, visuospatial skills, information processing speed, concentration, and logical reasoning. At the end of each session two additional exercises were provided as homework." Combined Physical and Cognitive Activity Intervention: "The combined intervention consisted of the physical plus cognitive interventions and took place twice a week. The cognitive training program was carried out at the first training session of the week, before the physical training. The total duration of the first training session each week therefore was 90 min, while the second session lasted only 60 min (consisting only of physical training)." Wait‐list Control: "An inactive waiting control group was selected to act as a comparison group. Study participants in the control group were asked to continue their daily routines as before. To increase the motivation to participate in the study, a 12‐week fitness class was offered after the follow‐up assessment." |
| Outcomes | Reasoning subtest of Leistungs‐Prüf‐System 50+ (LPS 50+) Spatial relations subtest of LPS 50+ d2: Test of Attention Trail Making Test Part A Digit‐Symbol Substitution Test (DSST from NAI) Word List test (NAI subtest) 2‐km walking test to estimate VO2 max |
| Notes | Testing was conducted at the Faculty of Sport Science at the University of Leipzig. Increase of cardiovascular fitness were not significantly different between the control and intervention groups. |
Contributions of authors
JY and MA: drafted reviews, obtained copies of trial reports, selected trials for inclusion and exclusion, extracted and entered data, and interpreted data analyses. NT: screened trials for inclusion and exclusion, extracted data and interpreted data analyses. JR: interpreted data analyses.
Consumer Editor: Judith Hoppesteyn‐Armstrong
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK.
This update was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
Jeremy Young ‐ none known Maaike Angevaren ‐ none known Jennifer Rusted ‐ none known Naji Tabet ‐ none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bakken 2001 {published data only}
- Bakken RC, Carey JR, Fabio RP, Erlandson TJ, Hake JL, Intihar TW. Effect of aerobic exercise on tracking performance in elderly people: a pilot study. Physical Therapy 2001;81(12):1870‐9. [PubMed] [Google Scholar]
Blumenthal 1989 {published data only}
- Blumenthal JA, Emery CF, Madden DJ, George LK, Coleman RE, Riddle MW, et al. Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. Journal of Gerontology 1989;44(5):M147‐57. [DOI] [PubMed] [Google Scholar]
Emery 1990a {published data only}
- Emery CF, Gatz M. Psychological and cognitive effects of an exercise program for community‐dwelling older adults. Gerontologist 1990;30(2):184‐8. [DOI] [PubMed] [Google Scholar]
Fabre 2002 {published data only}
- Fabre C, Chamari K, Mucci P, Massé‐Birron J, Préfaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. International Journal of Sports Medicine 2002;23(6):415‐21. [DOI] [PubMed] [Google Scholar]
Kramer 2001 {published data only}
- Kramer AF, Hahn S, McAuley E, Cohen NJ, Banich MT, Harrison C, et al. Exercise, aging and cognition: Healthy body, healthy mind?. In: Fisk AD, Rogers W editor(s). Human Factors Interventions for the Health Care of Older Adults. Hillsdale, NJ: Erlbaum, 2001:91‐120. [Google Scholar]
Langlois 2012 {published data only}
- Langlois F, Vu TTM, Chassé K, Dupuis G, Kergoat MJ, Bherer L. Benefits of physical exercise training on cognition and quality of life in frail older adults. Journals of Gerontology Series B: Psychological Sciences and Social Sciences 2012;68(3):400‐4. [DOI: ] [DOI] [PubMed] [Google Scholar]
Legault 2011 {published data only}
- Legault C, Jennings JM, Katula JA, Dagenbach D, Gaussoin SA, Sink KM, et al. Designing clinical trials for assessing the effects of cognitive training and physical activity interventions on cognitive outcomes: the Seniors Health and Activity Research Program Pilot (SHARP‐P) study, a randomized controlled trial. BMC Geriatrics 2011;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madden 1989 {published data only}
- Madden DJ, Blumenthal JA, Allen PA, Emery CF. Improving aerobic capacity in healthy older adults does not necessarily lead to improved cognitive performance. Psychology and Aging 1989;4(3):307‐20. [DOI] [PubMed] [Google Scholar]
Moul 1995 {published data only}
- Moul JL, Goldman B, Warren B. Physical activity and cognitive performance in the older population. Journal of Aging and Physical Activity 1995;3:135‐45. [Google Scholar]
Oken 2006 {published data only}
- Oken BS, Zajdel D, Kishiyama S, Flegal K, Dehen C, Haas M, et al. Randomized, controlled, six‐month trial of yoga in healthy seniors: effects on cognition and quality of life. Alternative Therapies in Health and Medicine 2006;12(1):40‐7. [PMC free article] [PubMed] [Google Scholar]
Panton 1990 {published data only}
- Panton LB, Graves JE, Pollock ML, Hagberg JM, Chen W. Effect of resistance training on fractionated reaction time and speed of movement. Journal of Gerontology 1990;45(1):M26‐31. [DOI] [PubMed] [Google Scholar]
Whitehurst 1991 {published data only}
- Whitehurst M. Reaction time unchanged in older women following aerobic training. Perception and Motor Skills 1991;72(1):251‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alessi 1999 {published data only}
- Alessi CA, Yoon EJ, Schnelle JF, Al‐Samarrai NR, Cruise PA. A randomized trial of a combined physical activity and environmental intervention in nursing home residents: do sleep and agitation improve?. Journal of the American Geriatrics Society 1999;47(7):784‐91. [DOI] [PubMed] [Google Scholar]
Alves 2012 {published data only}
- Alves CRR, Gualano B, Takao PP, Avakian P, Fernandes RM, Morine D, et al. Effects of acute physical exercise on executive functions: a comparison between aerobic and strength exercise. Journal of Sport & Exercise Psychology 2012;34(4):539‐49. [DOI] [PubMed] [Google Scholar]
Barry 1966 {published data only}
- Barry AJ, Steinmetz JR, Page HF, Rodahl K. The effects of physical conditioning on older individuals. II. Motor performance and cognitive function. Journal of Gerontology 1966;21(2):192‐9. [DOI] [PubMed] [Google Scholar]
Blumenthal 1988 {published data only}
- Blumenthal JA, Madden DJ. Effects of aerobic exercise training, age, and physical fitness on memory‐search performance. Psychology and Aging 1988;3(3):280‐5. [DOI] [PubMed] [Google Scholar]
Blumenthal 1991 {published data only}
- Blumenthal JA, Emery CF, Madden DJ, Schniebolk S, Walsh‐Riddle M, George LK, et al. Long‐term effects of exercise on psychological functioning in older men and women. Journal of Gerontology 1991;46(6):352‐61. [DOI] [PubMed] [Google Scholar]
Bream 1996 {unpublished data only}
- Bream JH. Interventions to aging: Immunologic and cognitive responses to 16 weeks of low‐intensity exercise training in older adults. Dissertation Abstracts International 1996. [DA9716196]
Carles 2007 {published data only}
- Carles S Jr, Curnier D, Pathak A, Roncalli J, Bousquet M, Garcia JL, et al. Effects of short‐term exercise and exercise training on cognitive function among patients with cardiac disease. Journal of Cardiopulmonary Rehabilitation and Prevention 2007;27(6):395‐9. [DOI] [PubMed] [Google Scholar]
Cassilhas 2007 {published data only}
- Cassilhas RC, Viana VA, Grassmann V, Santos RT, Santos RF, Tufik S, et al. The impact of resistance exercise on the cognitive function of the elderly. Medicine and Science in Sports and Exercise 2007;39(8):1401‐7. [DOI] [PubMed] [Google Scholar]
Colcombe 2006 {published data only}
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. Journals of Gerontology Series A‐Biological Sciences & Medical Sciences 2006;61(11):1166‐70. [DOI] [PubMed] [Google Scholar]
Deary 2006 {published data only}
- Deary IJ, Whalley LJ, Batty GD, Starr JM. Physical fitness and lifetime cognitive change. Neurology 2006;67(7):1195‐200. [DOI] [PubMed] [Google Scholar]
Dietrich 2004 {published data only}
- Dietrich A, Sparling PB. Endurance exercise selectively impairs prefrontal‐dependent cognition. Brain and Cognition 2004;55(3):516‐24. [DOI] [PubMed] [Google Scholar]
Dorner 2007 {published data only}
- Dorner T, Kranz A, Zettl‐Wiedner K, Ludwig C, Rieder A, Gisinger C. The effect of structured strength and balance training on cognitive function in frail, cognitive impaired elderly long‐term care residents. Aging Clinical and Experimental Research 2007;19(5):400‐5. [DOI] [PubMed] [Google Scholar]
Dustman 1984 {published data only}
- Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, et al. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiology of Aging 1984;5(1):35‐42. [DOI] [PubMed] [Google Scholar]
Emery 1990b {published data only}
- Emery CF, Blumenthal JA. Percieved change among participants in an exercise program for older adults. Gerontologist 1990;30(4):516‐21. [DOI] [PubMed] [Google Scholar]
Emery 1998 {published data only}
- Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychology 1998;17(3):232‐40. [DOI] [PubMed] [Google Scholar]
Emery 2003 {published data only}
- Emery CF, Hsiao ET, Hill SM, Frid DJ. Short‐term effects of exercise and music on cognitive performance among participants in a cardiac rehabilitation program. Heart & Lung: the Journal of Critical Care 2003;32(6):368‐73. [DOI] [PubMed] [Google Scholar]
Erickson 2007 {published data only}
- Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, et al. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiology of Aging 2007;28(2):179‐85. [DOI] [PubMed] [Google Scholar]
Etnier 1997a {published data only}
- Etnier JL, Landers DM. The influence of age and fitness on performance and learning. Journal of Aging and Physical Activity 1997;5:175‐89. [Google Scholar]
Etnier 2001 {published data only}
- Etnier JL, Berry M. Fluid intelligence in an older COPD sample after short‐ or long‐term exercise. Medicine and Science in Sports and Exercise 2001;33(10):1620‐8. [DOI] [PubMed] [Google Scholar]
Fabre 1999 {published data only}
- Fabre C, Massé‐Biron J, Chamari K, Varray A, Mucci P, Préfaut C. Evaluation of quality of life in elderly healthy subjects after aerobic and/or mental training. Archives of Gerontology and Geriatrics 1998;28(1):9‐22. [DOI] [PubMed] [Google Scholar]
Gates 2011 {published data only}
- Gates NJ, Valenzuela M, Sachdev PS, Singh NA, Baune BT, Brodaty H, et al. Study of Mental Activity and Regular Training (SMART) in at risk individuals: A randomised double blind, sham controlled, longitudinal trial. BMC Geriatrics 2011;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glisky 1997 {unpublished data only}
- Glisky ML. Interventions for cognitive and psychosocial functioning in older adults: A comparison of aerobic exercise and cognitive training. Dissertation Abstracts International 1997. [DA9806830]
Hassmén 1992 {published data only}
- Hassmén P, Ceci R, Bäckman L. Exercise for older women: a training method and its influences on physical and cognitive performance. European Journal of Applied Physiology and Occupational Physiology 1992;64(5):460‐6. [DOI] [PubMed] [Google Scholar]
Hassmén 1997 {published data only}
- Hassmén P, Koivula N. Mood, physical working capacity and cognitive performance in the elderly as related to physical activity. Aging 1997;9(1‐2):136‐42. [DOI] [PubMed] [Google Scholar]
Hawkins 1992 {published data only}
- Hawkins HL, Kramer AF, Capaldi D. Aging, exercise, and attention. Psychology and Aging 1992;7(4):643‐53. [DOI] [PubMed] [Google Scholar]
Hill 1993 {published data only}
- Hill RD, Storandt M, Malley M. The impact of long‐term exercise training on psychological function in older adults. Journal of Gerontology 1993;48(1):P12‐7. [DOI] [PubMed] [Google Scholar]
Ijuin 2013 {published data only}
- Ijuin M, Sugiyama M, Sakuma N, Inagaki H, Miyamae F, Ito K, et al. Walking exercise and cognitive functions in community‐dwelling older adults: preliminary results of a randomized controlled trial. International Journal of Geriatric Psychiatry 2013;28(1):109‐10. [DOI] [PubMed] [Google Scholar]
Jacobson 2007 {unpublished data only}
- Jacobson A. Specificity of Exercise on Enhancing Cognitive Abilities: Argentine Tango and Walking. Montreal, Canada: McGill University, 2007. [Google Scholar]
Jedrziewski 2007 {published data only}
- Jedrziewski MK, Lee VM‐Y, Trojanowski JQ. Physical activity and cognitive health. Alzheimer's and Dementia 2007;3(2):98‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerschan 2002 {published data only}
- Kerschan‐Schindl K, Wiesinger G, Zauner‐Dungl A, Kollmitzer J, Fialka‐Moser V, Quittan M. Step aerobic vs. cycle ergometer training: Effects on aerobic capacity, coordinative tasks, and pleasure in untrained adults ‐ A randomized controlled trial. Wiener Klinische Wochenschrift 2002;114(23‐24):992‐8. [PubMed] [Google Scholar]
Kharti 2001 {published data only}
- Khatri P, Blumenthal JA, Babyak MA, Craighead WE, Herman S, Baldewicz T, et al. Effects of exercise training on cognitive functioning among depressed older men and women. Journal of Aging and Physical Activity 2001;9(1):43‐57. [Google Scholar]
Kramer 1999 {published data only}
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, et al. Ageing, fitness and neurocognitive function. Nature 1999;400(6743):418‐9. [DOI] [PubMed] [Google Scholar]
Kramer 2007 {published data only}
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends in Cognitive Sciences 2007;11(8):342‐8. [DOI] [PubMed] [Google Scholar]
Larson 2006 {published data only}
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Annals of Internal Medicine 2006;144(2):73‐81. [DOI] [PubMed] [Google Scholar]
Lautenschlager 2008 {published data only}
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, Bockxmeer FM, Xiao J, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008;300(9):1027‐37. [DOI] [PubMed] [Google Scholar]
Leinonen 2007 {published data only}
- Leinonen R, Heikkinen E, Hirvensalo M, Lintunen T, Rasinaho M, Sakari‐Rantala R, et al. Customer‐oriented counseling for physical activity in older people: study protocol and selected baseline results of a randomized‐controlled trial (ISRCTN 07330512). Scandinavian Journal of Medicine & Science in Sports 2007;17(2):156‐64. [DOI] [PubMed] [Google Scholar]
Littbrand 2006 {published data only}
- Littbrand H, Rosendahl E, Lindelöf N, Lundin‐Olsson L, Gustafson Y, Nyberg L. A high‐intensity functional weight‐bearing exercise program for older people dependent in activities of daily living and living in residential care facilities: evaluation of the applicability with focus on cognitive function. Physical Therapy 2006;86(4):489‐98. [PubMed] [Google Scholar]
Liu‐Ambrose 2010 {published data only}
- Liu‐Ambrose T, Davis JC, Nagamatsu LS, Hsu CL, Katarynych LA, Khan KM. Changes in executive functions and self‐efficacy are independently associated with improved usual gait speed in older women. BMC Geriatrics 2010;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masley 2009 {published data only}
- Masley S, Roetzheim R, Gualtieri T. Aerobic exercise enhances cognitive flexibility. Journal of Clinical Psychology in Medical Settings 2009;16(2):186‐93. [DOI] [PubMed] [Google Scholar]
McAuley 2006 {published data only}
- McAuley E, Konopack JF, Motl RW, Morris KS, Doerksen SE, Rosengren KR. Physical activity and quality of life in older adults: influence of health status and self‐efficacy. Annals of Behavioral Medicine 2006;31(1):99‐103. [DOI] [PubMed] [Google Scholar]
McKenzie 2000 {unpublished data only}
- McKenzie DL. An investigation of the relationship between exercise and the cognitive function of attention in adult students with leaning disabilities and deficit disorder. Dissertation Abstracts International 2000. [DA9972924]
Molloy 1988 {published data only}
- Molloy DW, Richardson LD, Crilly RG. The effects of a three‐month exercise programme on neuropsychological function in elderly institutionalized women: a randomized controlled trial. Age and Ageing 1988;17(5):303‐10. [DOI] [PubMed] [Google Scholar]
Mortimer 2012 {published data only}
- Mortimer JA, Ding D, Borenstein AR, DeCarli C, Guo Q, Wu Y, et al. Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community‐based sample of non‐demented Chinese elders. Journal of Alzheimer's Disease 2012;30(4):757‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munguía‐Izquierdo 2007 {published data only}
- Munguía‐Izquierdo D, Legaz‐Arrese A. Exercise in warm water decreases pain and improves cognitive function in middle‐aged women with fibromyalgia. Clinical and Experimental Rheumatology 2007;25(6):823‐30. [PubMed] [Google Scholar]
Netz 2007 {published data only}
- Netz Y, Tomer R, Axelrad S, Argov E, Inbar O. The effect of a single aerobic training session on cognitive flexibility in late middle‐aged adults. International Journal of Sports Medicine 2007;28(1):82‐7. [DOI] [PubMed] [Google Scholar]
O'Dwyer 2007 {published data only}
- O'Dwyer ST, Burton NW, Pachana NA, Brown WJ . Protocol for Fit Bodies, Fine Minds: a randomized controlled trial on the affect of exercise and cognitive training on cognitive functioning in older adults. BMC Geriatrics 2007;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oken 2004 {published data only}
- Oken BS, Kishiyama S, Zajdel D, Bourdette D, Carlsen J, Haas M, et al. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology 2004;62(11):2058‐64. [DOI] [PubMed] [Google Scholar]
Okumiya 1996 {published data only}
- Okumiya K, Matsubayashi K, Wada T, Kimura S, Doi Y, Ozawa T. Effects of exercise on neurobehavioral function in community‐dwelling older people more than 75 years of age. Journal of the American Geriatrics Society 1996;44(5):569‐72. [DOI] [PubMed] [Google Scholar]
Palleschi 1996 {published data only}
- Palleschi L, Vetta F, Gennaro E, Idone G, Sottosanti G, Gianni W, et al. Effect of aerobic training on the cognitive performance of elderly patients with senile dementia of Alzheimer type. Archives of Gerontology and Geriatrics 1996;22(Suppl 1):47‐50. [DOI] [PubMed] [Google Scholar]
Palmer 1995 {unpublished data only}
- Palmer AC. The effects of aerobic exercise on cognitive ability and creativity in senior citizens. Dissertation Abstracts International 1995. [DA9530364]
Perri 1984 {published data only}
- Perri S 2nd, Templer DI. The effects of an aerobic exercise program on psychological variables in older adults. International Journal of Aging and Human Development 1984;20(3):167‐72. [DOI] [PubMed] [Google Scholar]
Pierce 1993 {published data only}
- Pierce TW, Madden DJ, Siegel WC, Blumenthal JA. Effects of aerobic exercise on cognitive and psychosocial functioning in patients with mild hypertension. Health Psychology 1993;12(4):286‐91. [DOI] [PubMed] [Google Scholar]
Plati 2006 {published data only}
- Plati MC, Covre P, Lukasova K, Macedo EC. Depressive symptoms and cognitive performance of the elderly: relationship between institutionalization and activity programs. Revista Brasileira de Psiquiatria 2006;28(2):118‐21. [DOI] [PubMed] [Google Scholar]
Powell 1975 {published data only}
- Powell RR. Effects of exercise on mental functioning. Journal of sports medicine and physical fitness 1975;15(2):125‐31. [PubMed] [Google Scholar]
Powers 2007 {unpublished data only}
- Powers M. High‐intensity resistance training in older adults: Impact on physical and cognitive function. Dissertation Abstracts International 2007;68:2308. [Google Scholar]
Predovan 2012 {published data only}
- Predovan D, Fraser SA, Renaud M, Bherer L. The Effect of Three Months of Aerobic Training on Stroop Performance in Older Adults. Journal of Aging Research 2012;2012:269815. [DOI: 10.1155/2012/269815] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prohaska 2007 {published data only}
- Prohaska TR, Peters KE. Physical activity and cognitive functioning: translating research to practice with a public health approach. Alzheimer's & Dementia 2007;3(2 Suppl):S58‐64. [DOI] [PubMed] [Google Scholar]
Querry 1998 {unpublished data only}
- Querry PA. Effects of aerobic exercise training and cognitive function in aging adults. Dissertation Abstracts International 1998. [DA9901203]
Rikli 1991 {published data only}
- Rikli RE, Edwards DJ. Effects of a three‐year exercise program on motor function and cognitive processing speed in older women. Research Quarterly for Exercise and Sport 1991;62(1):61‐7. [DOI] [PubMed] [Google Scholar]
Rosendahl 2006 {published data only}
- Rosendahl E, Lindelöf N, Littbrand H, Yifter‐Lindgren E, Lundin‐Olsson L, Håglin L, et al. High‐intensity functional exercise program and protein‐enriched energy supplement for older persons dependent in activities of daily living: a randomised controlled trial. Australian Journal of Physiotherapy 2006;52(2):105‐13. [DOI] [PubMed] [Google Scholar]
Russell 1984 {unpublished data only}
Sato 2007 {published data only}
- Sato D, Kaneda K, Wakabayashi H, Nomura T. The water exercise improves health‐related quality of life of frail elderly people at day service facility. Quality of Life Research 2007;16(10):1577‐85. [DOI] [PubMed] [Google Scholar]
Shatil 2013 {published data only}
- Shatil E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four‐condition randomized controlled trial among healthy older adults. Frontiers in Aging Neuroscience 2013;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sibley 2007 {published data only}
- Sibley BA, Beilock SL. Exercise and working memory: an individual differences investigation. Journal of Sport and Exercise Psychology 2007;29(6):783‐91. [DOI] [PubMed] [Google Scholar]
Small 2006 {published data only}
- Small GW, Silverman DH, Siddarth P, Ercoli LM, Miller KJ, Lavretsky H, et al. Effects of a 14‐day healthy longevity lifestyle program on cognition and brain function. American Journal of Geriatric Psychiatry 2006;14(6):538‐45. [DOI] [PubMed] [Google Scholar]
Smiley‐Oyen 2008 {published data only}
- Smiley‐Oyen AL, Lowry KA, Francois SJ, Kohut ML, Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: the "selective improvement" and "cardiovascular fitness" hypotheses. Annals of Behavioral Medicine 2008;36(3):280‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevenson 1990 {published data only}
- Stevenson JS, Topp R. Effects of moderate and low intensity long‐term exercise by older adults. Research in Nursing & Health 1990;13(4):209‐18. [DOI] [PubMed] [Google Scholar]
van Uffelen 2007 {published data only}
- Uffelen JG, Chin A Paw MJ, Hopman‐Rock M, Mechelen W. The effect of walking and vitamin B supplementation on quality of life in community‐dwelling adults with mild cognitive impairment: a randomized, controlled trial. Quality of Life Research 2007;16(7):1137‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verghese 2006 {published data only}
- Verghese J, LeValley A, Derby C, Kuslansky G, Katz M, Hall C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology 2006;66(6):821‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallman 2004 {published data only}
- Wallman KE, Morton AR, Goodman C, Grove R, Guilfoyle AM. Randomised controlled trial of graded exercise in chronic fatigue syndrome. Medical Journal of Australia 2004;180(9):444‐8. [DOI] [PubMed] [Google Scholar]
Wilbur 2005 {published data only}
- Wilbur J, Miller AM, McDevitt J, Wang E, Miller J. Menopausal status, moderate‐intensity walking, and symptoms in midlife women. Research and Theory for Nursing Practice 2005;19(2):163‐80. [PubMed] [Google Scholar]
Williams 1997 {published data only}
- Williams P, Lord SR. Effects of group exercise on cognitive functioning and mood in older women. Australian and New Zealand Journal of Public Health 1997;21(1):45‐52. [DOI] [PubMed] [Google Scholar]
Williamson 2009 {published data only}
- Williamson JD, Espeland M, Kritchevsky SB, Newman AB, King AC, Pahor M, et al. Changes in cognitive function in a randomized trial of physical activity: results of the lifestyle interventions and independence for elders pilot study. Journals of Gerontology. Series A: Biological sciences and medical sciences 2009;64(6):688‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winter 2007 {published data only}
- Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, et al. High impact running improves learning. Neurobiology of Learning & Memory 2007;87(4):597‐609. [DOI] [PubMed] [Google Scholar]
Zlomanczuk 2006 {published data only}
- Zlomanczuk P, Milczarek B, Dmitruk K, Sikorski W, Adamczyk W, Zegarski T, et al. Improvement in the face/name association performance after three months of physical training in elderly women. Journal of Physiology & Pharmacology 2006;57(Suppl 4):417‐24. [PubMed] [Google Scholar]
References to studies awaiting assessment
Chapman 2013 {published data only}
- Chapman SB, Aslan S, Spence JS, Defina LF, Keebler MW, Didehbani N, et al. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Frontiers in Aging Neuroscience 2013;5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2014 {published data only}
- Linde K, Alfermann D. Single versus combined cognitive and physical activity effects on fluid cognitive abilities of healthy older adults: a 4‐month randomized controlled trial with follow‐up. Journal of Aging and Physical Activity 2014;22(3):302‐13. [DOI] [PubMed] [Google Scholar]
Additional references
Abbott 2004
- Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA 2004;292(12):1447‐53. [DOI] [PubMed] [Google Scholar]
Aleman 2000
- Aleman A, Haan EHF, Verhaar HJJ, Samson MM, Vries WR, Koppeschaar HP. Relationship between cognitive and physical function in healthy older men; a role for aerobic power?. Journal of the American Geriatrics Society 2000;48(1):104‐5. [DOI] [PubMed] [Google Scholar]
Angevaren 2007
- Angevaren M, Vanhees L, Wendel‐Vos W, Verhaar HJJ, Aufdemkampe G, Aleman A, et al. Intensity, but not duration, of physical activities is related to cognitive function. European Journal of Cardiovascular Prevention and Rehabilitation 2007;14(6):825‐30. [DOI] [PubMed] [Google Scholar]
Barnes 2003
- Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. Journal of the American Geriatrics Society 2003;51(4):459‐65. [DOI] [PubMed] [Google Scholar]
Boutron 2005
- Boutron I, Moher D, Tugwell P, Giraudeau B, Poiraudeau S, Nizard R, et al. A checklist to evaluate a report of a nonpharmaceutical trial (CLEAR NPT) was developed using consensus. Journal of Clinical Epidemiology 2005;58(12):1233‐40. [DOI] [PubMed] [Google Scholar]
Brown 2008
- Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiology of Aging 2008;31(12):2047‐57. [DOI] [PubMed] [Google Scholar]
Brown 2012
- Brown BM, Peiffer JJ, Sohrabi HR, Mondal A, Gupta VB, Rainey‐Smith SR, et al. Intense physical activity is associated with cognitive performance in the elderly. Translational Psychiatry 2012;2:e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 1993
- Christensen H, Mackinnon A. The association between mental, social and physical activity and cognitive performance in young and old subjects. Age and Ageing 1993;22(3):175‐82. [DOI] [PubMed] [Google Scholar]
Churchill 2002
- Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiology of Aging 2002;23(5):941‐55. [DOI] [PubMed] [Google Scholar]
Colcombe 2003
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults; a meta‐analytic study. Psychological Science 2003;14(2):125‐30. [DOI] [PubMed] [Google Scholar]
Colcombe 2004
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America 2004;101(9):3316‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotman 2002
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences 2002;25(6):295‐301. [DOI] [PubMed] [Google Scholar]
Cotman 2007
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in Neurosciences 2007;30(9):464‐72. [DOI] [PubMed] [Google Scholar]
Crooks 2008
- Crooks V, Lubben J, Petitti D, Little D, Chiu V. Social network, cognitive function, and dementia incidence among elderly women. American Journal of Public Health 2008;98(8):1221‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davenport 2012
- Davenport MH, Hogan DB, Eskes GA, Longman RS, Poulin MJ . Cerebrovascular reserve: the link between fitness and cognitive function?. Exercise and Sport Sciences Reviews 2012;40(3):153‐8. [DOI] [PubMed] [Google Scholar]
Erickson 2009
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009;19(10):1030‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Etgen 2010
- Etgen T, Sander D, Huntgeburth U, Poppert H, Förstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Archives of Internal Medicine 2010;170(2):186‐93. [DOI] [PubMed] [Google Scholar]
Etnier 1997b
- Etnier JL, Salazar W, Landers DM, Petruzzello SJ, Han M, Nowell P. The influence of physical fitness and exercise upon cognitive functioning; a meta‐analysis. Journal of Sports and Exercise Psychology 1997;19:249‐77. [Google Scholar]
Etnier 2006
- Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta‐regression to examine the relationship between aerobic fitness and cognitive performance. Brain Research Reviews 2006;52(1):119‐30. [DOI] [PubMed] [Google Scholar]
Etnier 2007
- Etnier JL, Caselli RJ, Reiman EM, Aleander GE, Sibley BA, Tessier D, et al. Cognitive performance in older women relative to ApoE‐e4 genotype and aerobic fitness. Medicine and Science in Sports and Exercise 2007;39(1):199‐207. [DOI] [PubMed] [Google Scholar]
Heyn 2004
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia; a meta‐analysis. Archives of Physical Medicine and Rehabilitation 2004;85(10):1694‐704. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors) [Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011]. Available at www.cochrane‐handbook.org.
Hultsch 1993
- Hultsch DF, Hammer M, Small BJ. Age differences in cognitive performance in later life: relationships to self‐reported health and activity life style. Journal of Gerontology 1993;48(1):P1‐11. [DOI] [PubMed] [Google Scholar]
Hultsch 1999
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging?. Psychology and Aging 1999;14(2):245‐63. [DOI] [PubMed] [Google Scholar]
Kessels 2000
- Kessels RPC, Aleman A, Verhagen WIM, Luijtelaar EL. Cognitive functioning after whiplash injury: a meta‐analysis. Journal of the International Neuropsychological Society 2000;6(3):271‐8. [DOI] [PubMed] [Google Scholar]
Lachman 2010
- Lachman ME, Agrigoroaei S, Murphy C, Tun PA. Frequent cognitive activity compensates for education differences in episodic memory. American Journal of Geriatric Psychiatry 2010;18(1):4‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Landis 1977
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33(1):159‐74. [PubMed] [Google Scholar]
Laurin 2001
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Archives of Neurology 2001;58(3):498‐504. [DOI] [PubMed] [Google Scholar]
Lemura 2000
- Lemura LM, Duvillard SP, Mookerjee S. The effects of physical training of functional capacity in adults. Ages 46 to 90: a meta‐analysis. Journal of Sports Medicine and Physical Fitness 2000;40(1):1‐10. [PubMed] [Google Scholar]
Lezak 2004
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York: Oxford University Press, 2004. [Google Scholar]
Lojovich 2010
- Lojovich JM. The relationship between aerobic exercise and cognition: is movement medicinal?. Journal of Head Trauma Rehabilitation 2010;25(3):184‐92. [DOI] [PubMed] [Google Scholar]
Marquine 2012
- Marquine MJ, Segawa E, Wilson RS, Bennett DA, Barnes LL. Association between cognitive activity and cognitive function in older Hispanics. Journal of the International Neuropsychological Society 2012;18(6):1041‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2003
- Martin M, Zimprich D. Are changes in cognitive functioning in older adults related to changes in subjective complaints?. Experimental Aging Research 2003;29(3):335‐52. [DOI] [PubMed] [Google Scholar]
McAuley 2004
- McAuley E, Kramer AF, Colcombe SJ. Cardiovascular fitness and neurocognitive function in older adults: a brief review. Brain, Behavior and Immunity 2004;18(3):214‐20. [DOI] [PubMed] [Google Scholar]
Middleton 2011
- Middleton LE, Manini TM, Simonsick EM, Harris TB, Barnes DE, Tylavsky F, et al. Activity energy expenditure and incident cognitive impairment in older adults. Archives of Internal Medicine 2011;171(14):1251‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newson 2006
- Newson RS, Kemps EB. The nature of subjective cognitive complaints of older adults. International Journal of Aging and Human Development 2006;63(2):139‐51. [DOI] [PubMed] [Google Scholar]
Panagiotakos 2007
- Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Preventive Medicine 2007;44(4):335‐40. [DOI] [PubMed] [Google Scholar]
Podewils 2005
- Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. American Journal of Epidemiology 2005;161(7):639‐51. [DOI] [PubMed] [Google Scholar]
Prins 2002
- Prins ND, Heijer T, Hofman A, Koudstaal PJ, Jolles J, Clarke R, et al. Homocysteine and cognitive function in the elderly: the Rotterdam Scan Study. Neurology 2002;59(9):1375‐80. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 2003
- Richards M, Hardy R, Wadsworth ME. Does active leisure protect cognition? Evidence from a national birth cohort. Social Science & Medicine 2003;56(4):785‐92. [DOI] [PubMed] [Google Scholar]
Salthouse 1996
- Salthouse TA. General and specific speed mediation of adult age differences in memory. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 1996;51(1):30‐42. [DOI] [PubMed] [Google Scholar]
Salthouse 2003
- Salthouse TA. Memory aging from 18 to 80. Alzheimer's Disease and Associated Disorders 2003;17(3):162‐7. [DOI] [PubMed] [Google Scholar]
Schuit 2001
- Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Medical Science and Sports Exercise 2001;33(5):772‐7. [DOI] [PubMed] [Google Scholar]
Seeman 2001
- Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high‐functioning older adults: MacArthur studies of successful aging. Health Psychology 2001;20(4):243‐55. [DOI] [PubMed] [Google Scholar]
Smith 2010
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh‐Bohmer K, et al. Aerobic exercise and neurocognitive performance: a meta‐analytic review of randomized controlled trials. Psychosomatic Medicine 2010;72(3):239‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sturman 2005
- Sturman MT, Morris MC, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Physical activity, cognitive activity, and cognitive decline in a biracial community population. Archives of Neurology 2005;62(11):1750‐4. [DOI] [PubMed] [Google Scholar]
Tangney 2011
- Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean‐type dietary pattern and cognitive decline in a community population. American Journal of Clinical Nutrition 2011;93(3):601‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2010
- Tierney MC, Moineddin R, Morra A, Manson J, Blake J. Intensity of recreational physical activity throughout life and later life cognitive functioning in women. Journal of Alzheimer's Disease 2010;22(4):1331‐8. [DOI] [PubMed] [Google Scholar]
van Gelder 2004
- Gelder BM, Tijhuis MAR, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology 2004;63(12):2316‐21. [DOI] [PubMed] [Google Scholar]
van Uffelen 2008
- Uffelen JGZ, Chin A Paw MJM, Hopman‐Rock M, Mechelen W. The effects of exercise on cognition in older adults with and without cognitive decline: a systematic review. Clinical Journal of Sport Medicine 2008;18(6):486‐500. [DOI] [PubMed] [Google Scholar]
Verhaeghen 1997
- Verhaeghen P, Salthouse TA. Meta‐analyses of age‐cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychological Bulletin 1997;122(3):231‐49. [DOI] [PubMed] [Google Scholar]
Wilson 1999
- Wilson RS, Bennett DA, Beckett LA, Morris MC, Gilley DW, Bienias JL, et al. Cognitive activity in older persons from a geographically defined population. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 1999;54(3):P155‐60. [DOI] [PubMed] [Google Scholar]
Wilson 2005
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society 2005;11(4):400‐7. [PubMed] [Google Scholar]
WPP 2006
- World Population Prospects, The 2006 Revision Population Database. United Nations. http://www.un.org/esa/population/publications/wpp2006/wpp2006.htm (accessed 15 November 2007).
References to other published versions of this review
Angevaren 2008a
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD005381.pub2] [DOI] [PubMed] [Google Scholar]
Angevaren 2008b
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews 2008, Issue 7. [DOI: 10.1002/14651858.CD005381.pub3] [DOI] [PubMed] [Google Scholar]


