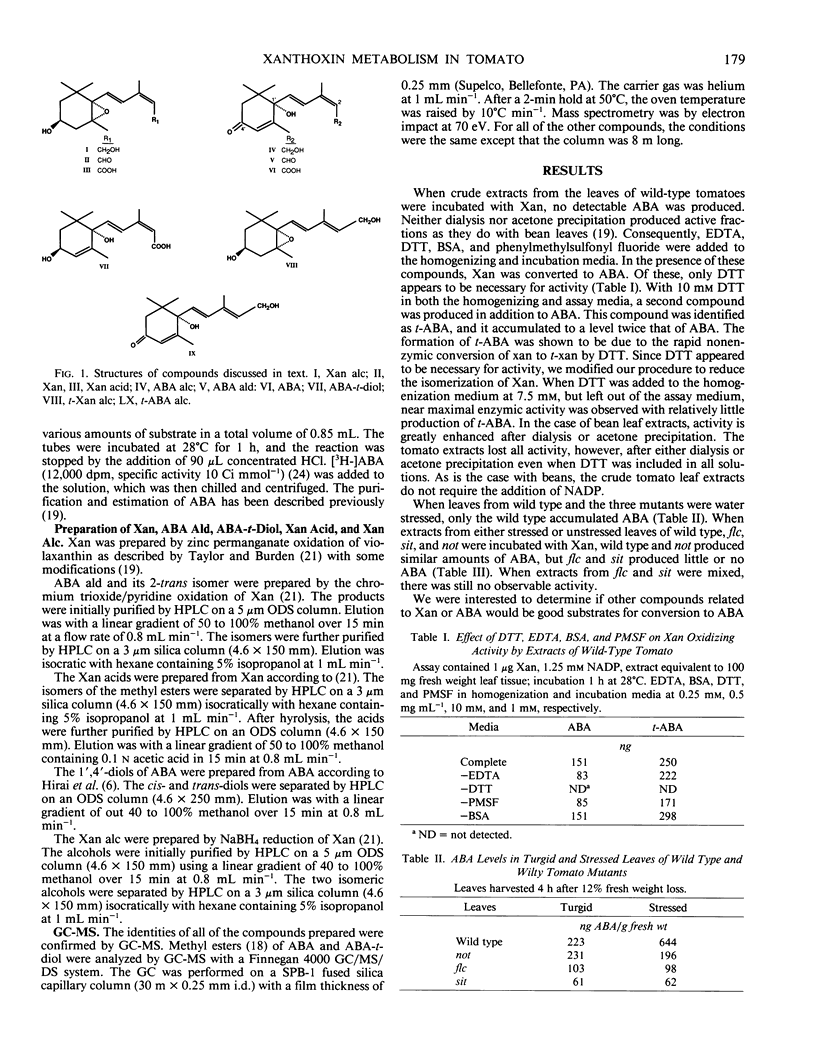
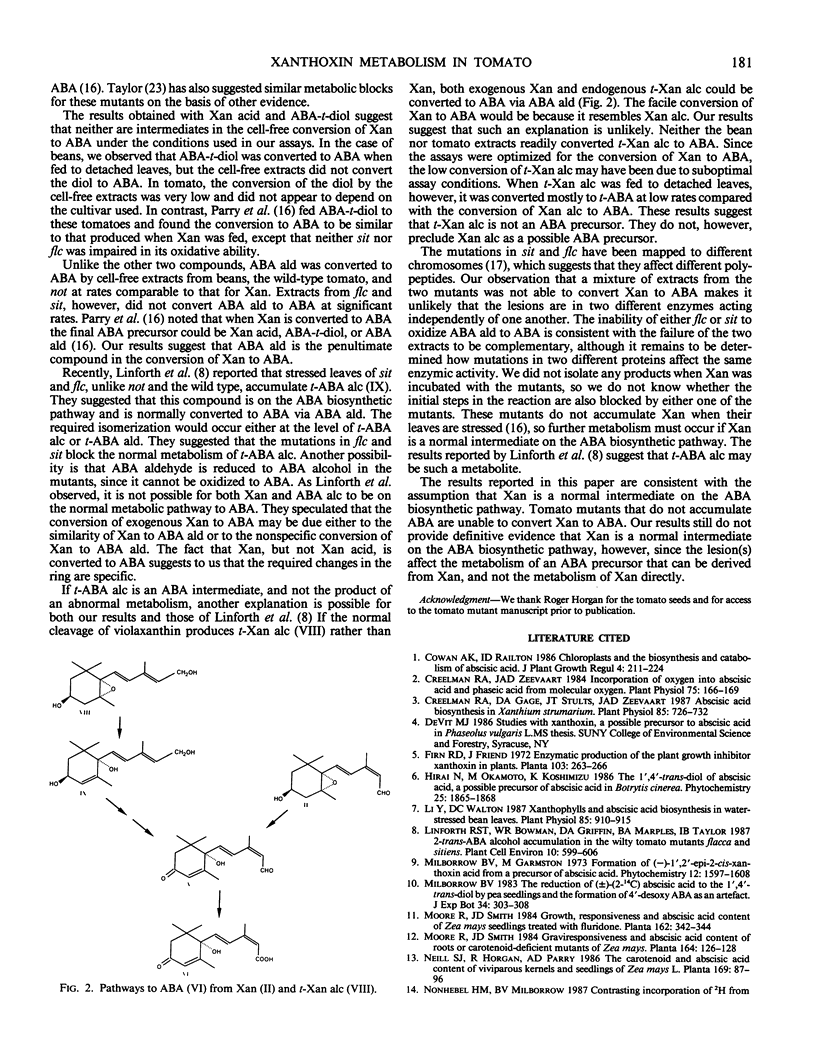
Abstract
Extracts prepared from the turgid and water-stressed leaves of wild-type tomato (Lycopersicon esculentum Mill cv Ailsa Craig) and the wilty mutants sitiens, notabilis, and flacca were tested for their ability to metabolize xanthoxin to ABA. Extracts from wild type and notabilis converted xanthoxin at similar rates, while extracts from sitiens and flacca showed little or no activity. We also observed no activity when extracts of sitiens and flacca were mixed. Similar results were obtained when ABA aldehyde was used as a substrate, in that extracts from wild type and notabilis were equally active, but extracts from flacca and sitiens showed little activity. None of the tomato extracts showed significant activity with xanthoxin acid, xanthoxin alcohol, or ABA-1′,4-′Trans-diol as substrates. Extracts from bean leaves (Phaseolus vulgaris L. cv Blue Lake) were similar to the wild-type tomato extracts in their ability to convert the various substrates to ABA, although excised bean leaves did convert ABA-1′,4′-trans-diol and xanthoxin alcohol to ABA when these substances were taken up through the petiole. These results are consistent with a role for xanthoxin as a normal intermediate on the ABA biosynthetic pathway, and they suggest that ABA aldehyde is the final ABA precursor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creelman R. A., Gage D. A., Stults J. T., Zeevaart J. A. Abscisic Acid Biosynthesis in Leaves and Roots of Xanthium strumarium. Plant Physiol. 1987 Nov;85(3):726–732. doi: 10.1104/pp.85.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Zeevaart J. A. Incorporation of oxygen into abscisic Acid and phaseic Acid from molecular oxygen. Plant Physiol. 1984 May;75(1):166–169. doi: 10.1104/pp.75.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Walton D. C. Xanthophylls and abscisic Acid biosynthesis in water-stressed bean leaves. Plant Physiol. 1987 Dec;85(4):910–915. doi: 10.1104/pp.85.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Smith J. D. Graviresponsiveness and abscisic-acid content of roots of carotenoid-deficient mutants of Zea mays L. Planta. 1985;164:126–128. [PubMed] [Google Scholar]
- Moore R., Smith J. D. Growth, graviresponsiveness and abscisic-acid content of Zea mays seedlings treated with fluridone. Planta. 1984;162:342–344. [PubMed] [Google Scholar]
- Sindhu R. K., Walton D. C. Conversion of xanthoxin to abscisic Acid by cell-free preparations from bean leaves. Plant Physiol. 1987 Dec;85(4):916–921. doi: 10.1104/pp.85.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. F., Burden R. S. Xanthoxin, a new naturally occurring plant growth inhibitor. Nature. 1970 Jul 18;227(5255):302–304. doi: 10.1038/227302a0. [DOI] [PubMed] [Google Scholar]
- Taylor H. F., Burden R. S. Xanthoxin, a recently discovered plant growth inhibitor. Proc R Soc Lond B Biol Sci. 1972 Mar 14;180(1060):317–346. doi: 10.1098/rspb.1972.0020. [DOI] [PubMed] [Google Scholar]


