Abstract
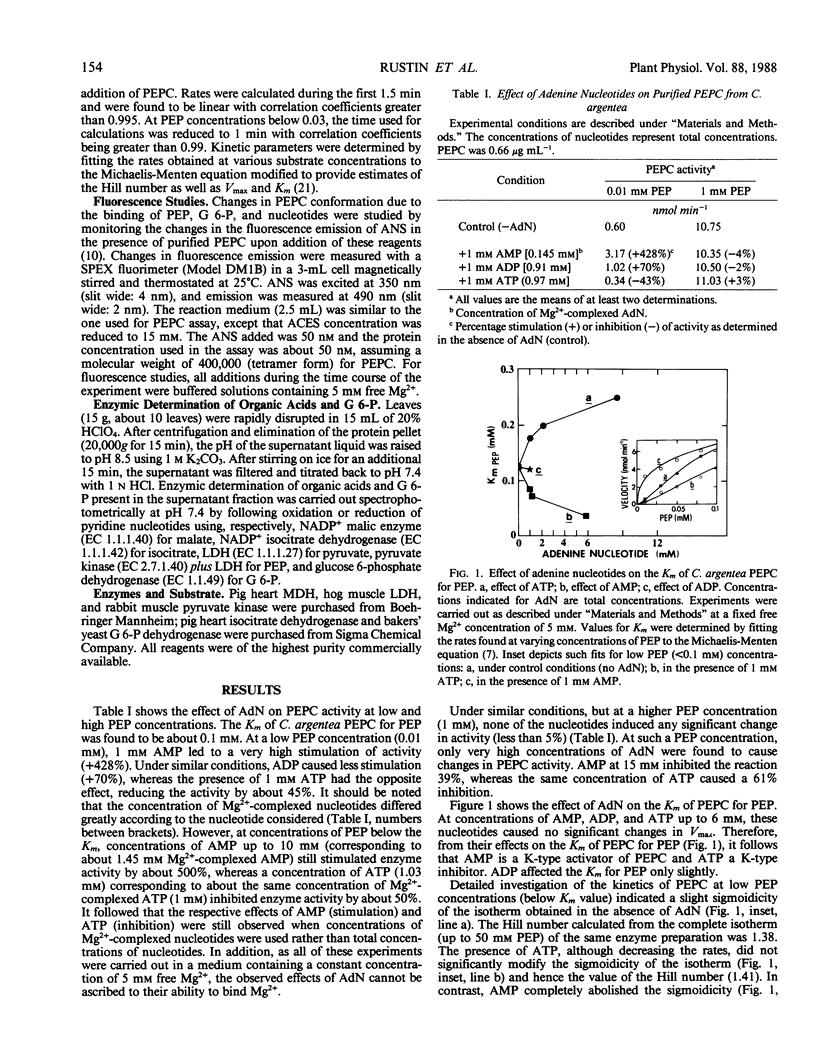
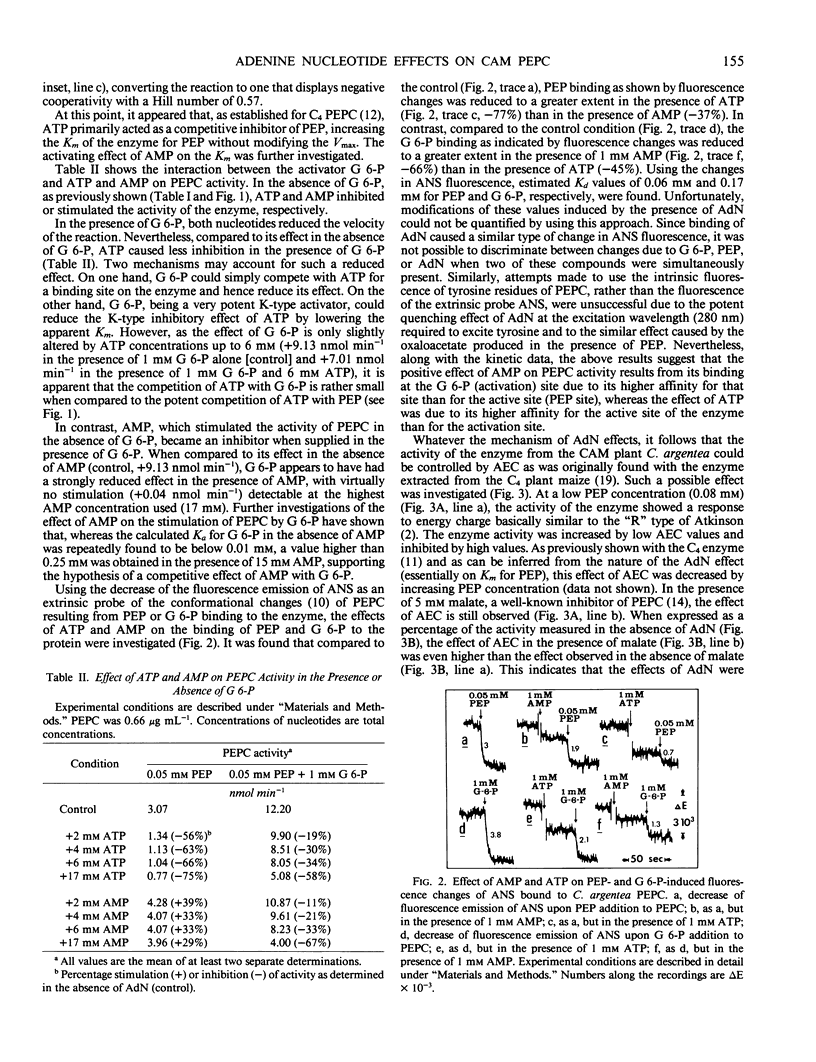
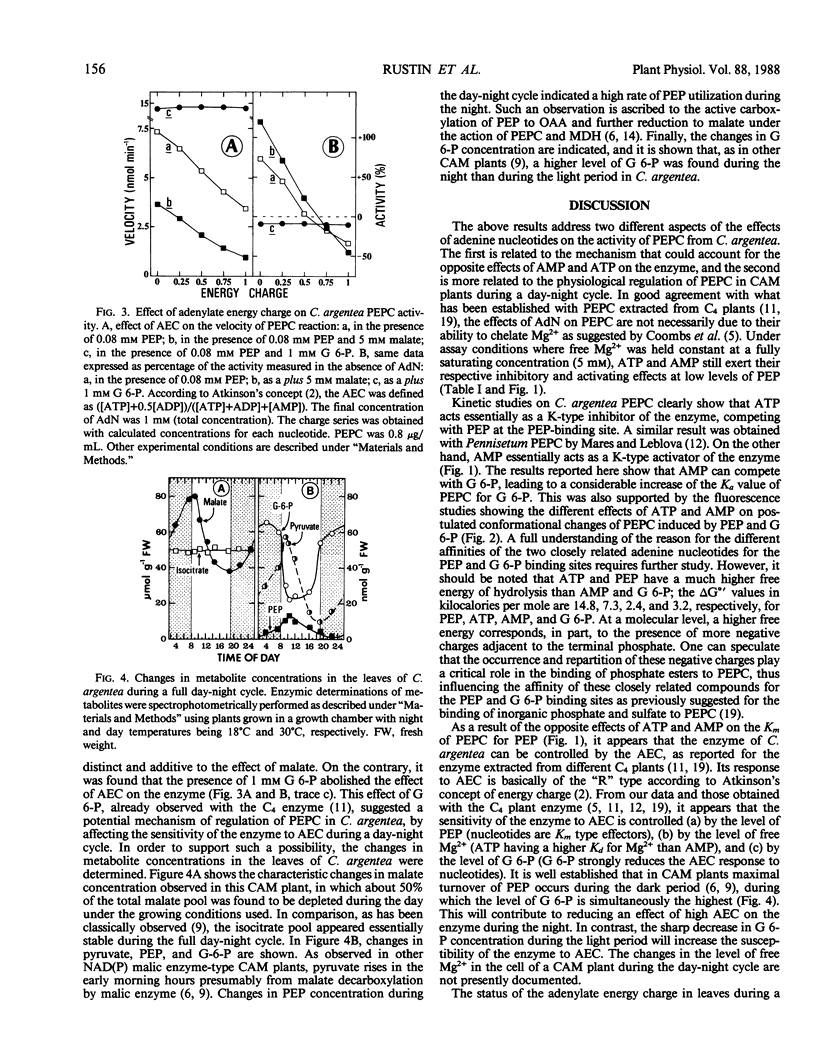
The effects of adenine nucleotides on phosphoenolypyruvate carboxylase were investigated using purified enzyme from the CAM plant, Crassula argentea. At 1 millimolar total concentration and with limiting phosphoenolpyruvate, AMP had a stimulatory effect, lowering the Km for phosphoenolpyruvate, ADP caused less stimulation, and ATP decreased the activity by increasing the Km for phosphoenolpyruvate. Activation by AMP was not additive to the stimulation by glucose 6-phosphate. Furthermore, AMP increased the Ka for glucose 6-phosphate. Inhibition by ATP was competitive with phosphoenolpyruvate. In support of the kinetic data, fluorescence binding studies indicated that ATP had a stronger effect than AMP on phosphoenolpyruvate binding, while AMP was more efficient in reducing glucose 6-phosphate binding. As free Mg2+ was held constant and saturating, these effects cannot be ascribed to Mg2+ chelation. Accordingly, the enzyme response to the adenylate energy charge was basically of the “R” type (involving enzymes of ATP regenerating sequences) according to D. E. Atkinson's (1968 Biochemistry 7: 4030-4034) concept of energy charge regulation. The effect of energy charge was abolished by 1 millimolar glucose 6-phosphate. Levels of glucose 6-phosphate and of other putative regulatory compounds of phosphoenolpyruvate carboxylase were determined in total leaf extracts during a day-night cycle. The level of glucose 6-phosphate rose at night and dropped sharply during the day. Such a decrease in glucose 6-phosphate concentration could permit an increased control of phosphoenolpyruvate carboxylase by energy charge during the day.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Boyer P. D. The inhibition of pyruvate kinase by ATP: a Mg++ buffer system for use in enzyme studies. Biochem Biophys Res Commun. 1969 Mar 10;34(5):702–706. doi: 10.1016/0006-291x(69)90795-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Grover S. D., Canellas P. F., Wedding R. T. Purification of NAD malic enzyme from potato and investigation of some physical and kinetic properties. Arch Biochem Biophys. 1981 Jul;209(2):396–407. doi: 10.1016/0003-9861(81)90297-6. [DOI] [PubMed] [Google Scholar]
- Hampp R., Goller M., Ziegler H. Adenylate Levels, Energy Charge, and Phosphorylation Potential during Dark-Light and Light-Dark Transition in Chloroplasts, Mitochondria, and Cytosol of Mesophyll Protoplasts from Avena sativa L. Plant Physiol. 1982 Feb;69(2):448–455. doi: 10.1104/pp.69.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon W. H., Holaday A. S., Black C. C. Diurnal Changes in Metabolite Levels and Crassulacean Acid Metabolism in Kalanchoë daigremontiana Leaves. Plant Physiol. 1981 Nov;68(5):1002–1007. doi: 10.1104/pp.68.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtley M. E., Koshland D. E., Jr Environmentally sensitive groups attached to proteins. Methods Enzymol. 1972;26:578–601. doi: 10.1016/s0076-6879(72)26027-x. [DOI] [PubMed] [Google Scholar]
- Meyer C. R., Rustin P., Wedding R. T. A simple and accurate spectrophotometric assay for phosphoenolpyruvate carboxylase activity. Plant Physiol. 1988 Feb;86(2):325–328. doi: 10.1104/pp.86.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Lane A. N., Clark R. A., Nieman R. H. Relationships between the rate of synthesis of ATP and the concentrations of reactants and products of ATP hydrolysis in maize root tips, determined by 31P nuclear magnetic resonance. Arch Biochem Biophys. 1985 Aug 1;240(2):712–722. doi: 10.1016/0003-9861(85)90080-3. [DOI] [PubMed] [Google Scholar]
- Stitt M., Lilley R. M., Heldt H. W. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982 Oct;70(4):971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. H., Ku M. S., Edwards G. E. Activity of maize leaf phosphoenolpyruvate carboxylase in relation to tautomerization and nonenzymatic decarboxylation of oxaloacetate. Arch Biochem Biophys. 1986 Aug 1;248(2):489–501. doi: 10.1016/0003-9861(86)90502-3. [DOI] [PubMed] [Google Scholar]
- Wong K. F., Davies D. D. Regulation of phosphoenolpyruvate carboxylase of Zea mays by metabolites. Biochem J. 1973 Mar;131(3):451–458. doi: 10.1042/bj1310451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Diurnal regulation of phosphoenolpyruvate carboxylase from crassula. Plant Physiol. 1985 Mar;77(3):667–675. doi: 10.1104/pp.77.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Temperature Effects on Phosphoenolpyruvate Carboxylase from a CAM and a C(4) Plant : A Comparative Study. Plant Physiol. 1987 Oct;85(2):497–501. doi: 10.1104/pp.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]


