Abstract
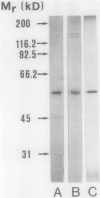
l-Tyrosine decarboxylase (EC 4.1.1.25) activity was induced in cell suspension cultures of Thalictrum rugosum Ait. and Eschscholtzia californica Cham. with a yeast polysaccharide preparation (elicitor). The highest l-tyrosine decarboxylase activity in extracts from 7-day-old cell cultures of E. californica was observed 5 hours after addition of 30 to 40 micrograms elicitor per gram cell fresh weight. The enzyme extracted from cells of E. californica was purified 1540-fold to a specific activity of 2.6 micromoles CO2 produced per minute per milligram protein at pH 8.4 and 30°C. Purified enzyme from T. rugosum showed a specific activity of 0.18 micromoles per minute per milligram protein. The purification procedure involved ammonium sulfate fractionation, anion-exchange fast protein liquid chromatography, ultrafiltration, and hydrophobic interaction chromatography. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that the enzyme from the two plant cell cultures had subunits of identical molecular weight (56,300 ± 300 daltons.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christenson J. G., Dairman W., Udenfriend S. Preparation and properties of a homogeneous aromatic L-amino acid decarboxylase from hog kidney. Arch Biochem Biophys. 1970 Nov;141(1):356–367. doi: 10.1016/0003-9861(70)90144-x. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Gügler K., Funk C., Brodelius P. Elicitor-induced tyrosine decarboxylase in berberine-synthesizing suspension cultures of Thalictrum rugosum. Eur J Biochem. 1988 Jan 4;170(3):661–666. doi: 10.1111/j.1432-1033.1988.tb13748.x. [DOI] [PubMed] [Google Scholar]
- Hahn M. G., Albersheim P. Host-Pathogen Interactions: XIV. Isolation and Partial Characterization of an Elicitor from Yeast Extract. Plant Physiol. 1978 Jul;62(1):107–111. doi: 10.1104/pp.62.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]