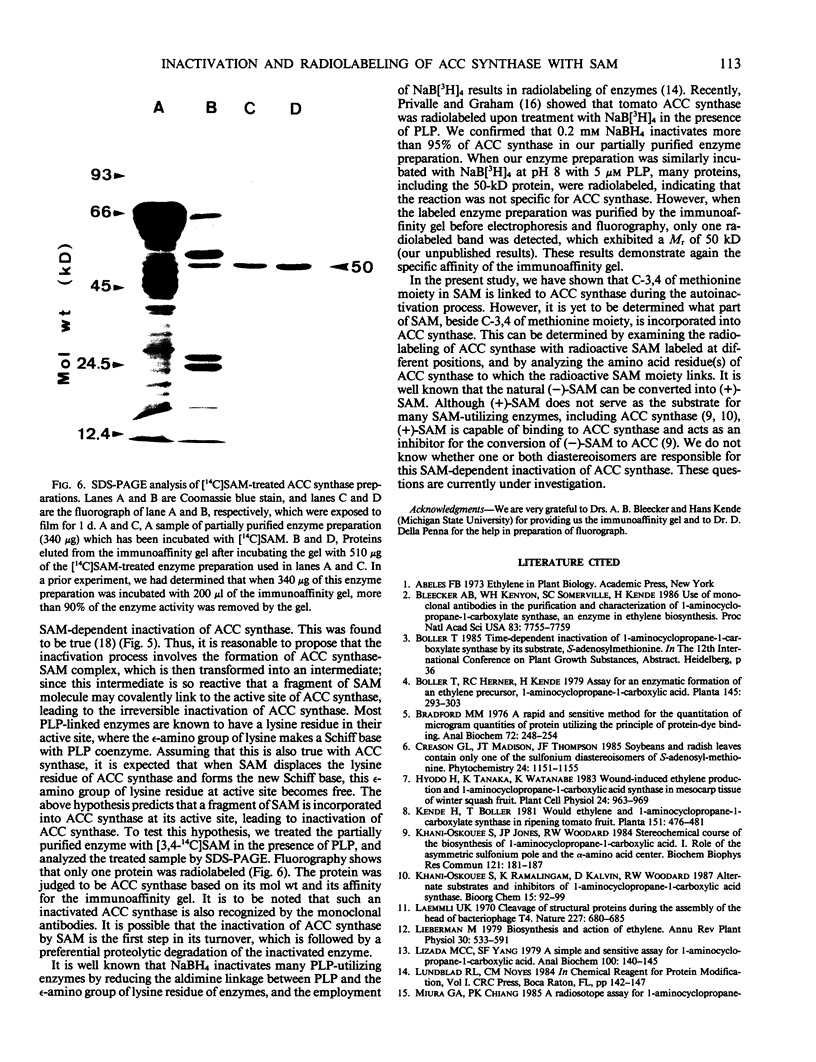
Abstract
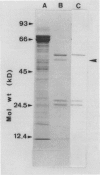
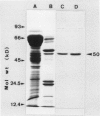
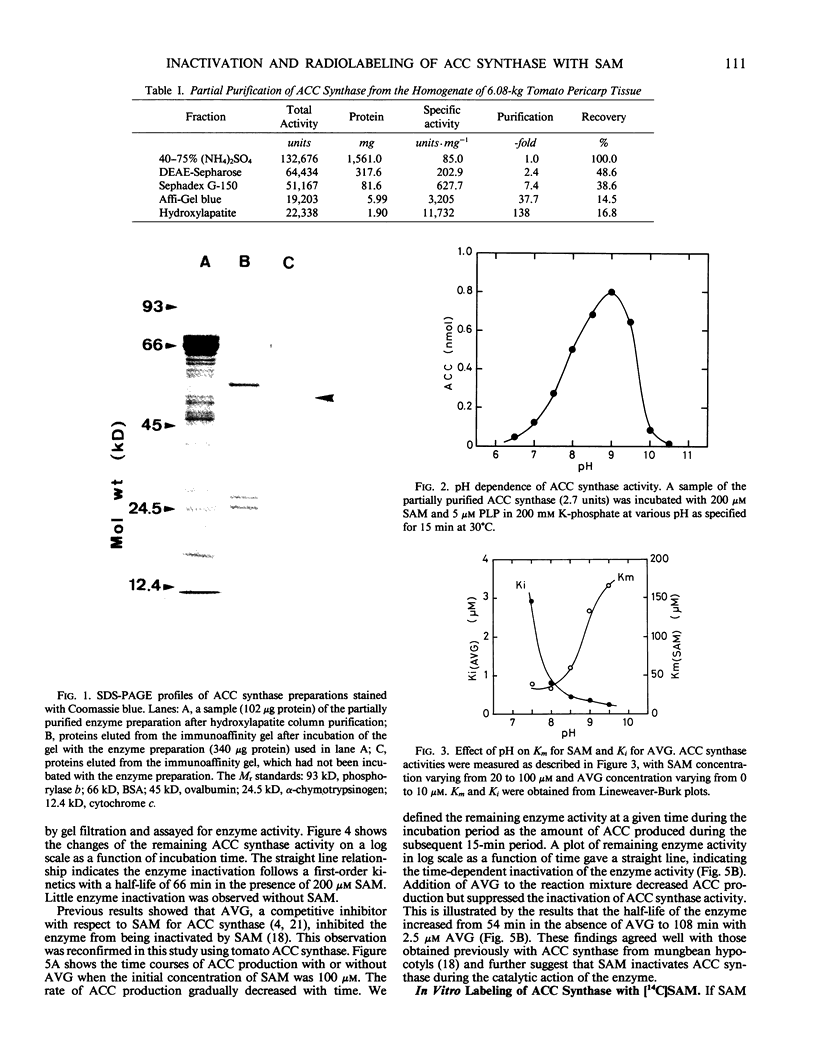
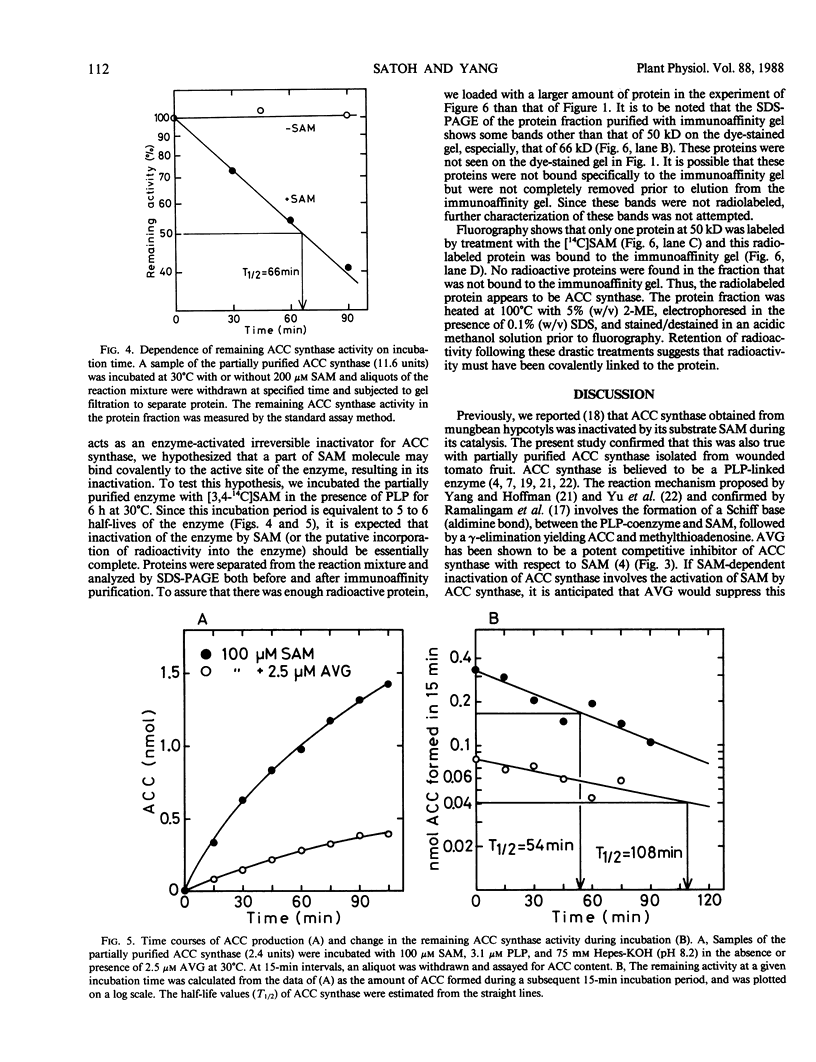
1-Aminocyclopropane-1-carboxylic acid (ACC) synthase was partially purified from the homogenate of wounded tomato (Lycoperiscon esculentum Mill.) pericarp tissue by (NH4)2SO4 fractionation followed by conventional column chromatography with diethylaminoethyl-Sepharose, Sephadex G-150, Affi-Gel blue and hydroxylapatite. The partially purified ACC synthase preparation attained a specific activity of about 12,000 nmoles per hour per milligram protein. Employing this enzyme preparation, we confirmed that the ACC synthase was inactivated by its substrate, S-adenosyl-l-methionine (SAM), during its catalytic action. When the partially purified enzyme preparation was incubated with [3,4-14C]SAM and the resulting proteins were analyzed by sodium dodecyl sulfate-gel electrophoresis, only one radioactive protein band was observed. This protein was thought to be ACC synthase based on its molecular mass of 50 kD and on the fact that it was specifically bound to a monoclonal antibody against ACC synthase (AB Bleecker et al. 1986 Proc Natl Acad Sci USA 83, 7755-7759). These results suggest that the substrate SAM acts as an enzyme-activated inactivator of ACC synthase by covalently linking a fragment of SAM molecule to the active site of ACC synthase, resulting in the inactivation of the enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleecker A. B., Kenyon W. H., Somerville S. C., Kende H. Use of monoclonal antibodies in the purification and characterization of 1-aminocyclopropane-1-carboxylate synthase, an enzyme in ethylene biosynthesis. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7755–7759. doi: 10.1073/pnas.83.20.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Khani-Oskouee S., Jones J. P., Woodard R. W. Stereochemical course of the biosynthesis of 1-aminocyclopropane-1-carboxylic acid. I. Role of the asymmetric sulfonium pole and the alpha-amino acid center. Biochem Biophys Res Commun. 1984 May 31;121(1):181–187. doi: 10.1016/0006-291x(84)90704-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Privalle L. S., Graham J. S. Radiolabeling of a wound-inducible pyridoxal phosphate-utilizing enzyme: evidence for its identification as ACC synthase. Arch Biochem Biophys. 1987 Mar;253(2):333–340. doi: 10.1016/0003-9861(87)90186-x. [DOI] [PubMed] [Google Scholar]
- Ramalingam K., Lee K. M., Woodard R. W., Bleecker A. B., Kende H. Stereochemical course of the reaction catalyzed by the pyridoxal phosphate-dependent enzyme 1-aminocyclopropane-1-carboxylate synthase. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7820–7824. doi: 10.1073/pnas.82.23.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Biosynthesis of wound ethylene. Plant Physiol. 1980 Aug;66(2):281–285. doi: 10.1104/pp.66.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]