Abstract
The susceptibilities of three bovine and two human Babesia divergens isolates to antimicrobial agents were evaluated in vitro by a tritiated hypoxanthine incorporation assay. The MICs at which 50% of isolates are inhibited (MIC50s) for mefloquine (chlorhydrate), chloroquine (sulfate), quinine (chlorhydrate), clindamycin (phosphate), pentamidine (isethionate), phenamidine (isethionate) plus oxomemazine (chlorhydrate), lincomycin (chlorhydrate monohydrate), and imidocarb (dipropionate) were determined. Except for imidocarb, the MIC50s observed for the different isolates were close. Imidocarb and the combination of phenamidine plus oxomemazine exhibited the highest in vitro activity, while antimalarial agents such as mefloquine, choroquine, and quinine were inactive. Other drugs had intermediate activities. The data support further in vitro evaluation of antimicrobial agents active against B. divergens for the improvement of therapeutic strategies.
Babesiosis is due to an intraerythrocytic protozoan parasite transmitted by tick bites to a wide variety of wild and domestic animals, including cattle, horses, dogs, and rodents. In Europe, Babesia divergens is considered the most pathogenic agent of bovine babesiosis and is responsible for important economic losses in beef- and milk-producing breeds. Current therapy includes the use of imidocarb dipropionate, but it remains debatable whether differences in outcomes among individual animals are due to variations in the sensitivities of the isolates to drugs or to host factors in animals which are not highly inbred (11).
In humans, 19 of the 24 cases of babesiosis reported in Europe were due to B. divergens, and all occurred in splenectomized patients. The initial therapeutic trials with antimalarial drugs for the treatment of this condition, characterized by severe symptoms and high mortality rates, have had limited success. More recently, complete parasite clearance and recovery were obtained with the combination of clindamycin and quinine in association with exchange transfusion (6).
Limited information is available on the antimicrobial susceptibility of Babesia spp. in vitro. The first reports of the use of tritiated nucleic acid precursors for the screening of drugs (7) showed a positive correlation between in vitro and in vivo activities in animals (10). More recently, in vitro assays have been proposed for determination of the susceptibilities of Babesia bovis and Babesia bigemina to drugs (4, 12). The aim of this work was to compare the antimicrobial susceptibilities of B. divergens isolates of bovine and human origin by a triatiated hypoxanthine incorporation assay.
MATERIALS AND METHODS
B. divergens isolates.
Five B. divergens isolates were tested. Three isolates were of bovine origin and were obtained from different geographical areas in Europe: Muenchen (Munich, Germany), Weybridge 8843 (Weybridge, United Kingdom), and Taylor (Ireland). Two isolates were obtained in France from patients admitted to Rouen University (isolate Rouen) and Le Mans (isolate Le Mans) hospitals. Isolates were maintained in human erythrocyte (RBC) cultures and infected 10 to 20% of the RBCs. The isolates were then suspended in RPMI 1640 medium (Gibco, Cergy, France) supplemented with 40% (vol/vol) fetal calf serum and 15% (vol/vol) dimethyl sulfoxide and frozen in liquid nitrogen.
B. divergens cultures.
After thawing and washing, 0.2 ml of packed infected RBCs was injected intraperitoneally into 2-month-old female gerbils (Meriones unguiculatus) (Centre D’elevage R. Janvier, Le Genest-Saint-Isle, France) 2 days after an intraperitoneal injection of 2 mg of dexamethasone (Soludecadron; Merck-Sharp-Dohme, Paris, France). When the level of parasitemia reached 50% (i.e., 2 to 3 days later), parasitized RBCs were collected by cardiac puncture. Human RBCs were collected from healthy type O- and Rh-positive volunteer blood donors, placed in preservative-free heparin (Heparine Leo, Saint-Quentin-Les-Yvelines, France), and washed by serial centrifugations in RPMI 1640 medium. The buffy coat and the upper part of the RBC pellets were discarded to avoid leukocyte contamination, and 0.2 ml of packed human RBCs was poured into 50-ml plastic flasks (Nunc, Roskilde, Denmark) containing 5 ml of RPMI 1640 medium supplemented with HEPES (25 mM; pH 7.4; Sigma, St. Louis, Mo.), gentamicin (12 μg/ml), and 10% (vol/vol) decomplemented pooled human serum from type O- and Rh-positive donors. A volume of 0.01 ml of parasitized packed gerbil RBCs was added. The cultures were maintained at 37°C in a 5% CO2 atmosphere and were supplemented with human RBCs twice a week to adjust the level of parasitemia to between 1 and 2%.
In vitro assays for drug susceptibility.
Drug susceptibility assays were performed in 96-well culture microplates (Falcon, Meylan, France) in the presence of a final RBC concentration of 1.8% (vol/vol) and serial dilutions of drugs. Each duplicate well received 200 μl of an RBC suspension and 50 μl of each drug dilution in RPMI 1640 medium. For each experiment, controls consisted of four wells containing infected RBCs without drug (i.e., wells to which 50 μl of RPMI 1640 alone was added) and two wells containing 200 μl of noninfected RBCs.
[3H]hypoxanthine (specific activity, 4 Ci/mmol; Amersham, Les Ulis, France) was added (0.4 μCi/well). The plates were incubated at 37°C for 48 h in a 5% CO2 atmosphere, the cells were harvested on glass fiber filters (Whatman GF/C) with a cell harvester, and the incorporated radioactivity (in counts per minute) was counted in a liquid scintillation spectrometer.
Five milliliters of RBCs from each well were stained with Giemsa stain, and 5,000 RBCs were microscopically examined to determine morphologically the percentage of infected RBCs.
Antimicrobial agents.
From a stock solution in 70% ethanol, nine dilutions of the following antimicrobial agents were prepared in RPMI 1640 medium: mefloquine chlorhydrate (0.3 to 0.017 μg/ml; Hoffmann-LaRoche-Basle, Basel, Switzerland), chloroquine sulfate (6 to 0.023 μg/ml; Rhône Poulenc, Paris, France), quinine chlorhydrate (1.2 to 0.047 μg/ml; Assistance Publique, Paris, France), clindamycin phosphate (250 to 0.98 μg/ml; Upjohn, Val de Reuil, France), pentamidine isethionate (10 to 0.07 μg/ml; Rhône Mérieux, Toulouse, France), phenamidine isethionate and oxomemazine chlorhydrate (2 to 0.008 μg/ml; Oxopirvédine; Rhône Mérieux, Lyon, France), lincomycin chlorhydrate monohydrate (4,000 to 15.6 μg/ml; Lincocine; Upjohn, Val de Reuil, France), and imidocarb dipropionate (25 to 0.098 μg/ml; Carbesia; Pitman-Moore, Meaux, France). A volume of 50 μl of each dilution was added to the wells. Control wells were filled with 50 μl of medium without drug.
Expression of results and statistical analysis.
The amount of parasite-dependent tritiated hypoxanthine incorporated was obtained by subtracting the amount of background incorporation observed in noninfected cultures. For each drug, a concentration-versus-[3H]hypoxanthine incorporation curve was established, and the inhibitory activity was expressed as the minimum concentration (wt/vol) of drug which either inhibited tritiated hypoxanthine incorporation or decreased the proportion of morphologically infected RBCs by 50% of the value in control cultures without drug (the concentration at which 50% of isolates are inhibited [MIC50]). MIC50s were expressed in micrograms per liter of blood with a 50% hematocrit.
Variance analysis was performed by the Fisher and Yates chi-square test or the Student t test, thus assuming normal, like distributions of values. The significance of r values was used to study linear correlation.
RESULTS
In preliminary experiments, tritiated hypoxanthine incorporation was measured in cultures maintained in the absence of antimicrobial agents (15 microplates, 24 wells/microplate). For the noninfected wells, the mean ± 1 standard deviation (SD) level of incorporation was 191 ± 52 cpm/well, and for infected wells without drug, the mean ± 1 SD level of incorporation was 11,707 ± 2,575 cpm/well. No significant difference was found between isolates (P > 0.1; data not shown). One SD accounted for less than 5 and 17% of the mean for each microplate and for the pooled data for the 15 microplates, respectively. The variations for the cultures maintained in the presence of the different antimicrobial agents are summarized in Fig. 1 to 3. In the case of clindamycin, it was verified with the Le Mans isolate that a good correlation was found between MIC50s obtained with 18 independent microplates (r = 0.7; P < 0.01).
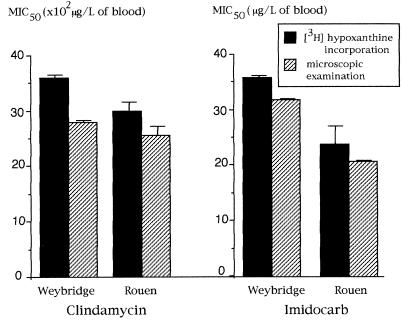
FIG. 1.
Comparison of MIC50s of clindamycin and imidocarb for the Weybridge 8843 bovine isolate and the Rouen human isolate obtained in the [3H]hypoxanthine incorporation assay and by microscopic examination. Results are expressed as mean values for 15 independent experiments (one bar indicates 1 SD).
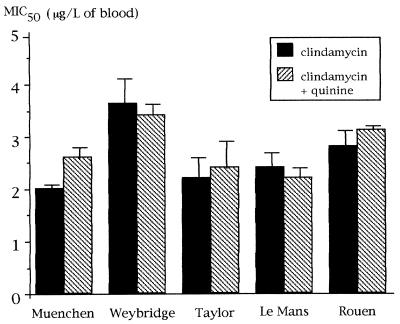
FIG. 3.
MIC50s of clindamycin and clindamycin plus quinine for bovine and human isolates of B. divergens in 18 independent experiments. Results are expressed as mean values for 18 independent experiments (one bar indicates 1 SD).
A detailed comparison of the MIC50s obtained by the tritiated hypoxanthine incorporation assay and microscopic counting was performed for the Weybridge 8843 and Rouen isolates in the presence of clindamycin or imidocarb. As indicated in Fig. 1, no significant difference in the MIC50s was observed in 15 independent experiments (P > 0.1). This was confirmed for all isolates in the presence of the other antimicrobial agents (P > 0.1; data not shown).
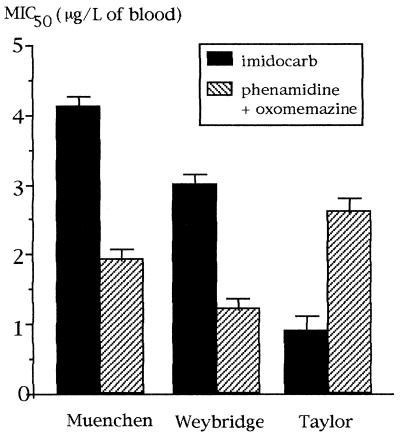
From these data, the MIC50s of clindamycin and imidocarb varied from 2,200 to 3,400 μg/liter and from 27 to 34 μg/liter, respectively. Imidocarb, a drug restricted to the treatment of babesiosis in animals, was efficient against both human and bovine B. divergens isolates (Fig. 1 and 2). The MIC50s of imidocarb and clindamycin for bovine and human isolates were not significantly different; however, MIC99s determined by using probit/log concentration regression curves suggested that they had higher levels of activity against human isolates than against bovine isolates (14,100 and 28,800 μg/liter, respectively). The combination of phenamidine plus oxomemazine was more effective than imidocarb against the bovine isolates Muenchen and Weybridge 8843 but not against the bovine isolate Taylor (Fig. 3). None of the B. divergens isolates tested was found to be sensitive to antimalarial agents (Table 1). Pentamidine isethionate inhibited B. divergens in vitro, and lincomycin was active and had a high MIC50 (Table 1).
FIG. 2.
MIC50s of phenamidine plus oxomemazine and of imidocarb for bovine B. divergens isolates. Results are expressed as mean values for eight independent experiments (one bar indicates 1 SD).
TABLE 1.
In vitro activities of mefloquine, chloroquine, quinine, pentamidine, and lincomycin against bovine and human B. divergens isolates
| Isolate source and isolate | MIC (μg/liter of blood)a
|
||||
|---|---|---|---|---|---|
| Meflo- quine | Chloro- quine | Quinine | Pent- amidine | Lincomycin | |
| Bovine isolates | |||||
| Muenchen | >24 | >48 | >96 | 5.1 ± 1 | 70,000 ± 2,100 |
| Weybridge 8843 | ND | ND | >96 | ND | ND |
| Taylor | >24 | >48 | >96 | 2.5 ± 0.4 | 32,000 ± 3,800 |
| Human isolates | |||||
| Le Mans | >24 | >48 | >96 | 2.2 ± 0.0 | 37,000 ± 4,700 |
| Rouen | ND | ND | >96 | ND | 38,000 ± 2,500 |
Results are for two to six triplicate experiments. ND, not determined.
DISCUSSION
In this report a 48-h in vitro assay was used to evaluate the susceptibilities of human and bovine B. divergens isolates to antimicrobial agents. Continuous cultures of B. divergens were maintained as described previously (7), and the level of incorporation of tritiated hypoxanthine was measured in the presence of different concentrations of drugs. This assay was easier to perform than microscopic counting and was found to be reliable and sensitive. The MIC50s did not differ significantly from the values obtained by microscopic examination. Uptake of tritiated purines such as hypoxanthine was previously proposed for the screening of drug activities against Babesia spp. with short-term cultures of blood from experimentally infected animals (8, 9). In the present culture assay, which uses human instead of bovine RBCs, higher levels of tritiated hypoxanthine incorporation were obtained.
Bovine babesiosis is generally treated with imidocarb dipropionate. For bovine isolates, the MIC50 of this compound was significantly lower than the maximum (Cmax) concentration in serum of 6.6 mg/liter observed in cattle receiving a dose of 17 mg/100 kg of body weight. This is consistent with the high level of activity of imidocarb against bovine babesiosis. Similar MIC50s, i.e., 0.87 μg/ml of medium, have been reported for B. bovis (15). In addition, the comparable results obtained for human and bovine isolates suggest that imidocarb is possibly a candidate agent for the treatment of human B. divergens babesiosis.
The combination of phenamidine isethionate plus oxomemazine chlorhydrate, which is currently restricted for use in the treatment of canine babesiosis, was found to be more active than imidocarb in vitro.
The treatment of human B. divergens babesiosis with antimalarial drugs has had limited success. Of the seven patients treated with chloroquine for several weeks, four died and three recovered (1, 5, 10, 14). A treatment based on blood exchange and the administration of clindamycin plus quinine has been successfully used in six patients. Our results confirm the susceptibility of B. divergens to clindamycin in vitro. The in vivo efficacy of quinine is controversial (3), since some of the patients treated with quinine alone died and others recovered after several weeks. The very high MIC50s of quinine found in this study suggest that this compound is not useful for treatment. The MIC50s of antimalarial drugs found in this study were higher than the cutoff limits of sensitivity for Plasmodium falciparum (8.6, 2.7, and 1 μg/liter of blood for quinine, chloroquine, and mefloquine, respectively). Interestingly, it has been shown in a recent study that atovaquone, which has antimalarial activity, was more active than imidocarb for the treatment of bovine B. divergens babesiosis (13).
All isolates were susceptible to pentamidine isethionate in vitro. For the Rouen isolate, the MIC50 (2.2 ± 0.08 μg/liter of blood) was higher than the Cmax (0.2 to 0.5 μg/liter of blood) resulting from an injection of 4 mg/kg, which is the maximum dose that can be used in humans due to the renal toxicity of this compound (16). This may explain the treatment failures reported for patients treated with pentamidine alone (17) or with pentamidine and chloroquine (2). No data on the pharmacokinetics of pentamidine isethionate in cattle are available, and it cannot be stated whether pentamidine isethionate can be proposed for use in the treatment of bovine babesiosis. The MIC50 of lincomycin found in this study is higher than the Cmax values obtained in humans (2 to 7 μg/liter of blood), which suggests that lincomycin probably has limited use for the treatment of this disease.
Data suggest that tritiated hypoxanthine incorporation by B. divergens in human RBC cultures provides an accurate and convenient means of evaluating the antibabesial activities of drugs, further drug screening, detecting resistant isolates, and comparing in vitro activities with in vivo activities.
REFERENCES
- 1.Bayle J, Dumon H, Verdot J J, Muratore R. Human piroplasmosis. One case. Presse Med. 1979;8:3674. [PubMed] [Google Scholar]
- 2.Beck P, Gorenflot A, Estebe J P, Marchand A, Roquilly A. Insuffisance respiratoire aigüe d’un cas de babésiose humaine. Bull Soc Fr Parasitol. 1987;5:16. [Google Scholar]
- 3.Brasseur P, Lecoublet S, Kapel N, Favennec L, Ballet J J. Quinine in the treatment of Babesia divergens infections in humans. Eur J Clin Microbiol Infect Dis. 1996;15:840–841. doi: 10.1007/BF01701533. [DOI] [PubMed] [Google Scholar]
- 4.Brockelman C R, Tan-Ariya P. Development of an in vitro microtest to assess drug susceptibility of Babesia bovis and Babesia bigemina. J Parasitol. 1991;77:994–997. [PubMed] [Google Scholar]
- 5.Entrican J H, Williams H, Cook I A, Lancaster W M, Clark J C, Joyner L P. Babesiosis in man: report of a case from Scotland with observations on the infecting strain. J Infect. 1979;1:227–234. [Google Scholar]
- 6.Gorenflot A, Brasseur P, Bonmarchand G, Laneele D, Simonin D. Two cases of severe human babesiosis treated with success. Presse Med. 1988;19:335. [PubMed] [Google Scholar]
- 7.Gorenflot A, Brasseur P, Precigout E, L’Hostis M, Marchand A, Schrevel J. Cytological and immunological responses to Babesia divergens in different hosts: ox, gerbil, man. Parasitol Res. 1991;73:3–12. doi: 10.1007/BF00934377. [DOI] [PubMed] [Google Scholar]
- 8.Irvin A D, Young E R. Possible in vitro test for screening drugs for activity against Babesia and other blood protozoa. Nature (London) 1977;169:407–409. doi: 10.1038/269407a0. [DOI] [PubMed] [Google Scholar]
- 9.Irvin A D, Young E R. Further studies on the uptake of tritiated nucleic acid precursors by Babesia spp. of cattle and mice. Int J Parasitol. 1979;9:109–112. doi: 10.1016/0020-7519(78)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Jadin J B, Giroult P. Society of Protozoologists (ed.), Parasitological topics special publication no. 1. Lawrence, Kans: Society of Protozoologists; 1981. Babesiosis and rickettsiosis; pp. 132–135. [Google Scholar]
- 11.Mallick K P, Dwivedi S K, Srivastana N K. A report on the occurrence of hemoprotozoan infections in rural livestock. Indiana J Parasitol. 1987;11:25–26. [Google Scholar]
- 12.Nott S E, O’Sullivan W J, Gero A M, Bagnara A S. Routine screening for potential babesicides using cultures of Babesia bovis. Int J Parasitol. 1990;20:797–802. doi: 10.1016/0020-7519(90)90014-e. [DOI] [PubMed] [Google Scholar]
- 13.Pudney M, Gray J S. Therapeutic efficacy of atovaquone against the bovine intraerythrocytic parasite, Babesia divergens. J Parasitol. 1997;83:307–310. [PubMed] [Google Scholar]
- 14.Rabinovich S A, Voronina Z K, Stepanova N I. Première découverte de babésiose humaine en URSS; courte analyse des cas décrits dans la littérature. Mosk Med Parasitol. 1978;47:97–107. [Google Scholar]
- 15.Rodriguez R I, Trees A J. In vitro responsiveness of Babesia bovis to imidocarb dipropionate and the selection of a drug-adapted line. Vet Parasitol. 1996;62:35–41. doi: 10.1016/0304-4017(95)00850-0. [DOI] [PubMed] [Google Scholar]
- 16.Ruebush T K, Rubin R H, Wolpw E R, Cassaday P B, Schultz M G. Neurologic complications following treatment of human Babesia microti infection with dimidazene aceturate. American J Med Hyg. 1979;28:184–189. doi: 10.4269/ajtmh.1979.28.184. [DOI] [PubMed] [Google Scholar]
- 17.Telford S R, Gorenflot A, Brasseur P, Spielman A. Babesial infections in humans and wildlife. In: Kreier J P, editor. Parasitic protozoa. New York, N.Y: Academic Press, Inc.; 1992. p. 17. [Google Scholar]