Abstract
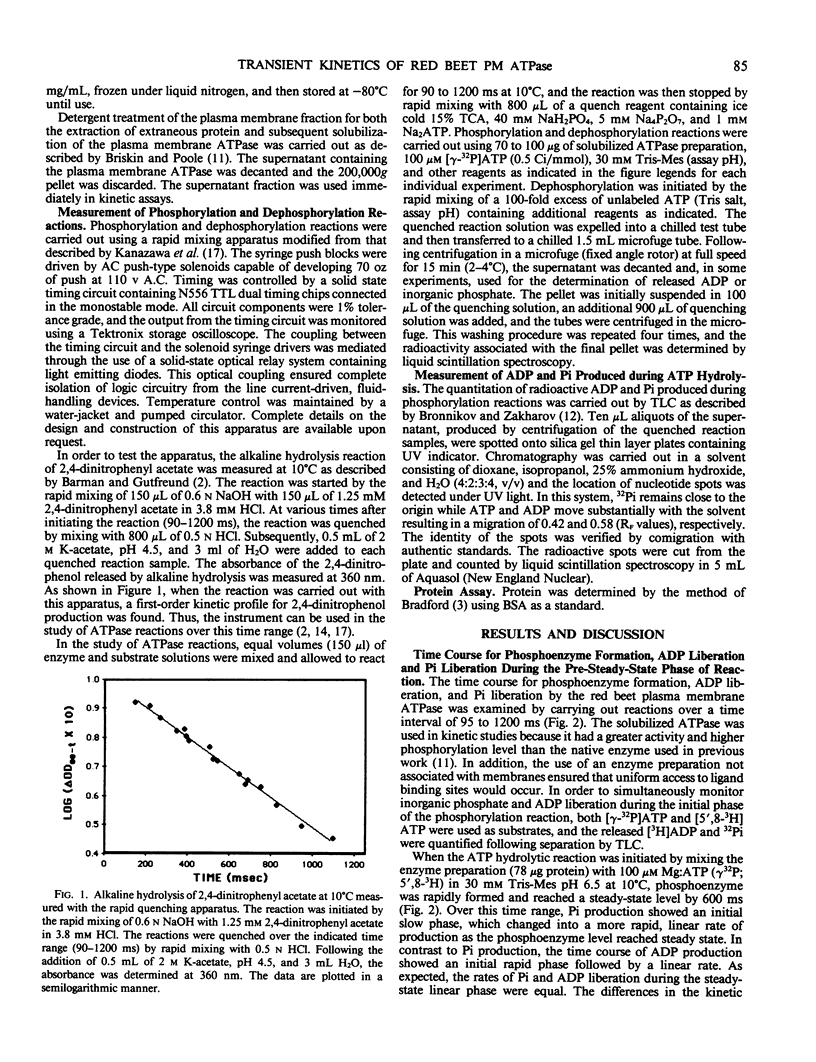
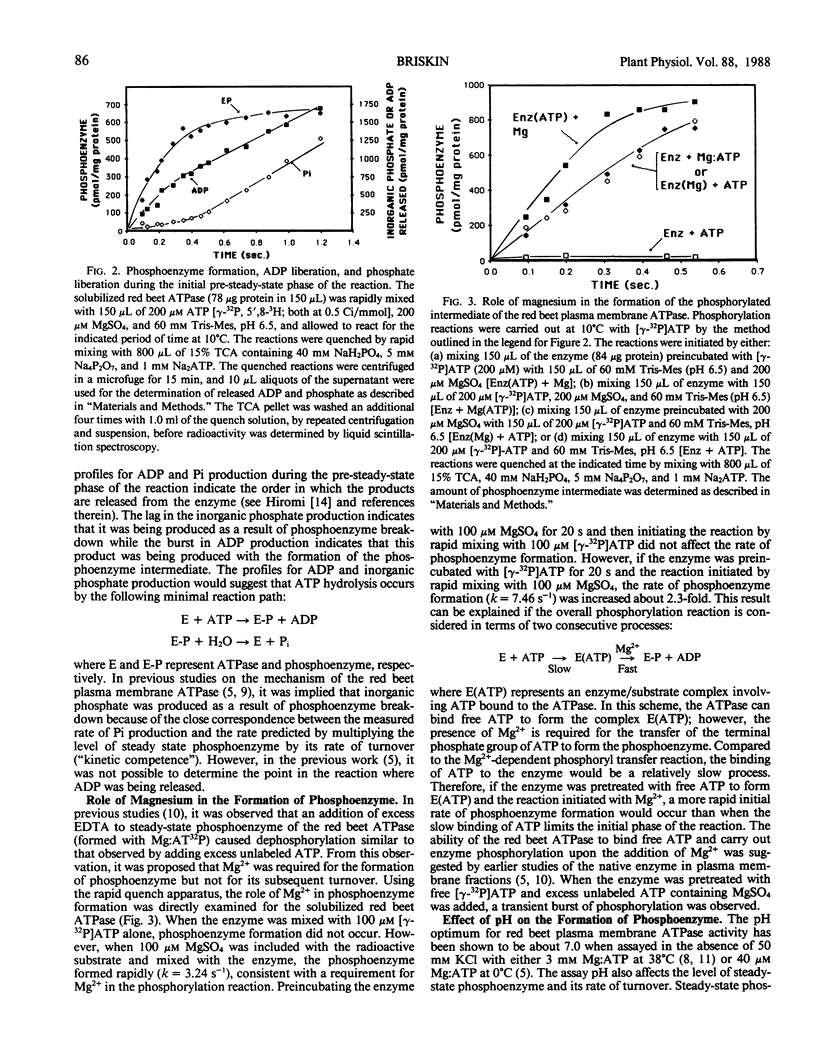
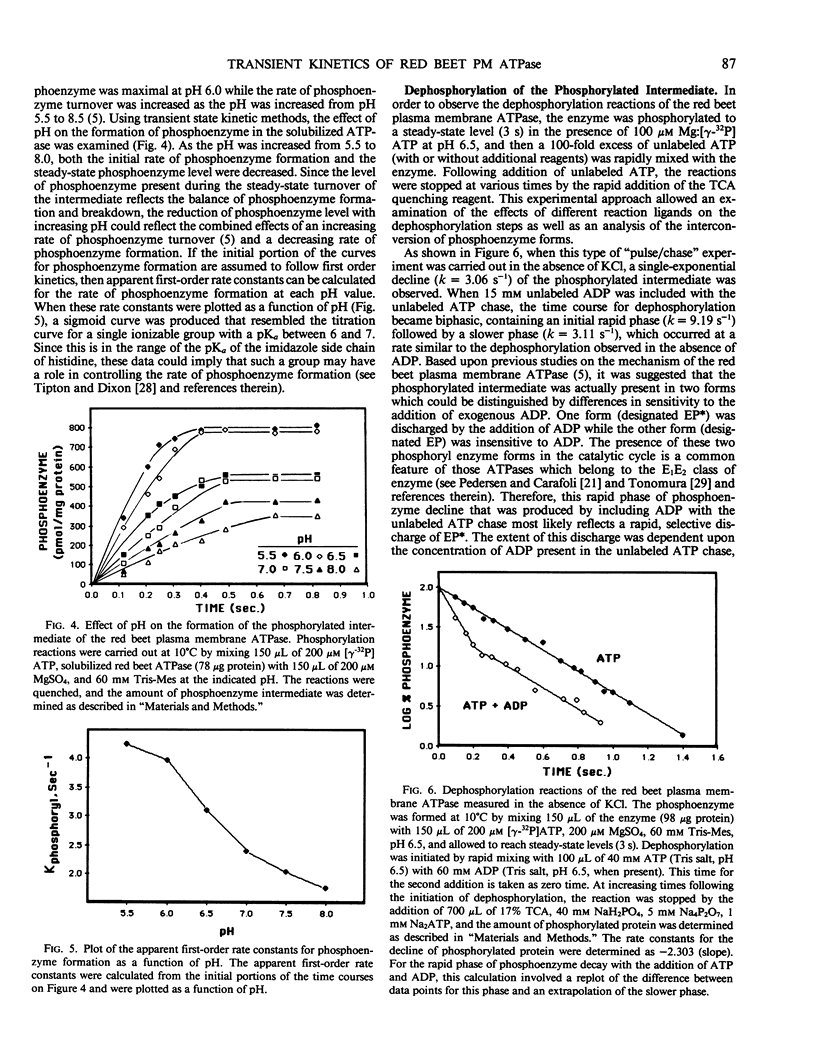
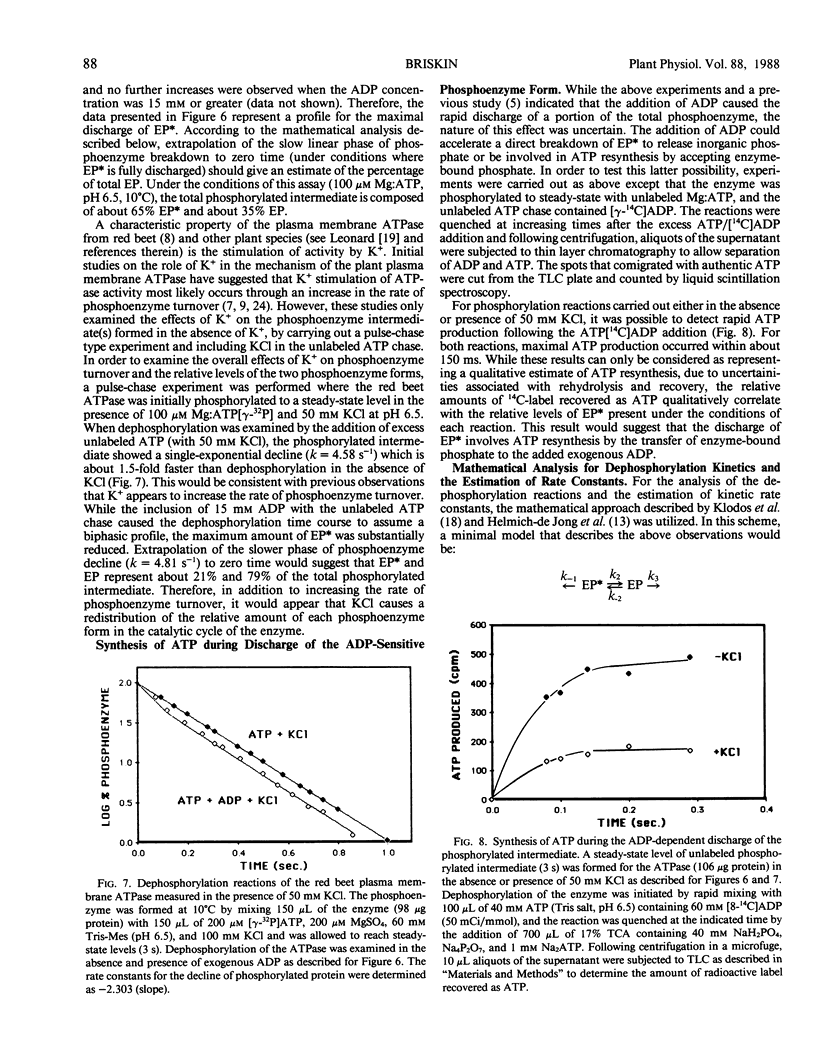
The reaction mechanism of the solubilized red beet (Beta vulgaris L.) plasma membrane ATPase was studied with a rapid quenching apparatus. Using a dual-labeled substrate ([γ-32P]ATP and [5′,8-3H]ATP), the presteady-state time course of phosphoenzyme formation, phosphate liberation and ADP liberation was examined. The time course for both phosphoenzyme formation and ADP liberation showed a rapid, initial rise while the timecourse for phosphate liberation showed an initial lag. This indicated that ADP was released with formation of the phosphoenzyme while phosphate was released with phosphoenzyme breakdown. Phosphoenzyme formation was Mg2+-dependent and preincubation of the enzyme with free ATP followed by the addition of Mg2+ increased the rate of phosphoenzyme formation 2.3-fold. This implied that phosphoenzyme formation could result from a slow reaction of ATP binding followed by a more rapid reaction of phosphate group transfer. Phosphoenzyme formation was accelerated as the pH was decreased, and the relationship between pH and the apparent first-order rate constants for phosphoenzyme formation suggested the role of a histidyl residue in this process. Transient kinetics of phosphoenzyme breakdown confirmed the presence of two phosphoenzyme forms, and the discharge of the ADP-sensitive form by ADP correlated with ATP synthesis. Potassium chloride increased the rate of phosphoenzyme turnover and shifted the steady-state distribution of phosphoenzyme forms. From these results, a minimal catalytic mechanism is proposed for the red beet plasma membrane ATPase, and rate constants for several reaction steps are estimated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke N. E., Hodges T. K. Plasma membrane adenosine triphosphatase of oat roots: activation and inhibition by mg and ATP. Plant Physiol. 1975 Jan;55(1):83–86. doi: 10.1104/pp.55.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Briskin D. P. Intermediate reaction states of the red beet plasma membrane ATPase. Arch Biochem Biophys. 1986 Jul;248(1):106–115. doi: 10.1016/0003-9861(86)90406-6. [DOI] [PubMed] [Google Scholar]
- Briskin D. P., Leonard R. T. Partial characterization of a phosphorylated intermediate associated with the plasma membrane ATPase of corn roots. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6922–6926. doi: 10.1073/pnas.79.22.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Characterization of a k-stimulated adenosine triphosphatase associated with the plasma membrane of red beet. Plant Physiol. 1983 Feb;71(2):350–355. doi: 10.1104/pp.71.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Characterization of the solubilized plasma membrane ATPase of red beet. Plant Physiol. 1984 Sep;76(1):26–30. doi: 10.1104/pp.76.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Plasma membrane ATPase of red beet forms a phosphorylated intermediate. Plant Physiol. 1983 Mar;71(3):507–512. doi: 10.1104/pp.71.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Role of magnesium in the plasma membrane ATPase of red beet. Plant Physiol. 1983 Apr;71(4):969–971. doi: 10.1104/pp.71.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronnikov G. E., Zakharov S. D. Microquantitative determination of Pi-ATP and ADP-ATP exchange kinetics using thin-layer chromatography on silica gel. Anal Biochem. 1983 May;131(1):69–74. doi: 10.1016/0003-2697(83)90136-7. [DOI] [PubMed] [Google Scholar]
- Helmich-de Jong M. L., van Emst-de Vries S. E., De Pont J. J., Schuurmans Stekhoven F. M., Bonting S. L. Direct evidence for an ADP-sensitive phosphointermediate of (K+ + H+)-ATPase. Biochim Biophys Acta. 1985 Dec 19;821(3):377–383. doi: 10.1016/0005-2736(85)90041-0. [DOI] [PubMed] [Google Scholar]
- Inesi G., Watanabe T., Coan C., Murphy A. The mechanism of sarcoplasmic reticulum ATPase. Ann N Y Acad Sci. 1982;402:515–534. doi: 10.1111/j.1749-6632.1982.tb25772.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L. Mechanism of the Na+, K+ pump. Protein structure and conformations of the pure (Na+ +K+)-ATPase. Biochim Biophys Acta. 1982 Aug 11;694(1):27–68. doi: 10.1016/0304-4157(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Saito M., Tonomura Y. Formation and decomposition of a phosphorylated intermediate in the reaction of Na plus-K plus dependent ATPase. J Biochem. 1970 May;67(5):693–711. doi: 10.1093/oxfordjournals.jbchem.a129297. [DOI] [PubMed] [Google Scholar]
- Klodos I., Nørby J. G., Plesner I. W. The steady-state kinetic mechanism of ATP hydrolysis catalyzed by membrane-bound (Na+ + K+)-ATPase from ox brain. II. Kinetic characterization of phosphointermediates. Biochim Biophys Acta. 1981 May 6;643(2):463–482. doi: 10.1016/0005-2736(81)90089-4. [DOI] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs G., Berglindh T., Rabon E., Wallmark B., Barcellona M. L., Stewart H. B., Saccomani G. The interaction of K+ with gastric parietal cells and gastric ATPase. Ann N Y Acad Sci. 1980;358:118–137. doi: 10.1111/j.1749-6632.1980.tb15391.x. [DOI] [PubMed] [Google Scholar]
- Scalla R., Amory A., Rigaud J., Goffeau A. Phosphorylated intermediate of a transport ATPase and activity of protein kinase in membranes from corn roots. Eur J Biochem. 1983 May 16;132(3):525–530. doi: 10.1111/j.1432-1033.1983.tb07393.x. [DOI] [PubMed] [Google Scholar]
- Stewart B., Wallmark B., Sachs G. The interaction of H+ and K+ with the partial reactions of gastric (H+ + K+)-ATPase. J Biol Chem. 1981 Mar 25;256(6):2682–2690. [PubMed] [Google Scholar]
- Vara F., Serrano R. Partial purification and properties of the proton-translocating ATPase of plant plasma membranes. J Biol Chem. 1982 Nov 10;257(21):12826–12830. [PubMed] [Google Scholar]
- Vara F., Serrano R. Phosphorylated intermediate of the ATPase of plant plasma membranes. J Biol Chem. 1983 May 10;258(9):5334–5336. [PubMed] [Google Scholar]
- Wallmark B., Mårdh S. Phosphorylation and dephosphorylation kinetics of potassium-stimulated ATP phosphohydrolase from hog gastric mucosa. J Biol Chem. 1979 Dec 10;254(23):11899–11902. [PubMed] [Google Scholar]


