Abstract
A group of cefotaxime-resistant Citrobacter freundii and Escherichia coli isolates were collected by a clinical laboratory in a hospital in Warsaw, Poland, in July 1996. Detailed analysis has shown that all of these produced a β-lactamase (pI, 8.4) belonging to the CTX-M family, one of the minor extended-spectrum β-lactamase families with a strong cefotaxime-hydrolyzing activity. Sequencing has revealed that C. freundii isolates produced a new CTX-M-3 enzyme which is very closely related to the CTX-M-1/MEN-1 β-lactamase, sporadically identified in Europe over a period of 6 years. Amino acid sequences of these two β-lactamases differ at four positions: Val77Ala, Asp114Asn, Ser140Ala, and Asn288Asp (the first amino acid of each pair refers to CTX-M-1/MEN-1 and second refers to CTX-M-3). The partial sequence of the E. coli CTX-M gene was identical to the corresponding region of blaCTX-M-3, but a transconjugant of the E. coli isolate expressed higher levels of resistance to β-lactams than did C. freundii transconjugants. These resistance differences correlated with differences in plasmid DNA restriction patterns. Our results suggest that CTX-M genes have been spread among different species of the family Enterobacteriaceae in the hospital and that the CTX-M-3-expressing C. freundii strain causing routine urinary tract infections has been maintained for a relatively long time in the hospital environment.
Class A plasmidic CTX-M β-lactamases constitute one of the minor families of extended-spectrum β-lactamases (ESBLs) which are much more active against cefotaxime than ceftazidime (5). Three members of this group have been reported to date: CTX-M-1/MEN-1 (3, 5), CTX-M-2 (4), and Toho-1 (10). CTX-M-producing strains of the family Enterobacteriaceae were isolated sporadically between 1989 and 1994 but over an extremely wide geographic area including Europe, South America, and the Middle and Far East (6, 7, 10).
Very little is known about the possible origins, evolution, or structural determinants of the CTX-M enzyme activity. Comparative analysis of protein sequences has revealed the similarity of these enzymes to the β-lactamases of Klebsiella oxytoca and Citrobacter diversus (>70% homology), which, to date, are known to be exclusively chromosomally encoded enzymes (2, 3, 7, 15, 16). The K. oxytoca β-lactamase was reported to possess a weak activity against oxyimino-β-lactams (2, 16). The amino acid residue(s) and the position(s) critical for the high cefotaxime-hydrolyzing activity of the CTX-M enzymes are not known; however, the Ser-237 residue, common to members of the family and not to the K. oxytoca and C. diversus enzymes, has been proposed to play this role (3).
At the beginning of July 1996, a clinical microbiology laboratory in the Praski Hospital in Warsaw, Poland, started to routinely test for ESBL activity in isolates of Enterobacteriaceae by the double-disc method (11). During this month, three Citrobacter freundii isolates and one Escherichia coli isolate, collected from urine samples from different patients, were identified as ESBL producers expressing high resistance to cefotaxime and susceptibility to ceftazidime in in vitro susceptibility tests. In this paper, we present results of the detailed analysis of these strains which led to the identification of the next CTX-M variant and the first description for Europe of the persistence of CTX-M-producing microorganisms in the microflora of a given hospital environment.
MATERIALS AND METHODS
Bacterial strains.
Three C. freundii clinical isolates (2524/96, 2525/96, and 2526/96) and one E. coli clinical isolate (2527/96) resistant to cefotaxime and identified as ESBL producers were collected in July 1996 at the Praski Hospital from different patients with urinary tract infections: two from the internal medicine ward, one from the urological ward, and one from the surgical ward of the hospital. All the patients suffered from urological complications and/or were subjected to invasive procedures (catheterization, cystoscopy, and nephrostomy). Two patients were diabetic, and one had cancer. Species identification, routine susceptibility tests, and double-disc tests for ESBL activity (11) were performed by the hospital microbiology laboratory. Species identification was confirmed by the ID32E ATB test (bioMerieux).
Ribotyping and randomly amplified polymorphic DNA (RAPD).
Genomic DNA was extracted from 200 μl of the overnight cultures grown in tryptic soy broth (Oxoid) at 37°C with the Genomic DNA Prep Plus kit (A & A Biotechnology, Gdańsk, Poland). For ribotyping, a mixture of two DNA probes corresponding to the 23S ribosomal DNA (rDNA) and 16S rDNA sequences of E. coli (9) was used. The probes were obtained in PCRs with genomic DNA of E. coli ATCC 25922 as a template and the following primers: 5′-GGTTAAGCGACTAAGCGTAC-3′ and 5′-CAGCTTCGGCGTTGTAAGG-3′ for 23S rDNA amplification and 5′-GGGGGACCTTCGGGC-3′ and 5′-GGTGTGACGGGCGGTGTG-3′ for 16S rDNA amplification. The PCR products were gel purified and labelled with the DIG DNA labelling kit (Boehringer Mannheim) according to the manufacturer’s recommended procedure. Total DNA preparations of analyzed isolates were digested with EcoRI and HindIII restriction enzymes (MBI Fermentas), electrophoresed in a 1% agarose gel (FMC Bioproducts), and blotted onto a positively charged nylon membrane (Boehringer Mannheim). Chemiluminescent detection of the hybridization signal was performed with a DIG luminescent detection kit (Boehringer Mannheim) according to the manufacturer’s instructions.
The RAPD analysis was performed with the RAPD-7 (18) primer. About 10 ng of total DNA, 50 pmol of the primer, 100 μM deoxynucleoside triphosphates, 2.5 mM MgCl2, 10 μg of bovine serum albumin, 2 U of Taq polymerase (MBI Fermentas), and buffer supplied by the manufacturer of the enzyme were used for each single reaction mixture. Reactions were run under the following conditions: 5 min at 94°C; five cycles of 15 s at 94°C, 30 s at 35°C, and 1.5 min at 72°C; and 30 cycles of 15 s at 94°C, 15 s at 55°C, 30 s at 72°C, and finally 7 min at 72°C in a GeneAmp PCR System 2400 (Perkin-Elmer). PCR products were electrophoresed in 2% agarose gels (FMC Bioproducts). C. freundii L-601, belonging to the culture collection of the Sera and Vaccines Central Research Laboratory, Warsaw, Poland, was used as an epidemiologically nonrelated control in both typing analyses. (The L-601 strain was isolated in January 1995 at the University Hospital in Szczecin, Poland).
Susceptibility testing.
MICs of various antibiotics were determined by the agar dilution method according to National Committee for Clinical Laboratory Standards guidelines (14). The following antibiotics were used: ampicillin, cefotaxime, and gentamicin (Polfa, Tarchomin, Poland); aztreonam (Bristol-Myers Squibb, New Brunswick, N.J.); cefoxitin (Sigma Chemical Co., St. Louis, Mo.); ceftazidime (Glaxo Wellcome, Stevenage, United Kingdom); lithium clavulanate (SmithKline Beecham Pharmaceuticals, Betchworth, United Kingdom); imipenem (Merck, Sharp & Dohme Research, Rahway, N.J.); piperacillin (Lederle Piperacillin Inc., Carolina, Puerto Rico); tazobactam (Lederle Laboratories, Pearl River, N.Y.); and tobramycin (Eli Lilly, Indianapolis, Ind.). In all β-lactam–inhibitor combinations, the constant concentrations of clavulanate and tazobactam were 2 and 4 μg/ml, respectively. C. freundii 870/97, isolated at the Praski Hospital in May 1997, was used as a wild-type control strain for antimicrobial susceptibility testing of ESBL-producing C. freundii isolates. E. coli ATCC 25922 was used as the reference strain.
Resistance transfer.
One-milliliter volumes of cultures of the donor and recipient strains (109 CFU of each strain per ml) grown in tryptic soy broth (Oxoid) were mixed and incubated for 18 h at 35°C. E. coli A15 R− resistant to rifampin was used as the recipient strain. Transconjugants were selected on MacConkey agar (Oxoid) supplemented with cefotaxime (2 mg/liter) and rifampin (128 mg/liter).
Isoelectric focusing (IEF) of β-lactamases and detection of cefotaxime-hydrolyzing activity within the IEF gel lane.
Sonicates of clinical isolates and transconjugants were subjected to analytical IEF over the pH range of 3 to 10. IEF was performed according to the method of Matthew et al. (13) with modifications described previously (5), with a Multiphor apparatus (Pharmacia LKB). Following electrophoresis, gels were stained with nitrocefin (Oxoid). After IEF of transconjugant extracts, cefotaxime-hydrolyzing activity was assigned to specific β-lactamase bands by the bioassay approach as previously described (5).
Plasmid DNA preparation and plasmid fingerprinting.
Plasmid DNA was purified from transconjugant cells with the Qiagen Plasmid Midi kit (Qiagen, Hilden, Germany), according to the manufacturer’s recommended procedure, as described previously (8). For the fingerprinting analysis, about 5 μg of plasmid DNA was digested with 10 U of PstI restriction enzyme (Boehringer Mannheim) for 2 h at 37°C. DNA was electrophoresed in a 1% agarose gel (FMC Bioproducts).
PCR amplification of the blaCTX-M-3 gene.
For partial gene PCR amplification, primers P1 (5′-GCGATGTGCAGCACCAGTAA-3′) and P2 (5′-GGTTGAGGCTGGGTGAAGTA-3′), specific for the blaCTX-M-1 gene (7), were used in reactions with plasmid DNA preparations as templates. The entire coding sequence was amplified in a PCR with the P1C (5′-TCGTCTCTTCCAGA-3′) and P2D (5′-CAGCGCTTTTGCCGTCTAAG-3′) oligonucleotides as primers. A single reaction mixture contained about 1 μg of plasmid DNA, 50 pmol of each primer, 100 μM deoxynucleoside triphosphates, 1 U of Taq polymerase (Boehringer Mannheim), and buffer with 2.5 mM MgCl2 supplied by the manufacturer of the enzyme. A Perkin-Elmer 9600 apparatus was used, and reactions were run under the following conditions: 3 min at 95°C; 30 cycles of 30 s at 95°C, 30 s at 55°C, and 30 s at 72°C; and finally 3 min at 72°C. The resulting products were run in a 1% agarose gel (FMC Bioproducts) and purified for direct sequencing reactions with a QIAquick PCR purification kit (Qiagen).
DNA sequencing.
Sequencing reactions were performed with consecutive primers specific for the blaCTX-M-1 gene (7) according to the dideoxy chain termination method of Sanger et al. (17). An automatic sequencer (373A; Applied Biosystems, Weiterstadt, Germany) was used.
Nucleotide sequence accession number.
The blaCTX-M-3 gene nucleotide sequence data will appear in the EMBL database under accession no. Y10278.
RESULTS
Typing.
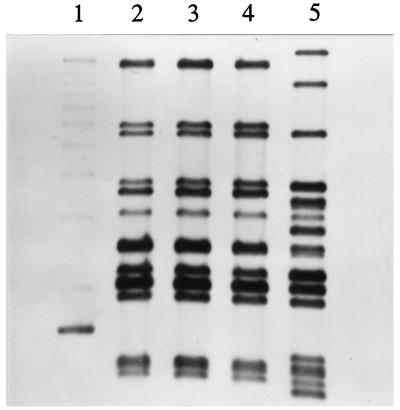
The three C. freundii isolates were typed by ribotyping and RAPD approaches. All the isolates were found to produce identical ribotyping patterns which were different from that of the epidemiologically nonrelated control strain (Fig. 1). These results were confirmed by the RAPD analysis (data not shown).
FIG. 1.
Ribotyping of C. freundii isolates. The three C. freundii isolates belonged to the same ribotype, which was different from that of the epidemiologically nonrelated control L-601 strain. Lanes: 1, 1-kb DNA ladder (Gibco BRL); 2, C. freundii 2524/96; 3, C. freundii 2525/96; 4, C. freundii 2526/96; 5, C. freundii L-601.
Antimicrobial susceptibilities of clinical isolates.
Results of the analysis are shown in Table 1. Of the β-lactams tested, all the ESBL-producing isolates were resistant to ampicillin (MICs, >512 μg/ml), piperacillin (MICs, >512 μg/ml), cefotaxime (MICs, ≥512 μg/ml), and aztreonam (MICs, 32 or 128 μg/ml) but remained susceptible or intermediate to ceftazidime (MICs, 4 or 16 μg/ml) and susceptible to imipenem (MICs, 0.125 μg/ml). β-Lactamase inhibitors restored activities of piperacillin, cefotaxime, and aztreonam. C. freundii isolates were resistant to cefoxitin (MICs, 128 μg/ml) whereas E. coli 2527/96 was intermediate to this antibiotic (MIC, 16 μg/ml). MICs of β-lactam antibiotics tested for the wild-type C. freundii strain (C. freundii 870/97) were substantially lower than those for the three ESBL-producing isolates, except for cefoxitin (MIC, 128 μg/ml) and imipenem (MIC, 0.25 μg/ml). All the ESBL-expressing isolates were found to be resistant to both aminoglycosides tested, gentamicin and tobramycin (MICs, 256 μg/ml). MICs obtained for the three C. freundii isolates were identical; E. coli 2527/96 was usually characterized by higher MICs.
TABLE 1.
Antimicrobial susceptibilities of clinical isolates, transconjugants, and control strains
| Antimicrobial | MIC (μg/ml) for strain or transconjugant:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C. freundii 2524/96 | C. freundii 2525/96 | C. freundii 2526/96 | E. coli 2527/96 | R+ [C. freundii 2524] | R+ [C. freundii 2525] | R+ [C. freundii 2526] | R+ [E. coli 2527] | E. coli A15 R− | C. freundii 870/97 (wild type) | |
| Ampicillin | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 2 | 32 |
| Piperacillin | >512 | >512 | >512 | >512 | 256 | 256 | 256 | >512 | 0.5 | 2 |
| Piperacillin-tazobactam | 8 | 8 | 8 | 16 | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 2 |
| Cefotaxime | >512 | >512 | >512 | >512 | 32 | 32 | 32 | 512 | ≤0.03 | 0.13 |
| Cefotaxime-clavulanate | 0.5 | 0.5 | 0.5 | 0.5 | ≤0.03 | ≤0.03 | ≤0.03 | 0.06 | ≤0.03 | NDa |
| Cefotaxime-tazobactam | 0.5 | 0.5 | 0.5 | 0.5 | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | ND |
| Ceftazidime | 4 | 4 | 4 | 16 | 0.5 | 0.5 | 0.5 | 4 | 0.06 | 0.5 |
| Ceftazidime-clavulanate | 1 | 1 | 1 | 2 | 0.06 | 0.06 | 0.06 | 0.25 | 0.06 | ND |
| Ceftazidime-tazobactam | 1 | 1 | 1 | 2 | 0.13 | 0.13 | 0.13 | 0.25 | ≤0.03 | ND |
| Aztreonam | 32 | 32 | 32 | 128 | 2 | 2 | 2 | 16 | 0.06 | 0.06 |
| Aztreonam-clavulanate | 0.25 | 0.25 | 0.25 | 0.25 | ≤0.03 | ≤0.03 | ≤0.03 | 0.06 | ≤0.03 | ND |
| Aztreonam-tazobactam | 0.25 | 0.25 | 0.25 | 0.5 | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | ND |
| Cefoxitin | 128 | 128 | 128 | 16 | 2 | 2 | 2 | 2 | 2 | 128 |
| Imipenem | 0.13 | 0.13 | 0.13 | 0.13 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Gentamicin | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | 0.13 | ND |
| Tobramycin | >256 | >256 | >256 | >256 | 256 | 256 | 256 | >256 | 0.25 | ND |
ND, not determined.
Resistance transfer and susceptibility testing of transconjugant strains.
Transconjugants were obtained for all the analyzed isolates. A very high frequency of conjugation (10−2 per donor cell) was observed with the C. freundii isolates. (For E. coli 2527/96, the frequency was 10−6 per donor cell.) Susceptibility testing (Table 1) has revealed that the transconjugant of E. coli 2527/96 was characterized by substantially higher MICs of piperacillin, cefotaxime, ceftazidime, and aztreonam than those for transconjugants of C. freundii isolates. The MICs of cefotaxime were higher than those of ceftazidime by 64 and 128 times for C. freundii and E. coli transconjugants, respectively. The cefoxitin resistance of C. freundii isolates was not transferred to recombinant strains. In all cases, resistance to gentamicin and tobramycin was cotransferred with the ESBL activity to the transconjugants.
IEF of β-lactamases and cefotaxime-hydrolyzing activity detection.
Two major nitrocefin-hydrolyzing bands were visualized in extracts of all clinical isolates and transconjugants (results not shown). One band with a pI of 8.4 comigrated with the CTX-M-1 enzyme produced by the original E. coli GRI CTX-M-1 strain (5); the second one was characterized by a pI value of 5.4. The subsequent bioassay experiment revealed that only the β-lactamase with a pI of 8.4 possessed the cefotaxime-hydrolyzing activity (results not shown).
Plasmid fingerprinting.
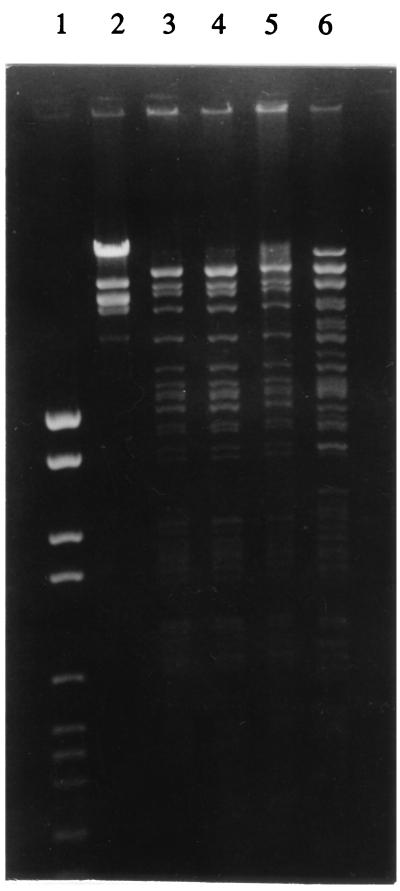
Plasmids isolated from transconjugants of all three C. freundii isolates revealed identical PstI restriction patterns which were different from that of the plasmid isolated from the E. coli 2527/96 transconjugant cells (Fig. 2).
FIG. 2.
Plasmid fingerprinting analysis. Plasmids isolated from transconjugants of C. freundii 2524/96, C. freundii 2525/96, and C. freundii 2526/96 were found to have identical PstI restriction patterns, and these were different from that of the R+ [E. coli 2527/96] strain. Lanes: 1, DNA molecular weight marker set VI (Boehringer Mannheim); 2, DNA molecular weight marker set I (Boehringer Mannheim); 3, R+ [C. freundii 2524/96]; 4, R+ [C. freundii 2525/96]; 5, R+ [C. freundii 2526/96]; 6, R+ [E. coli 2527/96].
PCR amplification and sequencing of the blaCTX-M-3 gene.
Partial gene amplification products of the expected size of ca. 600 bp were obtained in all cases (results not shown). Sequencing the PCR products has revealed that within the region of about 450 to 500 bp all four nucleotide sequences were identical except for six positions (two amino acid differences in deduced protein sequences: Asp114Asn and Ser140Ala) compared with the homologous part of the blaCTX-M-1 gene.
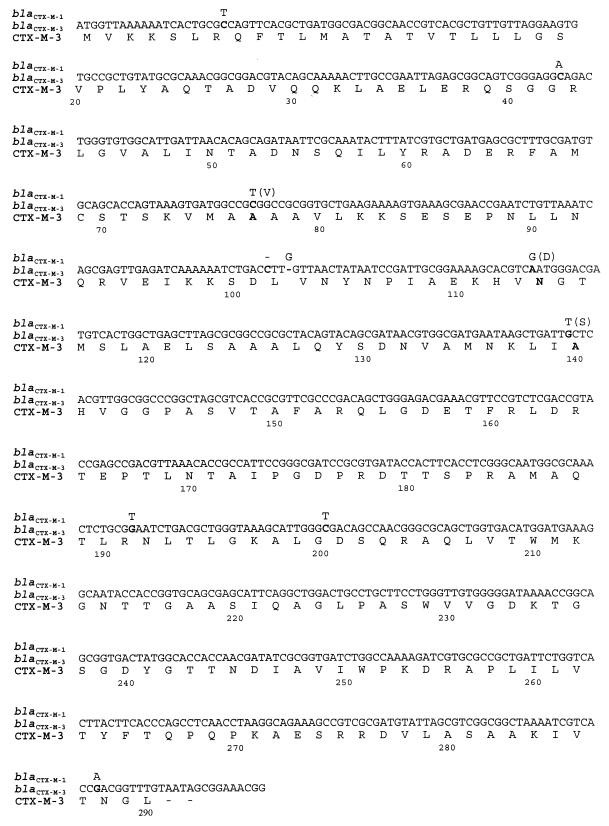
The entire β-lactamase coding sequence was amplified in the PCR with the plasmid preparation from the C. freundii 2526/96 transconjugant. The PCR product of the expected size of about 1 kb was obtained (result not shown) and sequenced. Comparative analysis has revealed that the CTX-M coding sequence differs from that of the blaCTX-M-1 gene at 10 nucleotide positions (Fig. 3). Four of the observed differences determine amino acid differences in deduced protein sequences. The analyzed enzyme was designated CTX-M-3, and its amino acid differences from the CTX-M-1 β-lactamase are as follows: Val77Ala, Asp114Asn, Ser140Ala, and Asn288Asp (the first amino acid in each pair refers to CTX-M-1, and the second refers to CTX-M-3).
FIG. 3.
Nucleotide sequence of the blaCTX-M-3 coding region. The deduced amino acid sequence of CTX-M-3 is presented below. Nucleotide residues different from those in the blaCTX-M-1 gene are shown in boldface; residues characteristic of the blaCTX-M-1 gene are presented above these. The CTX-M-1 amino acid residues different from those of CTX-M-3 are presented in parentheses next to the blaCTX-M-1-specific nucleotides. Amino acid numbering is according to the work of Ambler (1).
DISCUSSION
In this study, we report the identification of CTX-M-3, an enzyme which expands one of the minor families of ESBLs. The CTX-M family includes class A cefotaxime-hydrolyzing enzymes and to date consists of three members: the CTX-M-1/MEN-1 (3, 5), the CTX-M-2 (4), and the Toho-1 (10) β-lactamases. DNA regions coding for CTX-M-2 (7) and Toho-1 (10) differ by only one nucleotide, which determines a single amino acid difference in their deduced protein sequences. The CTX-M-1/MEN-1 enzyme is less similar to the others, with an amino acid sequence homology with CTX-M-2 of about 84% (7). CTX-M-3 differs from CTX-M-1/MEN-1 by four amino acid residues (10 nucleotide differences in protein coding regions) and is therefore closely related to this enzyme.
The pattern of antimicrobial susceptibility caused by the production of the CTX-M-3 β-lactamase (Table 1) corresponds very well to those observed for the CTX-M-1/MEN-1 (4, 5), CTX-M-2 (4), and Toho-1 (10) enzymes. Its activity for cefotaxime is approximately 2 orders of magnitude higher than that for ceftazidime as evaluated with MICs for transconjugant strains. Recombinant strains expressing CTX-M-1/MEN-1, CTX-M-2, and Toho-1 β-lactamases were characterized by MICs of cefotaxime 8, 32, and more than 128 times higher than that of ceftazidime, respectively (4, 5, 10). The Toho-1 enzyme hydrolyzes cefotaxime with a relative maximum rate about 100 times higher than that for ceftazidime (10). Comparison of antimicrobial susceptibilities (Table 1) demonstrates that CTX-M-3-expressing C. freundii isolates are clearly distinguishable from wild-type strains present in the same hospital by much higher MICs of β-lactam antibiotics tested, except for cefoxitin and imipenem. The observed cefoxitin resistance of C. freundii isolates was most probably due to induction of the chromosomal AmpC cephalosporinase (12) and certainly not to activity of CTX-M-3.
CTX-M ESBL-producing strains have been incidentally identified for 7 years as single clinical isolates in very distant geographic regions. CTX-M-2 was found in isolates from Argentina and Israel in 1992 and from Paraguay and again in two isolates from Argentina in 1994 (7). The almost identical Toho-1 enzyme was identified in 1993 in Japan (10). Isolation of CTX-M-1/MEN-1-producing strains has been restricted to Europe to date; the first isolates were found in 1989 in Germany (5) and in France (from a patient originating from Italy) (3), and the next two strains were collected in 1994 in two different cities in Germany (6). No outbreak caused by CTX-M-1/MEN-1-producing strains has ever been reported. The isolation of CTX-M-3-producing strains in Poland in 1996 suggests a wider distribution of CTX-M-1/MEN-1-related cefotaxime-hydrolyzing enzymes in Europe.
Isolates expressing the CTX-M-3 β-lactamase, collected in July 1996 in a single Warsaw hospital, may represent the first reported nosocomial epidemic caused by organisms producing a CTX-M-1/MEN-1-related enzyme in Europe. Ribotyping and RAPD approaches have suggested that the C. freundii isolates may be identical; moreover, this hypothesis is also supported by the same plasmid fingerprints, β-lactamase IEF patterns, and susceptibility data. These isolates were the only C. freundii isolates resistant to cefotaxime identified in the hospital in July 1996. Two were collected from patients hospitalized in the internal medicine ward, and one was from a patient in the urological ward. All patients were severely debilitated and predisposed to infections; the C. freundii isolates were identified as the etiological agents of urinary tract infections. It is impossible to say whether the CTX-M-3-producing C. freundii strain first appeared in the hospital in July 1996 or whether it was present prior to this time. Incidental isolations of cefotaxime-resistant C. freundii were reported by the hospital microbiological laboratory in the first half of 1996, but the isolates were neither collected nor typed at the time. The routine testing of Enterobacteriaceae isolates for the detection of ESBL activity was started in the laboratory at the beginning of July 1996. Over the next few months (August 1996 to January 1997), 19 new ESBL-producing, cefotaxime-resistant C. freundii isolates were identified in urine samples by the laboratory. Seven of them (November 1996 to January 1997) were collected and found to represent the same ribotypes and RAPD types as isolates 2524/96, 2525/96, and 2526/96 (data not shown). Most probably, the CTX-M-3-producing C. freundii strain has been persisting in the hospital environment for at least a year and regularly causing urinary tract infections in susceptible patients.
The second aspect of the CTX-M presence in the analyzed hospital is E. coli 2527/96. This strain is characterized by the same pattern of plasmid-mediated β-lactamases as those of the C. freundii isolates (enzymes with pIs of 8.4 and 5.4), and the partial (ca. 450 bp) nucleotide sequence of its CTX-M gene was identical to the corresponding region of the blaCTX-M-3 gene. However, MICs of piperacillin, cefotaxime, ceftazidime, and aztreonam characterizing the transconjugant R+ [E. coli 2527/96] were substantially higher than those for C. freundii transconjugants. This effect could be due to eventual differences in coding sequences apart from the sequenced region of the E. coli blaCTX-M gene or differences in levels of CTX-M-3 production. Plasmid DNA purified from R+ [E. coli 2527/96] was actually found to have a different PstI restriction map than that of plasmids extracted from C. freundii transconjugants, and so, the different sequence context of the β-lactamase gene might be responsible for quantitative differences in the phenotypes of the strains. It is noteworthy that at least two different plasmids carrying CTX-M genes have been spread among strains of different Enterobacteriaceae species present in the hospital and that these plasmids also contain a gene encoding another β-lactamase (with a pI of 5.4; probably the TEM-1 enzyme) and determine resistance to aminoglycosides.
ACKNOWLEDGMENTS
M.G. was partially supported by the FEMS fellowship.
We thank Ewa Wasińska for her contribution in susceptibility testing and Waleria Hryniewicz and Stephen Murchan for critical reading of the manuscript.
REFERENCES
- 1.Ambler R P. The structure of β-lactamases. Philos Trans R Soc Lond B. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa Y, Ohta M, Kido N, Mori M, Ito H, Komatsu T, Fujii Y, Kato N. Chromosomal β-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum β-lactam antibiotics. Antimicrob Agents Chemother. 1989;33:63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthélémy M, Peduzzi J, Bernard H, Tancrède C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended-spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind A, Casellas J M, Goldberg M, Holley M, Jungwirth R, Mangold P, Röhnisch T, Schweighart S, Wilhelm R. A new plasmidic cefotaximase from patients infected with Salmonella typhimurium. Infection. 1992;20:158–163. doi: 10.1007/BF01704610. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind A, Grimm H, Schweighart S. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection. 1990;18:294–298. doi: 10.1007/BF01647010. [DOI] [PubMed] [Google Scholar]
- 6.Bauernfeind, A., H. Grimm, I. Stemplinger, S. Ernst, and R. Jungwirth. 1995. Repeated incidence of CTX-M-1 (MEN) extended spectrum β-lactamase in Europe, abstr. 4210. Can. J. Infect. Dis. 6(Suppl. C):470C.
- 7.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequences of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other β-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauernfeind A, Stemplinger I, Jungwirth R, Mangold P, Amann S, Akalin E, Ang Ö, Bal C, Casellas J M. Characterization of β-lactamase gene blaPER-2, which encodes an extended-spectrum class A β-lactamase. Antimicrob Agents Chemother. 1996;40:616–620. doi: 10.1128/aac.40.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brosius J, Dull T J, Sleeter D D, Noller H F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981;148:107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- 10.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarlier V, Nicolas M, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 12.Livermore D M. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthew M, Harris A M, Marshall M J, Ross G W. The use of analytical isoelectric focussing for detection and identification of β-lactamases. J Gen Microbiol. 1975;88:169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 15.Perilli M, Franceschini N, Segatore B, Amicosante G, Oratore A, Duez C, Joris B, Frère J M. Cloning and sequencing of the gene encoding the β-lactamase from Citrobacter diversus. FEMS Microbiol Lett. 1991;83:79–84. doi: 10.1016/0378-1097(91)90448-j. [DOI] [PubMed] [Google Scholar]
- 16.Reynaud A, Peduzzi J, Barthélémy M, Labia R. Cefotaxime-hydrolysing activity of the β-lactamase of Klebsiella oxytoca D488 could be related to a threonine residue at position 140. FEMS Microbiol Lett. 1991;81:185–192. doi: 10.1016/0378-1097(91)90301-p. [DOI] [PubMed] [Google Scholar]
- 17.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Belkum A, Kluytmans J, van Leeuwen W, Bax R, Quint W, Peters E, Fluit A, Vandenbroucke-Grauls C, van den Brule A, Koeleman H, Melchers W, Meis J, Elaichouni A, Vaneechoutte M, Moonens F, Maes N, Struelens M, Tenover F, Verbrugh H. Multicenter evaluation of arbitrary primed PCR for typing of Staphylococcus aureus strains. J Clin Microbiol. 1995;33:1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]