Abstract
HIV-associated comorbidities, such as ischemic stroke, are prevalent in people with HIV (PWH). Several studies both in animal models and humans revealed an association between inflammasome activation in HIV-1 infection and stroke. The gut microbiota is an important component in controlling neuroinflammation in the central nervous system (CNS). It has also been proposed to be involved in the pathobiology of HIV-1 infection, and has been associated with an increase in inflammasome activation. In this review, we provide an overview of the microbiota-gut-inflammasome-brain axis, focusing on the NLRP3 inflammasome and microbiome dysregulation as risk factors that may contribute to ischemic stroke outcome and recovery in PWH. We also focus on the potential of targeting the NLRP3 inflammasome as a novel therapeutic approach for PWH who are at risk of developing cerebrovascular diseases.
Keywords: brain, AIDS, neurovascular, cerebrovascular, gut microbiome, gut microbiota
Ischemic stroke and HIV-1 Infection
In the early stages of HIV-1 infection, the virus infects CD4+ circulating cells in the blood, which may then pass through the blood-brain barrier (BBB) and infect cells of the central nervous system (CNS). Microglial cells and perivascular macrophages have been identified as targets for HIV-1 in the CNS; moreover, astrocytes and pericytes may be susceptible to viral infections [1-4] . The inefficient transfer of antiretroviral drugs across the BBB contributes to the persistent infection of CNS cells, leading to neuroinflammatory responses, the formation of HIV-1 reservoirs, and the development of cerebrovascular comorbidities [5].
Prior to the antiretroviral therapy (ART) era, ischemic stroke in people with HIV-1 (PWH) was found to be strongly correlated with viral load to occur mostly with advanced AIDS, and to be complicated by secondary infections, coagulopathies, and/or vasculitis [6]. The incidence of ischemic stroke was estimated to be about nine times higher in PWH than in healthy individuals [7]. After the introduction of ART, the incidence of ischemic stroke in PWH decreased; however, it is still approximately 3 times higher in PWH than in the uninfected population [8]. Moreover, stroke is one of the leading causes of death among PWH [6], and PWH typically experience worsened stroke outcomes and poststroke recovery, as compared to sex- and age-matched uninfected population [9-12] (Figure 1). ART with a high CNS penetration effectiveness (CPE) has been shown to better ameliorate the adverse side effects of stroke as compared to ART with a low CPE [6, 11, 13]. On the other hand, it was hypothesized that ART might also contribute to predisposition to stroke by accelerating vascular complications and indirectly by increasing life expectancy [13][14].
Figure 1. HIV-1 infection can worsen ischemic stroke outcomes.
HIV-1 infects CD4+ cells in the blood, allowing the virus to bypass the BBB and enter the brain via a ‘Trojan horse’ mechanism. The limited ability of antiretroviral drugs to bypass the BBB leads to insufficient antiretroviral protection, contributes to the formation of HIV reservoirs in the brain, and enhances the prevalence of cerebrovascular events, such as ischemic stroke. Moreover, PWH often experience more severe ischemic stroke outcomes. Figure created with BioRender (www.BioRender.com).
The mechanisms involved in the increased prevalence of ischemic stroke in HIV infection remain poorly understood. Here, we discuss the inflammasome and the gut microbiota as a potential link between these two pathologies, and the implications of this hypothesis for more targeted therapeutic strategies to treat ischemic stroke in PWH.
The NLRP3 inflammasome as a go-between in gut-brain axis communication
The inflammasome is an innate immune signaling complex of receptors and sensors that are activated in response to pathogens and environmental factors [15]. To date, the NLRP3 inflammasome is arguably the best characterized inflammasome complex, formed by the NLRP3 protein, the adapter apoptosis-associated speck-like protein containing CARD (ASC or Pycard), and the inflammatory pro-caspase-1. A two-stage process regulates NLRP3 inflammasome activation. The first is an initial priming stage, consisting of NLRP3 inflammasome formation mediated by pattern recognition receptors (PRRs) and toll-like receptors (TLRs). This stage concludes with the activation of the nuclear factor NFkappaB (NFκB), an increase in functional NLRP3, caspase-1 cleavage, and the expression of pro-interleukin-1β (pro-IL-1β) and pro-interleukin-18 (pro-IL-18) [16, 17]. The second stage is the activation stage, induced by multiple exogenous and endogenous factors like pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs) (Figure 2).
Figure 2. The NLRP3 inflammasome.
A. Components of the NLRP3, caspase recruitment domain-containing protein 8 (CARD8), and IFN-inducible protein 16 (IFI16) inflammasome. B. Schematic representation of the NLRP3, CARD8, and IFI16 inflammasome complexes. C. NLRP3 inflammasome activation. Signal 1: NLRP3 inflammasome priming. Signal 2: NLRP3 inflammasome activation. Description in text. Figure created with BioRender (www.BioRender.com).
The gut-brain axis refers to a complex, bidirectional communication between the nervous system and the gastrointestinal tract. Several pathways have been described as being involved in this axis, such as the hypothalamic-pituitary-adrenal axis, the endocrine system, and the immune system, which includes the bacteria metabolites and products from the intestinal microbiota [18, 19]. Indeed, the gut microbiota affects the innate immune system by expressing microbe-associated molecular patterns (MAMPs), which control the innate immune responses [20, 21]. The importance of the microbiota-gut-brain axis has been illustrated using several approaches such as the use of antibiotic-induced microbiota perturbation in mice [22], germ-free mice [23, 24], microbiota fecal transplantation [25] or probiotic treatments in both animal models and human populations, for instance in people with major depressive disorder [26-28]. In the past few years, more research has been conducted, highlighting the significant role the microbiota-gut-brain-axis plays in the maintenance of brain homeostasis and in the pathophysiology of neurological and psychiatric disorders [29] [30]. In studies in mice, it was demonstrated for instance that dysbiosis can trigger an increase in the mRNA expression of caspase-1, ASC, IL-18, and IL-1β in both intestinal and brain tissues [31]. Among different inflammasomes, the NLRP3 inflammasome has been proposed as a critical factor affecting the microbiota-gut-brain axis. Of note, several psychiatric and neurological disorders, including Alzheimer’s Disease, bipolar disorder, and Parkinson’s disease, are associated with upregulation of the NLRP3 inflammasome, and a role for the microbiota-gut-brain-inflammasome axis in this context has been proposed [30].
Gut dysbiosis and the activation of the NLRP3 inflammasome in HIV-1 infection
HIV-1 infects the gastrointestinal system during the onset of infection, leading to a large decrease in CD4+ T cells [32, 33]. Compelling evidence indicates that the virus diminishes the abundance and biodiversity of gut bacteria, and alters the composition of the gut microbiome, which may contribute to comorbidities and alterations of persistent immune responses seen in PWH [34, 35]. As the gastrointestinal tract plays a central role in the body’s immune system, gut dysbiosis likely contributes to an increase in opportunistic infections in PWH [32, 36], which can lead to the dysfunction of the epithelial barrier and leakage of bacteria into the circulatory system [34].
The role of inflammasome complexes in regulation of the innate immune system and inflammatory responses is particularly relevant to HIV-1 infection. The inflammasome complexes IFI16, CARD8, AIM2, NLRC4, NLRP3, NLRX1 and NLRP1 were shown to be activated upon HIV-1 infection in mice and PWH [37-43]. The NLRP3 inflammasome complex, however, is likely to be the most integral to these processes due to its role in viral entry, CD4+ T cell death, and chronic inflammation [44, 45]. The mechanisms of NLRP3 activation in HIV-1 infections remain largely unknown. At the start of viral entry into the host cell, NLRP3 acts as a factor that inhibits the conformational changes in F-actin polymers, which are required for HIV-1 to enter the cell. The involvement of a variety of alternative molecular mechanisms was suggested, including activation through HIV-1 RNAs and/or viral proteins [46-50] (Figure 3). As the infection progresses, the expression of NLRP3 in host cells increases over time [51-53]. The effector proteins of the inflammasome pathway, IL-18 and IL-1β, were suggested to play an excitatory role in viral replication and diminishing ART effectiveness in PWH [54].
Figure 3. HIV-1 induces NLRP3 inflammasome activation.
In the early stages of HIV-1 infection, the P2Y2 receptor is activated by ATP and induces PYK2 phosphorylation. PYK2, together with CBL, ubiquitinates and degrades NLRP3. In advanced HIV-1 infection, both nucleic acids and the virus contribute to the NLRP3 inflammasome activation. HIV-1 dsRNA is recognized by PKR and activates the NLRP3 inflammasome through ROS production and ERK1/2, JNK, and p38 activation. Through TLR8, HIV-1 ssRNA leads to an increase in ROS production and the protease cathepsin B release that induces NLRP3 upregulation and activation. Moreover, Vpr and Tat activate the NLRP3 inflammasome via the NFκB signaling pathway and/or by inhibiting miR-223 production. Furthermore, the envelope HIV-1 glycoprotein gp120 induces K+ efflux. The active NLRP3 complex results in caspase-1 cleavage and the production of proinflammatory cytokines IL-1β and IL-18. Abbreviations: PYK2, proline-rich tyrosine kinase 2; CBL, E3 ubiquitin ligase; PKR, IRNA-activated protein kinase; ROS, reactive oxygen species; MAPK, mitogen-activated protein kinases; Vpr, viral protein R; Tat, transactivator of transcription. Figure created with BioRender (www.BioRender.com).
Another possible pathway linking NLRP3 with HIV-1 infection is through the crosstalk between NFκB and the nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a key transcription factor in regulating oxidative stress and the expression of anti-oxidative enzymes [55, 56]. The fact that NLRP3 inflammasome function depends on the activation of the NFκB signaling pathway suggests Nrf2 as a key factor in regulating the NLRP3 inflammasome activation. Indeed, studies have indicated that reactive oxygen species (ROS) can regulate NLRP3 activity [57], pointing to Nfr2 as a potential regulator of NLRP3. Importantly, oxidative stress is a well-recognized factor of the CNS pathology in HIV-1 infection [58]. In this context, Nrf2 signaling can play a key role in regulating HIV infection. In macrophages, it has been reported that NRF2 activation modulated HIV-1 replication and apoptosis [59]. In HIV-1 transgenic rats, Nrf2 activation has been shown to restore barrier function in the alveolar epithelium [60].
Gut dysbiosis and the NLRP3 inflammasome in ischemic stroke
Multiple studies have related changes in gut microbiota to ischemic stroke. Both during and after the acute stage of ischemic stroke, the microbial composition of the gut is significantly altered as shown in both mouse models and in human studies [61-63]. In germ-free mice, transplantation of fecal microbiota normalized brain lesion-induced dysbiosis, improved stroke outcomes, and enhanced stroke recovery [61]. Moreover, animal model studies suggested that maintaining homeostasis of the gut microbiota could improve the efficacy of ischemic stroke treatment by regulating immunological, metabolic, and inflammatory responses through the microbiota-gut-brain axis [64, 65]. Augmented immune cell migration into the brain due to metabolic endotoxemia enhances neuroinflammation and contributes to chronic systemic inflammation in mouse models [61, 66]. On the other hand, studies performed in rodents showed that altered signaling to the gut-brain axis in stroke results in impaired intestinal barrier integrity, decreased mucus secretion, and intestinal bacterial translocation into the circulation and extraintestinal organs [67, 68]. Risk factors that worsen ischemic stroke, such as age, hypertension, diabetes, obesity, and vascular dysfunction, have also been linked to microbiome dysbiosis [69]. Interestingly, Treg and IL-17-producing γδ T cells have been linked to the development of ischemic injuries [70, 71].
Several inflammasome proteins, such as NLRP1, NLRP3, AIM2, and NLRC4, have been related to stroke outcome and post-stroke recovery [72-76]. However, the NLRP3 inflammasome appears to be the most significant inflammasome sensor in the brain of stroke patients [16, 76, 77]. Most critically, blocking the NLRP3 inflammasome in mice reduces endothelial cell death through a decrease in pyroptosis, which in turn reduces BBB breakdown [78]. Using mouse model of ischemic stroke, increasing BBB stability through NLRP3 inhibition has been proposed to prevent cognitive decline and neuroinflammatory responses following ischemic stroke, and reduce infarct volume [79-81]. Even though the mechanisms controlling NLRP3 inflammasome activation in ischemic stroke remain poorly understood, studies in rodents have demonstrated correlations between the activation of the NLRP3 inflammasome, potassium efflux, mitochondrial ROS release, and lysosomal damage [57, 82-84], providing clues into potential underlying mechanisms (Figure 4).
Figure 4. Ischemic stroke induces NLRP3 inflammasome activation.
Studies in rodent models of ischemic stroke indicate that following stroke, the accumulation and release of ROS, K+ efflux damage, and the lysosomal membrane rupture induce NLRP3 inflammasome activation [57, 82-84]. The active NLRP3 complex results in caspase-1 cleavage and the production of pro-inflammatory cytokines IL-1β and IL-18. Figure created with BioRender (www.BioRender.com).
As noted earlier, HIV infection results in alterations in the biodiversity and abundance of the gut microbiota; however, the proposed links between gut dysbiosis or inflammasome activation to ischemic stroke in the context of HIV infection remain to be tested, and to our knowledge, have not been directly examined so far. HIV-1 uses chemokine co-receptors CCR5 or CXCR4 to facilitate cell entry. While the transmission of the CCR5-tropic (R5) viral strains may be more efficient than the CXCR4-tropic (X4) strains, X4 viruses are associated with more pronounced depletion of CD4+ T cells and an accelerated rate of disease progression and mortality as compared to R5 viruses [85]. Importantly, X4 strains appear to be more potent in inducing NLRP3 inflammasome activation than R5 viruses, and this effect can be amplified by mitochondrial damage [86]. IL-18 production triggered by the NLRP3 inflammasome can cause further upregulation of the CXCR4 receptor and, consequently, favor X4 HIV-1 infection and replication in immune cells [87]. Increased CXCR4 signaling was shown to be implicated in neuronal death associated with ischemic stroke [88], although to our knowledge, no studies explored the involvement of the CXCR4 receptor in the NLRP3 inflammasome activation in the context of this pathology. Nevertheless, knockdown or pharmacological blocking of CCR5 improved motor recovery, attenuated cognitive decline, and reduced lesion area and hippocampal neuron loss in a mouse model of ischemic stroke [89, 90]. It would be interesting to explore whether the neuroprotective role of targeting CCR5 signaling in ischemic stroke is mediated by inhibition of inflammasome activation both in the context of HIV-1 infection and outside it.
Therapeutic interventions by targeting the NLRP3 inflammasome
There are several approaches available by which the NLRP3 inflammasome can be targeted. One strategy focuses on the thioredoxin-interacting protein (TXNIP), which is essential to NLRP3 assembly and activation. In preclinical studies, various molecules have been used to inhibit TXNIP, such as Nrf2, umbelliferon, and curcumin [91-93]. These compounds have broader effects and are not selective inhibitors of TXNIP. For example, treatment with umbelliferon reduced TXNIP and activated PPAR-γ, which has protective effect in focal cerebral ischemic in rats [91].
Another way to target the inflammasome is to inhibit ASC speck formation. MCC950 and β-hydroxybutyrate (BHB) are two molecules that have been extensively studied in mouse and rat models for their ability to inhibit the formation of the ASC speck [94-96]. In addition, CY-09 was shown to specifically inhibit ASC oligomerization in mice and patients with gout [97]. Moreover, targeting the signals that activate NLRP3, the use of agonists, the membrane-based purinergic 7 (P2X7), as well as protein modifications and translational regulation have been shown to inhibit NLRP3 in rodents [73, 79, 98-105], and can be envisioned as promising approaches to inhibit the NLRP3 inflammasome to mitigate neurological defects in stroke and HIV-1, although their relevance in this context and in clinical settings remains to be tested.
Other encouraging strategies are based on targeting the involved interleukins and/or their downstream signaling. Notably, inhibition of interleukin-1 receptor (IL-1R) by recombinant human IL-1R antagonist (rhIL-1Ra) in placebo controlled pilot studies in patients with acute stroke and subarachnoid hemorrhage indicated rhIL-1Ra as a potential therapeutic target for cerebrovascular diseases, including acute stroke [106, 107]. Anakinra is one of the IL-1R inhibitor products on the market. Pilot studies indicated its effectiveness in people with familiar cold autoinflammatory syndrome [108, 109]. Two additional IL-1R inhibitors used in pilots studies in people with cryopyrin-associated periodic syndromes [110] are Rilonacept and Canakinumab [109, 111-113]. Anakinra and Canakinumab entered clinical trials as potential therapeutic agents to reduce cardiovascular risk, intracerebral hemorrhage, and brain damage after hemorrhagic stroke i,ii,iii (Figure 5).
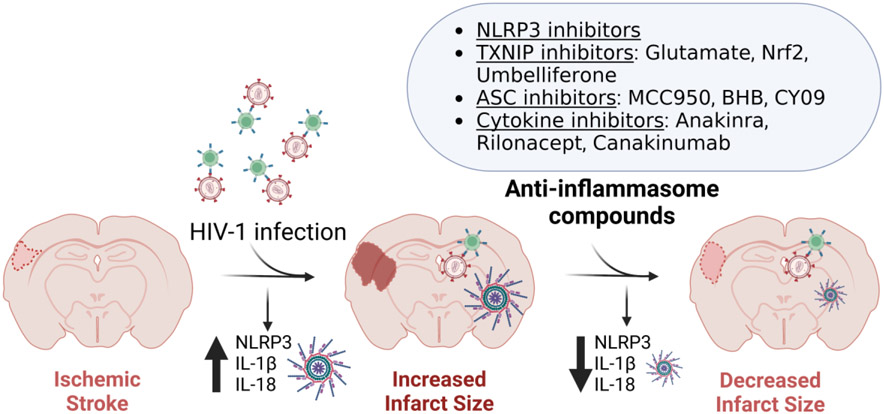
Figure 5. Inhibition of the NLRP3 inflammasome as a therapeutic target in ischemic stroke.
The NLRP3 inflammasome is activated after HIV-1 infection as shown in PWH and in animal models [37-43], which can then contribute to increased ischemic infarct size by promoting a proinflammatory environment via the production of IL1β and IL18. The use of anti-inflammasome factors that target NLRP3 inflammasome activation is an emerging therapeutic strategy that may improve ischemic stroke outcomes in PWH. Figure created with BioRender (www.BioRender.com).
Learning from therapeutic approaches that have been explored in people living with long COVID [114], the use of interferons and/or melatonin might be useful as potential anti-inflammatory options to examine in HIV infection. In terms of correcting gut dysbiosis, fecal microbial transplant and the use of probiotic supplements have been proposed [115, 116]. In a study performed on simian immunodeficiency virus (SIV)-infected rhesus macaques, fecal microbial transplant appeared to have a positive effect in mitigating gut dysbiosis [115]. In addition, a study on probiotic supplementation in PWH suggested that probiotics may decrease levels of IL-1β and other cytokines [116].
Concluding remarks and future perspectives
Several studies have described an increase in the incidence of cardiovascular and cerebrovascular diseases, such as ischemic stroke, in PWH. Yet, the mechanisms involved in the enhanced prevalence of stroke in PWH are not fully understood. Here, we discussed associations between gut dysbiosis, inflammasome activation and HIV-1 infection, and proposed gut dysbiosis and inflammasome activation as a potential link between HIV-1 infection and stroke outcomes. If validated in future studies, this framework highlights targeting the microbiota-gut-inflammasome-brain axis, and in particular the NLRP3 inflammasome as a potential approach to improve ischemic stroke outcomes and post-stroke recovery in PWH.
Among the various inflammasomes, NLRP3 has been recognized as a key mediator of stroke outcomes and post-ischemic inflammation. Furthermore, several studies have indicated connections between an increase in NLRP3 activation and gut dysbiosis. The NLRP3 inflammasome can recognize a variety of stimuli, including HIV-1, leading to an increase in the expression of proinflammatory cytokines and activation of inflammatory responses. These responses have been associated with neuronal damage and system-level outcomes.
HIV-1 infection has been linked to both activation of the NLRP3 inflammasome and the development of gut dysbiosis. However, there is only limited information on their mechanistic role in ischemic stroke in PWH (see Outstanding questions). While the gut-brain-axis plays an important role in stimulating neuroinflammatory responses, the exact components and the mechanisms of this influence remain poorly understood. Even more importantly, clinical studies are needed to address the question as to whether effective protection against gut dysbiosis can mitigate stroke complications and/or enhance post-stroke recovery in PWH.
Outstanding Questions.
Which signaling pathways contribute to the severity of ischemic stroke in HIV-1 infection?
How does the gut-brain axis influence the occurrence and progression of ischemic stroke in individuals with comorbid HIV-1 infection?
To what extent does the NLRP3 inflammasome play a role in facilitating the interaction between the central nervous system (CNS) and the microbiota through the gut-brain axis? Are there other inflammasomes involved in this communication? Furthermore, what is the comparative impact of inflammasome factors produced by the gut microbiota versus factors generated by the host?
What is the potential role of the NLRP3 inflammasome in the pathomechanism of ischemic stroke? How may a better understanding of the NLRP3 pathway contribute to the identification of novel therapeutic targets for the treatment of ischemic stroke, both in the context of HIV infection and more broadly?
In this review, we highlighted both HIV-induced microbiota dysbiosis and inflammasome activation as potential contributors to worsening ischemic stroke outcome and recovery (Figure 6, Key Figure). The growing interest in targeting the inflammasome as an approach for treating inflammatory and metabolic diseases, including stroke, is leading to an expansion of scientific knowledge in this area. In this context, the NLRP3 inflammasome appears to be a promising target for treating stroke in PWH.
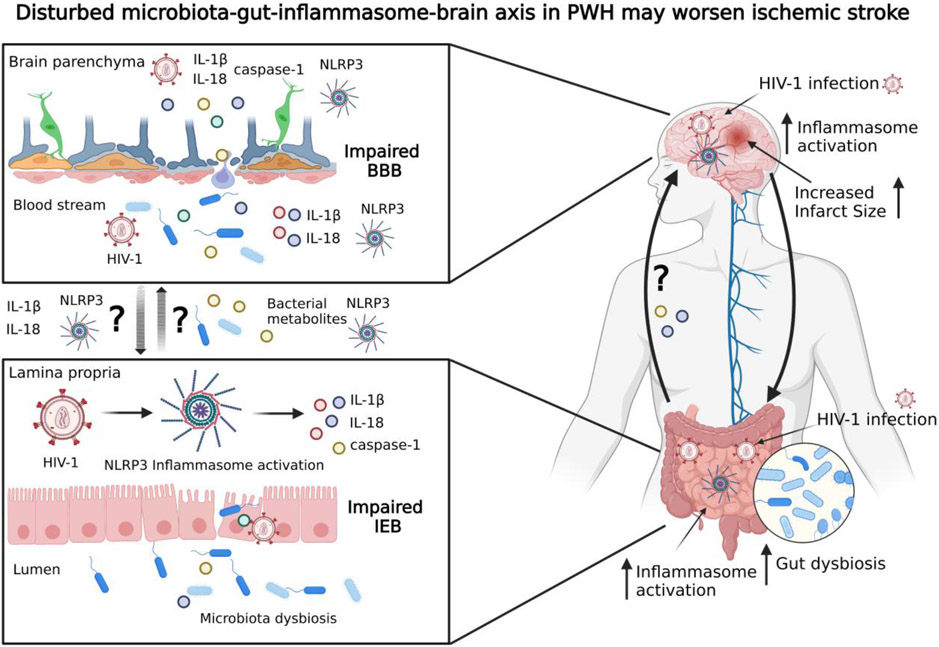
Figure 6, Key Figure. Dysregulation of the microbiota-gut-inflammasome-brain axis in HIV-1 infection may worsen ischemic stroke.
HIV-1 can infect both the CNS and the gut, activate the NLRP3 inflammasome, and induce dysregulation of the gut microbiota. Gut dysbiosis also induces NLRP3 inflammasome activation through a positive feedback loop. Some of the effector mediators of NLRP3 inflammasome activation are IL-1β, IL-18 and caspase-1, whose release may lead to impairments of the intestinal epithelial barrier. Bacterial metabolites and proinflammatory cytokines can travel from the gut to the brain via the gut-brain axis, where they may increase NLRP3 inflammasome activation and BBB damage. It has been proposed that activation of the NLRP3 inflammasome and enhanced inflammatory responses are important mechanisms contributing to worsening of ischemic stroke outcomes and post-stroke recovery in PWH. BBB, blood-brain barrier; IEB, intestinal epithelium barrier. Figure created with BioRender (www.BioRender.com).
Highlights.
Ischemic stroke is among the common comorbidities of HIV-1 infection. People with HIV (PWH) experience increased stroke susceptibility, more severe ischemic stroke outcomes, and delayed post-stroke recovery.
The gut microbiota and the central nervous system exhibit a bidirectional relationship, which is affected by both HIV-1 and ischemic stroke. The NLRP3 inflammasome is a key mediator in the microbiota-gut-brain axis.
HIV-1 infection can lead to gut dysbiosis and NLRP3 inflammasome activation, making people with HIV more susceptible to comorbidities and infections due to the integral involvement of the gastrointestinal tract in the body’s immune system.
Alterations of the gut microbiota may contribute to stroke severity. It is hypothesized that ischemic stroke treatment efficacy may be improved when coupled with maintenance of gut homeostasis. Blocking the NLRP3 inflammasome serves as a potential treatment for preventing neurological decline after stroke.
Acknowledgements
MT, TT, NF were supported by the National Institutes of Health (NIH) grants DA050528, DA044579, HL126559, MH128022, MH122235, and MH072567. ST was supported by the NIH/NIAID grant P30AI073961. ON was supported by the Research Supplement to Promote Diversity in Health-Related Research Program from NIDA grant DA050528-02S1. LD and LM were a part of the American Heart Association Hispanic Serving Institutions Scholars Program supported by Quest Diagnostics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors report no competing interests.
References
- 1.Bertrand L, Cho HJ, and Toborek M, Blood-brain barrier pericytes as a target for HIV-1 infection. Brain, 2019. 142(3): p. 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torices S, et al. , Expression of SARS-CoV-2-related receptors in cells of the neurovascular unit: implications for HIV-1 infection. J Neuroinflammation, 2021. 18(1): p. 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torices S, et al. , Occludin, caveolin-1, and Alix form a multi-protein complex and regulate HIV-1 infection of brain pericytes. FASEB J, 2020. 34(12): p. 16319–16332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElrath MJ, et al. , HIV-infected macrophages as efficient stimulator cells for detection of cytotoxic T cell responses to HIV in seronegative and seropositive vaccine recipients. AIDS Res Hum Retroviruses, 1994. 10(5): p. 541–9. [DOI] [PubMed] [Google Scholar]
- 5.Osborne O, et al. , The Paradox of HIV Blood-Brain Barrier Penetrance and Antiretroviral Drug Delivery Deficiencies. Trends Neurosci, 2020. 43(9): p. 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ismael S, et al. , HIV Associated Risk Factors for Ischemic Stroke and Future Perspectives. Int J Mol Sci, 2020. 21(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole JW, et al. , Acquired immunodeficiency syndrome and the risk of stroke. Stroke, 2004. 35(1): p. 51–6. [DOI] [PubMed] [Google Scholar]
- 8.Alonso A, et al. , HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. J Am Heart Assoc, 2019. 8(14): p. e012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertrand L, et al. , Targeting the HIV-infected brain to improve ischemic stroke outcome. Nat Commun, 2019. 10(1): p. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogorodskaya M, Chow FC, and Triant VA, Stroke in HIV. Can J Cardiol, 2019. 35(3): p. 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orviz E, et al. , HIV screening and its possible involvement in patients with stroke. Enferm Infecc Microbiol Clin (Engl Ed), 2020. 38(7): p. 350–351. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen I, Kim AS, and Chow FC, Prevention of stroke in people living with HIV. Prog Cardiovasc Dis, 2020. 63(2): p. 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertrand L, Dygert L, and Toborek M, Antiretroviral Treatment with Efavirenz Disrupts the Blood-Brain Barrier Integrity and Increases Stroke Severity. Sci Rep, 2016. 6: p. 39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin LA, et al. , HIV infection and stroke: current perspectives and future directions. Lancet Neurol, 2012. 11(10): p. 878–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broz P and Dixit VM, Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol, 2016. 16(7): p. 407–20. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, et al. , NLRP3 Inflammasome and Inflammatory Diseases. Oxid Med Cell Longev, 2020. 2020: p. 4063562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauernfeind FG, et al. , Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol, 2009. 183(2): p. 787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duvallet C, et al. , Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat Commun, 2017. 8(1): p. 1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burberry A, et al. , C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature, 2020. 582(7810): p. 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell C, et al. , Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines, 2023. 11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu WL, et al. , Microbiota regulate social behaviour via stress response neurons in the brain. Nature, 2021. 595(7867): p. 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guida F, et al. , Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav Immun, 2018. 67: p. 230–245. [DOI] [PubMed] [Google Scholar]
- 23.Luczynski P, et al. , Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int J Neuropsychopharmacol, 2016. 19(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luk B, et al. , Postnatal colonization with human "infant-type" Bifidobacterium species alters behavior of adult gnotobiotic mice. PLoS One, 2018. 13(5): p. e0196510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh R, et al. , Fecal microbiota transplantation against intestinal colonization by extended spectrum beta-lactamase producing Enterobacteriaceae: a proof of principle study. BMC Res Notes, 2018. 11(1): p. 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabouy L, et al. , Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain Behav Immun, 2018. 73: p. 310–319. [DOI] [PubMed] [Google Scholar]
- 27.Kazemi A, et al. , Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin Nutr, 2019. 38(2): p. 522–528. [DOI] [PubMed] [Google Scholar]
- 28.Fukui H, et al. , Effect of probiotic Bifidobacterium bifidum G9-1 on the relationship between gut microbiota profile and stress sensitivity in maternally separated rats. Sci Rep, 2018. 8(1): p. 12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatt S, et al. , Role of Brain-Gut-Microbiota Axis in Depression: Emerging Therapeutic Avenues. CNS Neurol Disord Drug Targets, 2023. 22(2): p. 276–288. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrini C, et al. , Microbiota-gut-brain axis in health and disease: Is NLRP3 inflammasome at the crossroads of microbiota-gut-brain communications? Prog Neurobiol, 2020. 191: p. 101806. [DOI] [PubMed] [Google Scholar]
- 31.Lowe PP, et al. , Reduced gut microbiome protects from alcohol-induced neuroinflammation and alters intestinal and brain inflammasome expression. J Neuroinflammation, 2018. 15(1): p. 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenchley JM, et al. , Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med, 2006. 12(12): p. 1365–71. [DOI] [PubMed] [Google Scholar]
- 33.Lu W, et al. , Association Between Gut Microbiota and CD4 Recovery in HIV-1 Infected Patients. Front Microbiol, 2018. 9: p. 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vujkovic-Cvijin I, et al. , HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat Commun, 2020. 11(1): p. 2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters BA, et al. , The Gut Microbiome, Microbial Metabolites, and Cardiovascular Disease in People Living with HIV. Curr HIV/AIDS Rep, 2023. [DOI] [PubMed] [Google Scholar]
- 36.Chege D, et al. , Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS, 2011. 25(6): p. 741–9. [DOI] [PubMed] [Google Scholar]
- 37.Feria MG, et al. , HIV replication is associated to inflammasomes activation, IL-1beta, IL-18 and caspase-1 expression in GALT and peripheral blood. PLoS One, 2018. 13(4): p. e0192845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bandera A, et al. , The NLRP3 Inflammasome Is Upregulated in HIV-Infected Antiretroviral Therapy-Treated Individuals with Defective Immune Recovery. Front Immunol, 2018. 9: p. 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monroe KM, et al. , IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science, 2014. 343(6169): p. 428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nissen SK, et al. , Innate DNA sensing is impaired in HIV patients and IFI16 expression correlates with chronic immune activation. Clin Exp Immunol, 2014. 177(1): p. 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Booiman T and Kootstra NA, Polymorphism in IFI16 affects CD4(+) T-cell counts in HIV-1 infection. Int J Immunogenet, 2014. 41(6): p. 518–20. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, et al. , CARD8 is an inflammasome sensor for HIV-1 protease activity. Science, 2021. 371(6535). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dos Reis EC, et al. , Flagellin/NLRC4 Pathway Rescues NLRP3-Inflammasome Defect in Dendritic Cells From HIV-Infected Patients: Perspective for New Adjuvant in Immunocompromised Individuals. Front Immunol, 2019. 10: p. 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, et al. , NLRP3 inflammasome induces CD4+ T cell loss in chronically HIV-1-infected patients. J Clin Invest, 2021. 131(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaccari M, et al. , HIV vaccine candidate activation of hypoxia and the inflammasome in CD14(+) monocytes is associated with a decreased risk of SIV(mac251) acquisition. Nat Med, 2018. 24(6): p. 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mamik MK, et al. , HIV-1 Viral Protein R Activates NLRP3 Inflammasome in Microglia: implications for HIV-1 Associated Neuroinflammation. J Neuroimmune Pharmacol, 2017. 12(2): p. 233–248. [DOI] [PubMed] [Google Scholar]
- 47.Li G, et al. , HIV-1 Vpr-Induced Proinflammatory Response and Apoptosis Are Mediated through the Sur1-Trpm4 Channel in Astrocytes. mBio, 2020. 11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chivero ET, et al. , HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J Neurosci, 2017. 37(13): p. 3599–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He X, et al. , NLRP3-dependent pyroptosis is required for HIV-1 gp120-induced neuropathology. Cell Mol Immunol, 2020. 17(3): p. 283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J and Chen ZJ, PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature, 2018. 564(7734): p. 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seror C, et al. , Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med, 2011. 208(9): p. 1823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray CJ, et al. , Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet, 2012. 380(9859): p. 2197–223. [DOI] [PubMed] [Google Scholar]
- 53.Paoletti A, et al. , HIV-1 Envelope Overcomes NLRP3-Mediated Inhibition of F-Actin Polymerization for Viral Entry. Cell Rep, 2019. 28(13): p. 3381–3394 e7. [DOI] [PubMed] [Google Scholar]
- 54.Stylianou E, et al. , Raised serum levels of interleukin-18 is associated with disease progression and may contribute to virological treatment failure in HIV-1-infected patients. Clin Exp Immunol, 2003. 132(3): p. 462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wardyn JD, Ponsford AH, and Sanderson CM, Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem Soc Trans, 2015. 43(4): p. 621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sohn SH, et al. , TRK inhibitors block NFKB and induce NRF2 in TRK fusion-positive colon cancer. J Cancer, 2021. 12(21): p. 6356–6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minutoli L, et al. , ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxid Med Cell Longev, 2016. 2016: p. 2183026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivanov AV, et al. , Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid Med Cell Longev, 2016. 2016: p. 8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han D, et al. , Activation of NRF2 blocks HIV replication and apoptosis in macrophages. Heliyon, 2023. 9(1): p. e12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan X, et al. , Activating the Nrf2-mediated antioxidant response element restores barrier function in the alveolar epithelium of HIV-1 transgenic rats. Am J Physiol Lung Cell Mol Physiol, 2013, 305(3): p. L267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh V, et al. , Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J Neurosci, 2016. 36(28): p. 7428–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu DJ, et al. , Compositional and functional alterations of gut microbiota in patients with stroke. Nutr Metab Cardiovasc Dis, 2021. 31(12): p. 3434–3448. [DOI] [PubMed] [Google Scholar]
- 63.Clottes P, et al. , Gut microbiota and stroke: new avenues to improve prevention and outcome. Eur J Neurol, 2023. [DOI] [PubMed] [Google Scholar]
- 64.Lin H, et al. , Integrated Analysis of the Cecal Microbiome and Plasma Metabolomics to Explore NaoMaiTong and Its Potential Role in Changing the Intestinal Flora and Their Metabolites in Ischemic Stroke. Front Pharmacol, 2021. 12: p. 773722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng Y, et al. , Effect of intestinal microbiota transplantation on cerebral ischemia reperfusion injury in aged mice via inhibition of IL-17. Neurogastroenterol Motil, 2022. 34(7): p. e14313. [DOI] [PubMed] [Google Scholar]
- 66.Benakis C, et al. , Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med, 2016. 22(5): p. 516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chidambaram SB, et al. , The Influence of Gut Dysbiosis in the Pathogenesis and Management of Ischemic Stroke. Cells, 2022. 11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh V, et al. , The gut microbiome primes a cerebroprotective immune response after stroke. J Cereb Blood Flow Metab, 2018. 38(8): p. 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Battaglini D, et al. , Gut Microbiota in Acute Ischemic Stroke: From Pathophysiology to Therapeutic Implications. Front Neurol, 2020. 11: p. 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lei TY, et al. , The immune response of T cells and therapeutic targets related to regulating the levels of T helper cells after ischaemic stroke. J Neuroinflammation, 2021. 18(1): p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu X, Li P, and Chen J, Pro: Regulatory T cells are protective in ischemic stroke. Stroke, 2013. 44(8): p. e85–6. [DOI] [PubMed] [Google Scholar]
- 72.Li SJ, et al. , The role of NLRP3 inflammasome in stroke and central poststroke pain. Medicine (Baltimore), 2018. 97(33): p. e11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lammerding L, et al. , Poststroke Inflammasome Expression and Regulation in the Peri-Infarct Area by Gonadal Steroids after Transient Focal Ischemia in the Rat Brain. Neuroendocrinology, 2016. 103(5): p. 460–75. [DOI] [PubMed] [Google Scholar]
- 74.Abulafia DP, et al. , Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab, 2009. 29(3): p. 534–44. [DOI] [PubMed] [Google Scholar]
- 75.Kim H, et al. , AIM2 inflammasome contributes to brain injury and chronic post-stroke cognitive impairment in mice. Brain Behav Immun, 2020. 87: p. 765–776. [DOI] [PubMed] [Google Scholar]
- 76.Fann DY, et al. , Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis, 2013. 4(9): p. e790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yaribeygi H, et al. , Effects of antidiabetic drugs on NLRP3 inflammasome activity, with a focus on diabetic kidneys. Drug Discov Today, 2019. 24(1): p. 256–262. [DOI] [PubMed] [Google Scholar]
- 78.Cao G, et al. , Ruscogenin Attenuates Cerebral Ischemia-Induced Blood-Brain Barrier Dysfunction by Suppressing TXNIP/NLRP3 Inflammasome Activation and the MAPK Pathway. Int J Mol Sci, 2016. 17(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang F, et al. , NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab, 2014. 34(4): p. 660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong P, et al. , NLRP3 inflammasome as a potential treatment in ischemic stroke concomitant with diabetes. J Neuroinflammation, 2019. 16(1): p. 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou S, et al. , Puerarin protects against sepsis-associated encephalopathy by inhibiting NLRP3/Caspase-1/GSDMD pyroptosis pathway and reducing blood-brain barrier damage. Eur J Pharmacol, 2023. 945: p. 175616. [DOI] [PubMed] [Google Scholar]
- 82.Tong Y, et al. , The NLRP3 inflammasome and stroke. Int J Clin Exp Med, 2015. 8(4): p. 4787–94. [PMC free article] [PubMed] [Google Scholar]
- 83.Mongin AA, Disruption of ionic and cell volume homeostasis in cerebral ischemia: The perfect storm. Pathophysiology, 2007. 14(3-4): p. 183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duewell P, et al. , NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature, 2010. 464(7293): p. 1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waters L, et al. , The impact of HIV tropism on decreases in CD4 cell count, clinical progression, and subsequent response to a first antiretroviral therapy regimen. Clin Infect Dis, 2008. 46(10): p. 1617–23. [DOI] [PubMed] [Google Scholar]
- 86.Ojeda DS, et al. , Cell Death Is Counteracted by Mitophagy in HIV-Productively Infected Astrocytes but Is Promoted by Inflammasome Activation Among Non-productively Infected Cells. Front Immunol, 2018. 9: p. 2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Triantafilou K, et al. , Differential recognition of HIV-stimulated IL-1beta and IL-18 secretion through NLR and NAIP signalling in monocyte-derived macrophages. PLoS Pathog, 2021. 17(4): p. e1009417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hill WD, et al. , SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol, 2004. 63(1): p. 84–96. [DOI] [PubMed] [Google Scholar]
- 89.Victoria ECG, et al. , Knockdown of C-C Chemokine Receptor 5 (CCR5) is Protective Against Cerebral Ischemia and Reperfusion Injury. Curr Neurovasc Res, 2017. 14(2): p. 125–131. [DOI] [PubMed] [Google Scholar]
- 90.Joy MT, et al. , CCR5 Is a Therapeutic Target for Recovery after Stroke and Traumatic Brain Injury. Cell, 2019. 176(5): p. 1143–1157 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X, et al. , Umbelliferone ameliorates cerebral ischemia-reperfusion injury via upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3 inflammasome. Neurosci Lett, 2015. 600: p. 182–7. [DOI] [PubMed] [Google Scholar]
- 92.Hou Y, et al. , Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav Brain Res, 2018. 336: p. 32–39. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, et al. , Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol Appl Pharmacol, 2015. 286(1): p. 53–63. [DOI] [PubMed] [Google Scholar]
- 94.Coll RC, et al. , A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med, 2015. 21(3): p. 248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Youm YH, et al. , The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med, 2015. 21(3): p. 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ward R, et al. , NLRP3 inflammasome inhibition with MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol Res, 2019. 142: p. 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang H, et al. , Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med, 2017. 214(11): p. 3219–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qiu J, et al. , The neuroprotection of Sinomenine against ischemic stroke in mice by suppressing NLRP3 inflammasome via AMPK signaling. Int Immunopharmacol, 2016. 40: p. 492–500. [DOI] [PubMed] [Google Scholar]
- 99.Fann DY, et al. , Intermittent fasting attenuates inflammasome activity in ischemic stroke. Exp Neurol, 2014. 257: p. 114–9. [DOI] [PubMed] [Google Scholar]
- 100.Deroide N, et al. , MFGE8 inhibits inflammasome-induced IL-1beta production and limits postischemic cerebral injury. J Clin Invest, 2013. 123(3): p. 1176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guarda G, et al. , Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity, 2011. 34(2): p. 213–23. [DOI] [PubMed] [Google Scholar]
- 102.Bauernfeind F, et al. , NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol, 2012. 189(8): p. 4175–81. [DOI] [PubMed] [Google Scholar]
- 103.Chang YP, et al. , Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J Cell Physiol, 2015. 230(7): p. 1567–79. [DOI] [PubMed] [Google Scholar]
- 104.Abderrazak A, et al. , Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation, 2015. 131(12): p. 1061–70. [DOI] [PubMed] [Google Scholar]
- 105.Shao BZ, et al. , Activating cannabinoid receptor 2 alleviates pathogenesis of experimental autoimmune encephalomyelitis via activation of autophagy and inhibiting NLRP3 inflammasome. CNS Neurosci Ther, 2014. 20(12): p. 1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galea J, et al. , Intravenous anakinra can achieve experimentally effective concentrations in the central nervous system within a therapeutic time window: results of a dose-ranging study. J Cereb Blood Flow Metab, 2011. 31(2): p. 439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Emsley HC, et al. , A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry, 2005. 76(10): p. 1366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ross JB, et al. , Use of anakinra (Kineret) in the treatment of familial cold autoinflammatory syndrome with a 16-month follow-up. J Cutan Med Surg, 2008. 12(1): p. 8–16. [DOI] [PubMed] [Google Scholar]
- 109.Goldbach-Mansky R, et al. , A pilot study to evaluate the safety and efficacy of the long-acting interleukin-1 inhibitor rilonacept (interleukin-1 Trap) in patients with familial cold autoinflammatory syndrome. Arthritis Rheum, 2008. 58(8): p. 2432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lachmann HJ, et al. , Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med, 2009. 360(23): p. 2416–25. [DOI] [PubMed] [Google Scholar]
- 111.Savic S and McDermott MF, Inflammation: canakinumab for the cryopyrin-associated periodic syndromes. Nat Rev Rheumatol, 2009. 5(10): p. 529–30. [DOI] [PubMed] [Google Scholar]
- 112.Hoffman HM, Rilonacept for the treatment of cryopyrin-associated periodic syndromes (CAPS). Expert Opin Biol Ther, 2009. 9(4): p. 519–31. [DOI] [PubMed] [Google Scholar]
- 113.Economides AN, et al. , Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med, 2003. 9(1): p. 47–52. [DOI] [PubMed] [Google Scholar]
- 114.Vlachou M, et al. , Pineal hormone melatonin as an adjuvant treatment for COVID-19 (Review). Int J Mol Med, 2021. 47(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hensley-McBain T, et al. , Effects of Fecal Microbial Transplantation on Microbiome and Immunity in Simian Immunodeficiency Virus-Infected Macaques. J Virol, 2016. 90(10): p. 4981–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Falasca K, et al. , Effect of Probiotic Supplement on Cytokine Levels in HIV-Infected Individuals: A Preliminary Study. Nutrients, 2015. 7(10): p. 8335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Q, Tang XN, and Yenari MA, The inflammatory response in stroke. J Neuroimmunol, 2007. 184(1-2): p. 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]