Abstract
The benefits of treating several types of cancers using immunotherapy have recently been established. The overexpression of nucleolin (NCL) in a number of types of cancer provides an attractive antigen target for the development of novel anticancer immunotherapeutic treatments. NCL is a multifunctional protein abundantly distributed in the nucleus, cytoplasm and cell membrane. It influences carcinogenesis, and the proliferation, survival and metastasis of cancer cells, leading to cancer progression. Additionally, the meta-analysis of total and cytoplasmic NCL overexpression indicates a poor prognosis of patients with breast cancer. The AS1411 aptamers currently appear to have therapeutic action in the phase II clinical trial. The authors' research group has recently explored the anticancer function of NCL through the activation of T cells by dendritic cell-based immunotherapy. The present review describes and discusses the mechanisms through which the multiple functions of NCL can participate in the progression of cancer. In addition, the studies that define the utility of NCL-dependent anticancer therapies are summarized, with specific focus being paid to cancer immunotherapeutic approaches.
Keywords: nucleolin, anticancer therapy, cancer, immunotherapy, targeted therapy
1. Introduction
Cancer currently ranks as either the first or second leading cause of mortality among people prior to the age of 70 years worldwide (1). Based on global cancer statistics collected in 2020, the large burden of cancer incidence and mortality is projected to grow, with a predicted 22 million new cancer cases and 13 million cancer-related deaths occurring annually by the year 2030 (2,3). Cancer is increasing in prominence as an effort is being made to increase the range of novel treatment approaches for cancer. The conventional therapeutic methods for the treatment of cancer, including chemotherapy and irradiation following conservative surgery, are not always effective and are associated with undesirable side-effects (4). Hence, additional intensive strategies are required to improve the chances of successful therapy.
The immune system in the tumor microenvironment is considered to be a key target for cancer therapy (5,6). Immune responses to tumor cells have been generally considered to eliminate the vast majority of incipient cancer cells and consequently, nascent tumors (5). Cancer immunotherapy aims to boost the power of the host's immune system to prevent, control and eliminate cancer (7). The use of tumor antigen-specific T-cells in preclinical and clinical studies on multiple types of cancer has the objective of inducing the activation of the patient's immune system, directing it specifically toward tumor-associated antigens (TAAs) (8). Due to their genetic instability, TAAs are antigens that are aberrantly expressed to a greater degree in tumors than in normal tissues (9). This feature contributes to the low systemic toxicity if a TAA is targeted for treatment, and since TAAs are common markers of cancer cells, they are attractive as specific targets for a range of cancer types (7).
Clinical trials are currently being conducted on TAAs to translate the discoveries of these new potential molecular targets into clinical cancer treatments. Several good examples include treatments for malignant pleural mesothelioma, pancreatic and ovarian cancer that target mesothelin antigens (phase I, ClinicalTrials.gov: NCT01355965 and ClinicalTrials.gov: NCT02159716), NKG2D-L antigen in phase I/II and treatments for ovarian, colorectal, pancreatic, bladder, triple-negative breast cancer (TNBC), acute myeloid leukemia and multiple myeloma that target the NKG2D-L antigen (phase I/II, ClinicalTrials.gov: NCT03018405). Several clinical studies are using human epidermal growth factor receptor 2 (HER-2) antigen in the treatment of sarcoma, including osteosarcomas, Ewing sarcoma and soft tissue sarcomas, such as synovial sarcoma or desmoplastic small round cell tumors, nonrhabdomyosarcoma and rhabdomyosarcoma (ClinicalTrials.gov: NCT01935843), and in the treatment of advanced sarcoma (ClinicalTrials.gov: NCT00902044), lung, gastrointestinal tract, ovarian, biliary tract, pancreatic and brain cancer (glioblastoma multiforme) (ClinicalTrials. gov: NCT01109095 and ClinicalTrials.gov: NCT02349724). Moreover, the treatment of New York esophageal squamous cell carcinoma 1 (NY-ESO-1; Wilms' tumor 1, WT1) is cited in an attempt to create off-the-shelf dendritic cell vaccines that could target multiple malignancies sharing the same antigen (NCT02387125).
Among the various known TAA options available for immunotherapy to combat cancers is nucleolin (NCL), which was first described as a nucleolar protein in Novikoff hepatoma cells and Chinese Hamster Ovary cells (10,11). NCL is one of the most abundant proteins in the nucleus and >90% of the total NCL levels in cells are located there (12). It is also found in the cytoplasm and on the cell membrane. Notably, NCL on the cell surface can bind to various ligands to affect numerous physiological functions, including ribosome biogenesis, chromatin organization and stability, DNA and RNA metabolism, cytokinesis, cell proliferation, angiogenesis, apoptosis regulation, stress response and microRNA processing (13). The shuttling of NCL between the nucleus, cytoplasm and plasmalemma is significantly higher in cancer cells than in normal cells (14). Previous studies have demonstrated the role of NCL in angiogenesis (15), epithelial-to-mesenchymal transition and stemness (16,17). Increasing evidence from several research articles and datasets associates an elevated NCL expression with a poor prognosis of patients with various types of cancer, including pediatric and adult ependymoma (18,19), hepatocellular carcinoma (20), non-small cell lung cancer (21), esophageal squamous cell carcinoma (22), B-cell lymphoma (23), gastric cancer (24) and endometrial cancer (25). Although NCL is mainly present in the nucleus, its functions in aggressive cancer progression have been reported to be associated with its presence in the cancer cell cytoplasm and membrane (26). The nuclear, cytoplasmic and cell membrane expression of NCL can be used as a prognostic marker and therapeutic target in various types of cancer (26-28) (Table I). A high cytoplasmic expression of NCL is associated with a poor prognosis of patients with breast cancer, endometrial carcinoma, gastric cancer and non-small cell lung cancer (24,25,29,30) whereas a high nuclear expression of NCL is an independent good prognostic marker in gastric cancer, endometrial carcinoma and pancreatic ductal adenocarcinoma (17,24,25) (Table I). However, the surface NCL has been linked to the poor survival of patients with gastric cancer, rhabdomyosarcoma, breast cancer, hepatocellular carcinoma, colon cancer and prostate cancer (26,31-33) (Table I). In agreement with the rational of a decrease in cell surface NCL expression or activity, several in vitro and in vivo studies showed that decreases in cell surface NCL expression or activity inhibited the growth of cancer cells and triggered apoptosis (34,35).
Table I.
The characterization of each compartment of NCL as the biomarkers for diagnosis, prognostic prediction, and therapeutic targets.
| Impact of NCL | Nuclear NCL (Refs.) | Cytoplasmic NCL (Refs.) | Cell surface NCL (Refs.) |
|---|---|---|---|
| 1. Diagnostic and prognostic values | High nuclear NCL was associated with a good prognosis in endometrial carcinoma, gastric cancer, and pancreatic ductal adenocarcinoma (17,24,25) | High cytoplasmic NCL was correlated with poor prognosis in breast cancer, endometrial carcinoma, gastric cancer, and non-small cell lung cancer (24-26,29,30) | High cell surface NCL was correlated with poor prognosis and might serve as a promising biomarker for cancer diagnosis (13,26,31-33) |
| 2. Therapeutic target | AS1411, F3 peptide and N6L targeted nuclear NCL and induce cancer cell death and decrease malignant transformation of prostate cancer (85,86,147,203) | AS1411, HB-19 and N6L targeted cytoplasmic NCL and induced cancer cell death of leukemia cells and breast cancer (132,148,203,204) | AS1411, F3 peptide, 4LB5 antibody, HB-19, and N6L targeted surface NCL and induced cancer cell death of gastric cancer, rhabdomyosarcoma, breast cancer and hepatocellular carcinoma (31-33) |
NCL, nucleolin.
Epigenetic regulation is well known for its effect on protein expression, which contributes to cancer initiation and progression, including DNA methylation, histone modification, nucleosome remodeling and RNA or microRNA-mediated targeting molecules (36,37). The epigenetic regulators are targetable and have become prognostic biomarkers for various types of cancers including brain, breast, liver, ovary, prostate, gastric, lung, colorectal cancers, melanoma and angiosarcoma (36-39). NCL was also identified as an epigenetic regulator of leukemogenesis (40). The overexpression of NCL has been shown to promote the survival of leukemic cells by enhancing DNA methyltransferase 1 expression and subsequently inducing DNA hypermethylation followed by the epigenetic silencing of tumor suppressor gene transcription (40). Therefore, targeting NCL as epigenetic therapy may improve the survival of patients with leukemia (40).
Thus, NCL is considered to be a promising target for anticancer immunotherapy. In the present review, the nature of NCL and the NCL-based targeting strategies under development are discussed. Special focus has been paid to recently developed cell-based immunotherapy approaches.
2. Structure and location of nucleolin
NCL is a 100-110-kDa protein that is a multifunctional phosphoprotein expressed in exponentially growing eukaryotic cells. The biophysical and biochemical research of NCL mainly results from its multidomain structure. The human NCL gene consists of 14 exons and 13 introns on chromosome 2q12-qter (41). Mammalian NCL consists of 707 amino acids and a predicted molecular mass of ~77 kDa (13,42). The biophysical and biochemical research of NCL has disclosed a multidomain structure comprising three structural domains: An N-terminal domain rich in acidic regions and containing multiple phosphorylation sites; a central domain containing four RNA-binding domains (RBDs); and a C-terminal domain containing multiple glycine, arginine and phenylalanine residues (27,43). The N-terminal domain contains acidic stretches (rich in glutamic acid and aspartic acid) and its length varies greatly between the different NCL-like proteins (43). This domain participates in functions during the cell cycle that result in a high degree of phosphorylation of cell division control protein 2 homolog (p34cdc2), casein kinase 2, protein kinase C and cyclin-dependent kinase 1 (13,44-46). The involvement of the N-terminal domain in several protein-protein interactions has also been shown, including interactions with components of the pre-rRNA processing complex, and interactions with chromatin and untranslated regions controlling rDNA transcription (47-50). The central domain has four domains with RNA-binding domains (RBDs) or RNA-recognition motifs, and their modulatory effects have been the focus of numerous studies on cancer research (13). These domains are known for their RNA-binding specificity (43). One of the functions of NCL RBDs is assisting in pre-RNA transcription where they interact with the stem-loop structure of 18S and 28S ribosomal RNA (51), helping the pre-rRNA to fold correctly (52). Moreover, studies have demonstrated that the RBDs of NCL function in RNA packaging, pre-mRNA splicing, poly-A tail synthesis, maturation, mRNA stability and translational control (53). The C-terminal domain is rich in arginine-glycine-glycine repeats interspersed with several aromatic amino acids (43,54), and can interact with a number of target mRNAs and proteins (55).
Fractionation studies have located >90% of the NCL in the nucleolar portion of the cellular pool (12,27). The distribution of NCL between the cytoplasm and membrane is difficult to estimate, as it is dependent on the quality of the fractionation or sensitivity of the detection techniques (56,57). However, NCL appears to be ubiquitously distributed, being not only in the nucleus, but also in the cytoplasm and cell membrane (22). The distribution of NCL between cellular compartments, shuttling between the nucleus, cytoplasm and membrane, plays a significant role in the diverse mechanisms of NCL involvement in cancer (26,27). Several methylations and phosphorylations of the NH2 terminus of the NCL protein are required for the nuclear export of NCL to the cytoplasm (58,59). Highly proliferative cells, such as metabolically active cancer cells (60), have the common features of NCL overexpression and localization in the cytoplasm and cell membrane (27).
In tumor tissues from patients with gastric cancer (24), colorectal cancer (61), breast cancer (62), non-small cell lung cancer (29), and endometrial cancer (25), a high cytoplasmic NCL expression is associated with a poor prognosis and unfavorable clinical outcomes (26). The involvement of cytoplasmic NCL in the transportation of diverse molecules required for biogenesis in the nucleolus has been high-lighted (63). For example, NCL can interact with the p53 mRNA 5′UTR and prevent its translation (64,65), and the overexpression of cytoplasmic NCL enhances the stability of BCL-2 mRNA in B-cells from patients with chronic lymphocytic leukemia (66). It may be possible that the modulation of protein availability by the interaction of cytoplasmic NCL with mRNAs allows tumor cells to rapidly proliferate and escape apoptotic cell death.
NCL on the cell surface serves as an anchor protein that binds various molecules implicated in cell differentiation, adhesion, trafficking, inflammation, angiogenesis and cancer development (12,67-69). The mechanism whereby NCL is translocated to the plasma membrane remains unclear. Accumulating evidence validates the conclusion that the NCL, which is localized on the surface of various types of cancer cells, but not on their normal counterparts, is an effective strategic target for the treatment of cancer (68). By binding with Fas (70), or with Ras via the C-terminal domain (71), NCL significantly promotes cancer cell proliferation and inhibits apoptosis by decreasing the expression of the pro-apoptotic BAX gene (72). Targeting cell-surface NCL might trigger multiple inhibitory effects, depending on the cell type (68,73). Taken together, to date, these observations highlight a critical role for NCL in tumorigenesis and tumor progression. However, potential methods with which to modulate NCL for cancer immunotherapeutic purposes have been challenging to implement.
3. Therapeutic strategies exploiting NCL-based targeting
Surface NCL as a target for intracellular drug delivery
NCL was described for the first time in the plasma membrane of HepG2 cells (74,75) and was reported to be overexpressed at the cell membrane of various cancer cell lines (24,25,76-80). Cell-surface NCL functions as a receptor for several oncogenic ligands and is implicated in various oncogenic processes, such as epithelial-mesenchymal transition, the stabilization of pro-survival protein, angiogenesis and lymphangiogenesis (16). It also plays a critical role in anti-apoptosis (70) associated with chemoresistance in B-cell lymphoma (70), cervical cancer (81), breast cancer (82) and acute lymphoblastic leukemia (83). Several studies have described the development of molecules that target NCL on the cell surface of cancer cells to suppress or block NCL, and thereby inhibit cancer cell proliferation, apoptosis and angiogenesis (15,26,31-33,47).
The overexpression of surface NCL on cancer cells, compared to normal cells, provides a very promising target for cancer therapy, since targeting it does not alter NCL expression in the nucleus and generates minimal toxicity (27). Among the various NCL-binding ligands that have been tested thus far, the F3 peptide and the AS1411 aptamer are those that have been explored the most extensively (47).
F3 peptide as targeting ligand for agent delivery
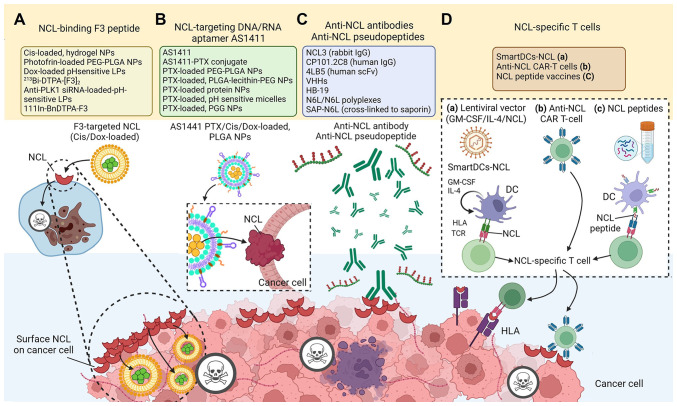
The NCL-binding F3 peptide is a 31-amino acid (KDEPQRRS ARLSAKPAPPKPEPKPKKAPAKK) (84) that was generated by a phage-displayed cDNA library, and selected using ex vivo screening on cell suspensions prepared from mouse bone marrow and in vivo screening for tumor homing (85). The use of NCL-binding F3 peptides has been encouraged for intracellular drug delivery (Table II and Fig. 1A). F3 homes to surface-expressed NCL as a receptor in cancer cells, neovasculature and endothelial cells (86). After the F3 peptide binds at the N-terminal domain of NCL, it is internalized into the targeted cell and translocated to the nucleus (85,86). The C-terminus of the F3 peptide has been utilized as an effective carrier of nanoparticles (NPs) for chemotherapeutic drugs, such as doxorubicin (87,88), cisplatin (Cis) (89), paclitaxel, the sphingolipid, C6-ceramide (inhibitor of the PI3K pathway) (90), or photodynamic therapy agents (91), and it has been chemically conjugated to radiotherapeutics (92). A range of cancer models has been utilized (77,87,93-116). Among notable findings, after intravenous administration of an F3 peptide-targeted Cis-loaded hydrogel NP there was decreased Cis-sensitive-derived or Cis-resistant-derived ovarian cancer progression (89). F3 peptide has also been used for the delivery of photodynamic therapeutic agents, dextran-coated iron oxide NPs, siRNAs and oligonucleotides to various tumor cell lines and tumor xenografts (117,118). In a previous study, results were obtained from institutional pathology reports and reviewed by experienced pathologists from the Portuguese Institute of Oncology, Coimbra, Portugal, which confirmed that the uptake of F3-targeted liposomes by cancer cells was associated only with the expression of the NCL receptor (88). Indeed, compared to treatments with phages and antibodies, the small mass of F3 peptide (~5 kDa) permitted wider distribution in cells (86). Moreover, an anti-NCL single variable domain has been generated on the variable domain of heavy-chain antibodies (VHH) (nanobodies) by grafting 10-amino acids of F3 peptide-derived NCL-binding sequences onto complementarity-determining regions (CDR)1 or CDR3 of a parental VHH to yield anti-NCL-CDR1 VHH and anti-NCL-CDR3 VHH. The two different anti-NCL VHHs exhibited maximal binding and increased cytotoxicity against an NCL-overexpressing breast cancer cell line (119,120). In a previous study, the treatment of Polo-like kinase 1 (PLK1)-overexpressing prostate cancer and angiogenic endothelial cells with an F3-targeted liposomal nanocarrier entrapping an anti-PLK1 siRNA significantly decreased cell viability (121). In preclinical models, targeted drug delivery into tumors has been achieved by the conjugation of anticancer drugs with F3 tumor-homing peptides. An F3 dimer coupled to the radiopharmaceutical DTPA chelated with 213Bi, (213Bi-DTPA-[F3]2), has been tested for binding affinity, bio-distribution, and anti-tumor activity in vitro and in vivo in a preclinical model of peritoneal carcinomatosis (122). In another study, The 213Bi-DTPA-[F3]2 was highly cytotoxic without severe side-effects in animals (122), significantly reduced clonogenic survival in vitro, and delayed tumor growth in vivo (84).
Table II.
Therapeutic strategies exploiting NCL-based targeting.
| Therapeutic strategies | Proposed functions | (Refs.) |
|---|---|---|
| F3 peptide-based agents | Peptides targeted to NCL on the cancer cell surface | (77,84,86-88,90,92-117) |
| AS1411-based agents | Aptamer targeted to DNA/RNA-binding domains of NCL on the cancer cell surface | (140-143) |
| Anti-NCL antibodies | Antigen-binding fragments (Fab) and the fragment crystallizable (Fc) region of NCL on the cancer cell surface | (31,47,111,154,155,201) |
| Anti-NCL pseudopeptides | A specific antagonist that binds the C-terminal RGG domain of NCL on the cancer cell surface | (15,33,157-180) |
| NCL-specific T-cells | Targeted to NCL on the cancer cell surface and cytoplasm (anti-NCL CAR T-cell and SmartDCs-NCL) | (181,190) |
NCL, nucleolin.
Figure 1.
Schematic diagram of NCL-targeting cancer therapies. (A) NCL-binding peptides, (B) NCL targeting aptamers, (C) Anti-NCL antibodies/pseudopeptides, and (D) NCL-specific T-cells including (a) SmartDCs-NCL, (b) anti-NCL CAR-T cell, and (c) NCL peptide vaccines. The figure was created using BioRender.com. NCL, nucleolin; SmartDCs-NCL, self-differentiated myeloid-derived APC reactive against tumors presenting NCL; Cis, cisplatin; PLGA-NPs, poly-lactic-co-glycolic acid-based nanoparticles; LPs, lipid polymers; GM-CSF, granulocyte-macrophage-colony stimulating factor; CAR, chimeric antigen receptor; DC, dendritic cell; IL-4, interleukin-4; HLA, human leukocyte antigen; TCR, T-cell receptor.
AS1411 aptamer as a targeting ligand for agent delivery
An aptamer is a short single-stranded nucleic acid sequence, either DNA or RNA, that can specifically target cellular and extracellular targets with high affinity (12). The aptamer AS1411, unmodified guanosine (G)-rich oligonucleotide (5′-d GGT GGT GGT GGT TGT GGT GGT GGT GG-3′), has a high affinity for NCL, binds to overexpressed NCL on the cell surface, and is then internalized (123,124), without adverse effects on normal cells (125-127). AS1411 functions as a molecular decoy, blocking and shortening the half-life of shuttle-NCL-regulated RNAs (128). The AS1411-based strategies have been used to target NCL with a delivery system of NPs containing agents such as siRNA, or small drugs (129,130) (Table II and Fig. 1B).
The first successful transition of an aptamer into clinical trials for cancer treatment was that of an NCL-targeting DNA aptamer AS1411 (119,120). In phase I clinical trials, three AS1411-based agents have been assessed for safety and efficacy in advanced solid tumors (ClinicalTrials.gov: NCT00881244) and acute myeloid leukemia (AML) (ClinicalTrials.gov: NCT00512083). The first, tested in NCT00881244, after it was demonstrated to significantly reduce tumor growth in xenograft models of both renal and lung cancers (131-133), demonstrated that 50% of 30 patients with advanced solid tumors had stable symptoms for 2-9 months after AS1411 administration without severe side-effects (134).
The phase 1 trial under NCT00512083, completed in 2006 (135), confirmed the safety and good tolerance of AS1411 in patients with AML. Furthermore, a phase II study [ClinicalTrials.gov: NCT00512083 (136,137)] demonstrated synergistic anticancer effects of AS1411 with cytarabine in the treatment of AML, thus demonstrating that it may be a promising candidate for AML therapy (131). In a previous study on patients with relapsed/refractory AML, high doses of cytarabine were associated improved response rates among patients who received combination therapy with continuous infusion of either 10 or 40 mg/kg/day doses of AS1411 compared to the control cytarabine-only group (138). Initial clinical assessments have been made of the responses in phase II trials in patients with metastatic refractory renal cell carcinoma (ClinicalTrials.gov: NCT00740441) (139) and AML (ClinicalTrials.gov: NCT01034410) (140-143). Although evidence of successful anticancer activity in phase I and II human clinical trials to treat AML was reported, and the aptamer has been renamed ACT-GRO-77, the company that developed it (Advanced Cancer Therapeutics), is no longer testing it against this type of cancer (27). A phase II clinical trial was also performed in patients with carcinoid/neuroendocrine tumors, and this drug is currently being developed by the company Tetragene.
During the development of clinical trials to further assess the efficacy of the AS1411 aptamer regarding the overall rate of response on a larger number of patients, the therapeutic effects of other types of AS1411 aptamer-conjugated agents were tested, including poly-lactic-co-glycolic acid (PLGA)-based NPs (PLGA-NPs) and lipid polymers (144). Several preclinical models are also being used to investigate the potential of the aptamer in transport mechanisms for other anticancer drugs (Table II, Fig. 1B). For example, AS1411-conjugated gold nanospheres have exhibited an enhanced cellular uptake, and increased anti-proliferative and cytotoxic effects in both in vitro and in vivo experiments (144). In lung cancer cells overexpressing NCL, AS1411-gemcitabine-NPs have been shown to exert an inhibitory effect on cell proliferation (145). Moreover, the AS1411-coated polymeric nanosystem can function as a potential drug delivery mechanism against various types of cancer, such as ovarian, pancreatic and lung cancer (146). Targeting surface NCL concurrently impairs the progression of several cancer cell types in vitro and in vivo. AS1411 targets nuclear NCL, leading to the re-localization of the protein arginine methyltransferase 5-NCL complex from the nucleus to the cytoplasm of prostate cancer, which may decrease malignant transformation (147). Moreover, the RNA-binding activity of cytoplasmic NCL to various mRNAs is inhibited by AS1411 aptamer, resulting in the induction of tumor cell death (148).
NCL in cancer immunotherapy
Active cancer immunotherapy uses the strategy of deploying the immune system of cancer patients to destroy tumors and prevent their recurrence (149). As proposed in Coley's toxins, agents with potent immunostimulatory properties (adjuvants) have achieved favorable responses in various types of cancer (150) and have significantly facilitated the advent of novel immunotherapy technologies. In recent years, cancer immunotherapy has made a significant breakthrough due to the development of adoptive T-cell therapies and the use of monoclonal antibodies that block the cytotoxic T-lymphocyte (CTL) antigen-4 (CTLA-4) and programmed cell death 1 (PD-1) immune checkpoints (151). Another approach that is often employed is the identification of TAA molecule(s) that are either selectively expressed or overexpressed by tumors (152). As discussed herein, NCL may be the most prominent eligible structure, which is stable on the cell surface of proliferating cells (153); hence, it represents an attractive target for immune-based anticancer agents.
Anti-nucleolin antibodies and pseudopeptides
Anti-NCL antibody production is the adaptive immune system response to the detection of the antigen (47). The antibody-based targeting of NCL has been previously explored (Table II and Fig. 1C). Treatment with anti-NCL3 antibodies has been shown to decrease the viability of both angiogenic endothelial and melanoma cells, and this is accompanied by the downregulation of BCL-2 expression (154). The same effect was observed in a murine model bearing breast cancer, in which anti-NCL3 antibody administration resulted in decreased tumor hypoxia (155). In another study using a leukemic xenograft mouse model, the anti-NCL3 antibody reduced cell viability without triggering immune responses (47,155), whereas CP101.2C8, which binds to the RBDs of NCL, led to a 30% greater mouse survival rate compared to the control IgG isotype group, and reduced the viability of leukemic cells (155).
Furthermore, the generation of anti-NCL antibodies has also been explored in the context of single-chain fragment variable (scFv) and VHH antibodies. The 4LB5 antibody, an scFv antibody that binds to the RNA-binding domain of cell-surface NCL, was previously found to decrease cell viability, clonogenicity and tumor growth in xenografts, while inducing the apoptosis of breast cancer and hepatocellular carcinoma (31). Indeed, a decreased tumor growth was observed in the in vivo model following the 4LB5 administration with no evidence of adverse effects (31). Novel anti-NCL VHHs have also been developed using a strategy of grafting F3 peptide-derived NCL-binding sequences onto a VHH CDR1 or CDR3 (111). These VHHs are more compact and smaller (~15 kDa) than scFv, with a stable immunoglobulin fold (156). The CDR3-grafted VHH enabled a significant antibody-dependent cell-mediated cytotoxicity effect against breast cancer cells with a 2-fold increase in cell death (111).
HB-19 is a surface NCL-antagonistic pseudopeptide with a pentavalent structure comprising the tripeptide lysine-glycine-proline (with a reduced bond between the lysine and proline residues) coupled to an 8-amino-acid template (15,33,157-180) (Fig. 1C). This pseudopeptide is translocated to the cytoplasm, but not to the nucleus, leading to a reduction in NCL levels in the cytoplasm and at the cell surface (33,157,172-175). The binding of HB-19 to surface NCL can form an irreversible complex that inhibits both tumor progression and angiogenesis (171). HB-19 treatment has been shown to suppress the tumor growth of established human breast cancer xenografts and exhibits low toxicity to normal tissue (15). HB-19 has been shown to impair angiogenesis, the formation of capillary-like structures, and blood vessel branching in chick embryo chorioallantoic membranes (15). Moreover, the colony formation capacity of several cancer cell lines in soft agar was decreased, which underscored the anticancer activities of HB-19 (15,167,169).
Multivalent pseudopeptide NucAnt 6L (N6L) also specifically binds to the surface NCL of cancer and endothelial cells and displays antitumor activities (176). It was synthesized to improve the biological actions of HB-19 by using a specific antagonist that binds the RGG domain of the NCL C-terminal tail (170). The normalization of tumor vessels and improved delivery and efficacy of chemotherapeutic drugs were observed as a consequence of NCL inhibition by N6L (17). The combination of N6L and mTOR inhibitors was found to synergistically inhibit the proliferation and viability of pancreatic cancer in both 2 and 3-D preclinical models (179). In another study, the pseudopeptide N6L cross-linked to saporin-S6 (SAP-N6L) induced the internalization of toxin to glioblastoma cancer cells and enhanced the toxic activity 1,000-fold compared to saporin alone in glioblastoma primary cells. The conjugated saporin and N6L induced glioblastoma cell death at low nanomolar concentrations and induced more apoptotic cell death than saporin alone (177). Moreover, N6L incorporated as polyplexes with nanoparticles (N6L-polyplexes) exhibited enhanced anti-tumor activities against pancreatic cancer. Gemcitabine treatment provides a standard of care for this cancer type. However, in an in vivo model of pancreatic ductal adenocarcinoma, the administration of N6L-polyplexes led to a significantly greater reduction of tumor volume than the administration of gemcitabine (175).
Adoptive T-cells by antigen-presenting cells (APCs)
It has been found that >75% of patients with TNBC highly express NCL (181). Yangngam et al (26) performed a meta-analysis to assess the prognostic value of NCL expression. A high level of total NCL was significantly associated with a poor overall survival (OS) and a shorter disease-free survival (DFS). A high level of cytoplasmic NCL was significantly associated with a poor OS and a shorter DFS. By contrast, a high nuclear expression of NCL was associated with an increased patient OS (26). These findings are consistent with the potential of upregulated NCL as a TAA.
An active approach to obtaining NCL-specific T-cells takes advantage of the potent antigen-presenting capacity of dendritic cells (DCs) loaded with NCL antigen (181) (Table II and Fig. 1D-a). The self-differentiated myeloid-derived APC reactive against tumors presenting NCL (SmartDCs-NCL) are monocyte-derived DC transduced with lentivirus harboring tricistronic complementary DNA sequences encoding cytokines (granulocyte-macrophage-colony stimulating factor; interleukin-4) and NCL antigen. A high copy number of integrated lentiviral vectors per cell in the SmartDCs platform led to ~1.5-3.0-fold self-differentiation more than the control vector (182), based on the endogenous load on the major histocompatibility complex (MHC) class I complex at the cell surface of APCs. DCs that acquire APC-like function provide the majority of endogenous MHC class I ligands for association with CD8+ CTLs (183). SmartDCs-NCL expressed all DCs markers, including the downregulation of CD14 and the upregulation of CD11c, CD40, CD80, CD83, CD86 and human leukocyte antigen (HLA)-DR, as compared with monocytes. The T-cells established following co-culture with SmartDCs-NCL comprised markedly increased CD3+/CD8+/CD45RA−/CD62L− effector memory T-cells (184), when compared to those generated by conventionally produced DCs. Furthermore, MHC class I-restricted stimulation of allogenic T-cells by the NCL-specific T-cells was verified by higher levels of interferon (IFN)-γ production. NCL-specific T-cells activated with SmartDCs-NCL gave significantly increased percentages of specific cell lysis at effector:target ratios of 1:1, 5:1, and 10:1 against NCLHigh MDA-MB-231 and HCC70 TNBC cells. No killing activities of NCL-specific T-cells were detected against normal mammary cells expressing no NCL (181).
Among the immunotherapeutic strategies that are recurrently being conducted worldwide in preclinical studies or clinical trials, the use of immune checkpoint inhibitors is particularly prominent (185). In a previous study, the combined treatment with SmartDCs-NCL-activated NCL-specific T-cells and anti-programmed cell death ligand 1 (PD-L1) peptide (CLQKTPKQC) (186) yielded significant killing of NCLHigh/PD-L1High MDA-MB-231 and NCLHigh/PD-L1High HCC70 TNBC cells. NCL-specific T-cell-mediated TNBC cell killing was mediated through both apoptotic and autophagic pathways. The higher lytic potential was also revealed by exposing TNBC cells to an autophagy inducer, the medicinal herb curcumin, which attenuated PD-L1 expression (181). The degradation of PD-L1 via the autophagy pathway has been reported (187-189). Indeed, curcumin can suppress PD-L1 production in cancer cells through p62-mediated autophagy. This is the first small-scale study that opens the door for potential clinical approaches targeting not only TNBC but also NCLHigh/PD-L1High cancer cells with NCL-specific T-cells in combination with a PD-L1 inhibitor or autophagic stimulator (181). Further intensive studies may lead to the incorporation of the adoptive transfer of NCL-specific T-cells into the standard of care protocol for clinical applications.
Chimeric antigen receptor (CAR) T-cells
The development of anti-NCL CAR T-cells has been previously reported (190) (Table II and Fig. 1D-b). Lentiviral-based CAR-transduction was previously used to generate anti-NCL CAR T-cells from PBMCs of healthy donors. The transduction efficacy of T-cells assessed by packaging:rev:envelope plasmid ratio revealed the highest transduction rate of 50% of tdTomato fluorescent protein and surface CAR expression assessed by flow cytometry assay (Table II and Fig. 1D-b). The anticancer activity of the NCL CAR T-cell was described (190). Importantly, similar to studies with SmartDCs-NCL, there has been only one publication on NCL CAR T-cells and it illustrates the potential of NCL as a target for anticancer immunotherapy (190). However, anti-NCL CAR T-cells and SmartDCs-NCL have opened a new field of novel strategies for NCL-based targeting in cancer treatment in terms of anti-NCL immunotherapeutic approaches.
Nucleolin peptide vaccines
Currently, the identification of TAA and the subsequent identification of T-cell epitopes render 'reverse immunology' applicable to the treatment of malignant tumors (191). This approach consists in raising T-cells against specific peptides corresponding to fragments of conventional proteins. New opportunities for cancer prevention and treatment can arise from peptides that promise a novel class of anticancer agents that can specifically target cancer cells, while remaining non-toxic to normal tissues (192). The ability of some TAA peptides to specifically recognize and bind to the membrane proteins of cancer cells could overcome the issues associated with the high molecular weight, low tissue diffusion, slow delivery and the poor cellular uptake of therapeutic antibodies (193-195). Moreover, peptide-based therapies are attractive over other treatment forms as peptides are easily synthesized on a large scale, chemically stable, have a high in vivo biocompatibility, are free of contaminating substances such as bacterial pathogens and are devoid of oncogenic potential (193,196). In breast cancer, HER-2/neu is overexpressed in 15 to 30% of patients and is associated with a poor prognosis (197). The E75 and GP2 HER-2/neu binding peptides were identified by their antigen-specific T-cell responses and tested in phase 1 clinical trial in patients with metastatic breast and ovarian cancer (197,198). Moreover, the identification of two novel 9-mer peptides, M1.1 and M1.2, with high binding affinity to HLA-A*02, could lead to their application in vaccine therapies for breast and ovarian cancer, based on the finding that >90% of the cancer cells of patient overexpress human mucin 1 (MUC-1) protein (199,200). More importantly, MUC1-specific CTLs were induced in patients with breast and ovarian cancer after vaccination with DC that had been pulsed with these peptides (200). Very recently, in silico predictive computational algorithms have revealed 9-mer NCL epitope peptides with major HLA-A*02-binding motifs (KMAPPPKEV15-23 and VLSNLSYSN488-496) in the full-length NCL sequence (181) (Fig. 1D-c). In in vitro experiments using these 2 NCL peptides, high IFN-γ-producing NCL-specific T-cells that kill NCL-positive cancer cells were observed (unpublished data).
4. Conclusions and future perspectives
NCL is an ubiquitous multifunctional protein with a tripartite domain structure that includes an acidic histone-like N-terminus, a central domain containing four RBDs, and an arginine and glycine-rich C-terminus (27,43). It functions as a shuttle phosphoprotein between nucleolus and cytoplasm in its diverse roles in cell growth, proliferation, carcinogenesis, angiogenesis, metastasis and cell death (13,43). Surface NCL is overexpressed in cancer cells, which renders it an attractive target for cancer treatment (12,26,67-69). A high level of cytoplasmic NCL, or cytoplasmic NCL and total NCL together, can provide a potent predictive marker for a shorter OS, whereas a high level of nuclear NCL presents a predictive marker for a long OS and DFS of patients (26). On the whole, this evidence indicates that NCL not only provides a potential biomarker for cancer diagnosis and prognosis, but also that anti-NCL therapies could represent a novel opportunity for cancer treatment.
A broad range of studies now supports this potential for cancer immunotherapy. Targeting surface NCL impaired the progression of several cancer cell types in vitro and in vivo. Furthermore, a wide variety of ligands targeting cell-surface NCL has been reported to block cancer cell growth and progression, including aptamer AS1411 (140-143), F3 peptides (77,84,86-88,90,92-117), antibodies against NCL (31,47,111,154,155,201) and the multivalent pseudopeptides (HB-19 and N6L) (15,33,157-180). The pace of clinical trials based on this approach is accelerating. Four aptamers AS1411 have been evaluated in clinical phase I/II including ClinicalTrials.gov: NCT00881244, NCT00512083, NCT00740441 and NCT01034410 (140-143). Two anti-NCL antibodies have been evaluated in clinical trials with the outcome of proliferation reduction. The antagonistic pseudo peptide N6L showed good results against solid tumors during a phase I/IIa clinical trial [ClinicalTrials.gov: NCT01711398 (202)], and a phase II trial is currently underway.
An important criterion for the selection of TAAs for personalized immunization is that it should be expressed by more than two-fold compared to para-tumor tissues (152). This property could allow for the induction of antigen-specific T-cells for patients with relatively low expression levels in normal tissues since immunization induced anti-TAA-specific CD4+ and CD8+ T-cell responses, which were enhanced by 0.1 to 10.0% (152). This highlights the potential of using protein overexpression, which is associated with a favorable OS for personalized TAA immunization. Sensitivity to NCL-specific T-cells is dependent on the level of NCL expressed in cancer cells (181). The in vitro data showed that NCL-specific T-cells activated by the DC-based platform decreased the viability of NCL-overexpressing breast cancer cells. Notably, the effects of NCL-specific T-cells, apoptosis and autophagic cell death, can be enhanced by an anti-PD-L1 peptide or autophagy modulator. Accordingly, treatment with NCL-specific T-cells is compatible with a combined approach, including immune checkpoint inhibitors for effective cancer patient treatment (181,190). However, at this time, the number of experiments is too limited to draw firm conclusions. Large-scale in vitro and clinical trials are required to validate the safety and effectiveness of this approach.
It is widely accepted that NCL has a functional role in cancer regulation. The present up-to-date review provides data to support the notion of targeting NCL among the developing anticancer approaches, including the use of anti-NCL peptides, aptamers, anti-NCL antibodies/pseudopeptides and NCL-specific T-cells. The NCL-specific T-cells generated by APCs or NCL peptides illustrate the use of the platforms that open a window for using immunotherapy targeted at NCL as another very promising approach in NCL-positive cancers. Further studies involving a specific target for each compartment of NCL are required in order to obtain a better understanding of the mechanisms through which different compartments of NCL contribute to the distinct prognoses of patients with cancer. Importantly, precision NCL-based targeting approaches with immunological potential and low toxicity need to be tested in both preclinical and clinical research to extend the value of NCL-targeting strategies.
Acknowledgments
The authors gratefully acknowledge Dr Jan O'Day Davies, Mahidol University, Bangkok, Thailand, for the English editing of the manuscript.
Abbreviations
- APC
antigen-presenting cells
- AML
acute myeloid leukemia
- CAR
chimeric antigen receptor
- CDR
complementarity-determining regions
- Cis
cisplatin
- CTL
cytotoxic T-lymphocyte
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- DCs
dendritic cells
- DFS
disease-free survival
- HLA
human leukocyte antigen
- IFN
interferon
- MHC
major histocompatibility complex
- MUC-1
mucin 1
- NCL
nucleolin
- OS
overall survival
- PD-1
programmed cell death 1
- PD-L1
programmed cell death ligand 1
- PLGA-NPs
poly-lactic-co-glycolic acid-based nanoparticles
- TAA
tumor-associated antigen
- TNBC
triple-negative breast cancer
- RBDs
RNA-binding domains
- scFv
single-chain fragment variable
- SmartDCs-NCL
self-differentiated myeloid-derived APC reactive against tumors presenting NCL
Funding Statement
The present study was funded by the New Researcher Grant, Mahidol University (grant no. R016420006), the Siriraj Research Fund, Faculty of Medicine Siriraj Hospital, Mahidol University (grant no. R016334002), a Research Grant, National Research Council of Thailand, and Mid-Research Grant (N42A650343), National Research Council of Thailand and Mahidol University (grant no. R016541043).
Availability of data and materials
Not applicable.
Authors' contributions
ST, PT, PTY and CT were involved in the conceptualization of the study. ST, KA and CT were involved in the writing and preparation of the original draft. CT critically reviewed and finalized the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
In the section '3. Therapeutic strategies exploiting NCL-based targeting', the in vitro experiments mentioned (unpublished data) were performed using biological samples of both healthy donors and patients with breast cancer. These experiments were approved by Siriraj Institutional Review Board (COA no. Si 580/2018). Informed consent was obtained from all subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Fidler MM, Bray F, Soerjomataram I. The global cancer burden and human development: A review. Scand J Public Health. 2018;46:27–36. doi: 10.1177/1403494817715400. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S, Sabatos-Peyton C, Petruzzelli L, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Velcheti V, Schalper K. Basic overview of current immunotherapy approaches in cancer. Am Soc Clin Oncol Educ Book. 2016;36:298–308. doi: 10.1200/EDBK_156572. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez Pérez Á, Campillo-Davo D, Van Tendeloo V, Benitez-Ribas D. Cellular immunotherapy: A clinical state-of-the-art of a new paradigm for cancer treatment. Clin Transl Oncol. 2020;22:1923–1937. doi: 10.1007/s12094-020-02344-4. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster M, Nechansky A, Kircheis R. Cancer immunotherapy. Biotechnol. 2006;1:138–147. doi: 10.1002/biot.200500044. [DOI] [PubMed] [Google Scholar]
- 10.Bugler B, Caizergues-Ferrer M, Bouche G, Bourbon H, Amalric F. Detection and localization of a class of proteins immunologically related to a 100-kDa nucleolar protein. Eur J Biochem. 1982;128:475–480. doi: 10.1111/j.1432-1033.1982.tb06989.x. [DOI] [PubMed] [Google Scholar]
- 11.Orrick LR, Olson MO, Busch H. Comparison of nucleolar proteins of normal rat liver and Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA. 1973;70:1316–1320. doi: 10.1073/pnas.70.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, Xu X. Roles of nucleolin: Focus on cancer and anti-cancer therapy. Saudi Med J. 2016;37:1312–1318. doi: 10.15537/smj.2016.12.15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia W, Yao Z, Zhao J, Guan Q, Gao L. New perspectives of physiological and pathological functions of nucleolin (NCL) Life Sci. 2017;186:1–10. doi: 10.1016/j.lfs.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Qiu W, Wang G, Sun X, Ye J, Wei F, Shi X, Lv G. The involvement of cell surface nucleolin in the initiation of CCR6 signaling in human hepatocellular carcinoma. Med Oncol. 2015;32:75. doi: 10.1007/s12032-015-0530-1. [DOI] [PubMed] [Google Scholar]
- 15.Destouches D, El Khoury D, Hamma-Kourbali Y, Krust B, Albanese P, Katsoris P, Guichard G, Briand JP, Courty J, Hovanessian AG. Suppression of tumor growth and angiogenesis by a specific antagonist of the cell-surface expressed nucleolin. PLoS One. 2008;3:e2518. doi: 10.1371/journal.pone.0002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ugrinova I, Petrova M, Chalabi-Dchar M, Bouvet P. Multifaceted nucleolin protein and its molecular partners in oncogenesis. Adv Protein Chem Struct Biol. 2018;111:133–164. doi: 10.1016/bs.apcsb.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Gilles ME, Maione F, Cossutta M, Carpentier G, Caruana L, Di Maria S, Houppe C, Destouches D, Shchors K, Prochasson C, et al. Nucleolin targeting impairs the progression of pancreatic cancer and promotes the normalization of tumor vasculature. Cancer Res. 2016;76:7181–7193. doi: 10.1158/0008-5472.CAN-16-0300. [DOI] [PubMed] [Google Scholar]
- 18.Ridley L, Rahman R, Brundler MA, Ellison D, Lowe J, Robson K, Prebble E, Luckett I, Gilbertson RJ, Parkes S, et al. Multifactorial analysis of predictors of outcome in pediatric intracranial ependymoma. Neuro Oncol. 2008;10:675–689. doi: 10.1215/15228517-2008-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modena P, Buttarelli FR, Miceli R, Piccinin E, Baldi C, Antonelli M, Morra I, Lauriola L, Di Rocco C, Garrè ML, et al. Predictors of outcome in an AIEOP series of childhood ependymomas: A multifactorial analysis. Neuro Oncol. 2012;14:1346–1356. doi: 10.1093/neuonc/nos245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo X, Xiong L, Yu L, Li R, Wang Z, Ren B, Dong J, Li B, Wang D. Increased level of nucleolin confers to aggressive tumor progression and poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Diagn Pathol. 2014;9:175. doi: 10.1186/s13000-014-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang F, Wu Y, Tan H, Guo T, Zhang K, Li D, Tong Z. Phosphorylation of nucleolin is indispensable to its involvement in the proliferation and migration of non-small cell lung cancer cells. Oncol Rep. 2019;41:590–598. doi: 10.3892/or.2018.6787. [DOI] [PubMed] [Google Scholar]
- 22.Qi J, Li H, Liu N, Xing Y, Zhou G, Wu Y, Liu Y, Chen W, Yue J, Han B, et al. The implications and mechanisms of the extra-nuclear nucleolin in the esophageal squamous cell carcinomas. Med Oncol. 2015;32:45. doi: 10.1007/s12032-015-0484-3. [DOI] [PubMed] [Google Scholar]
- 23.Jain N, Zhu H, Khashab T, Ye Q, George B, Mathur R, Singh RK, Berkova Z, Wise JF, Braun FK, et al. Targeting nucleolin for better survival in diffuse large B-cell lymphoma. Leukemia. 2018;32:663–674. doi: 10.1038/leu.2017.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu W, Zhou F, Zhang Q, Sun X, Shi X, Liang Y, Wang X, Yue L. Overexpression of nucleolin and different expression sites both related to the prognosis of gastric cancer. APMIS. 2013;121:919–925. doi: 10.1111/apm.12131. [DOI] [PubMed] [Google Scholar]
- 25.Lin Q, Ma X, Hu S, Li R, Wei X, Han B, Ma Y, Liu P, Pang Y. Overexpression of nucleolin is a potential prognostic marker in endometrial carcinoma. Cancer Manag Res. 2021;13:1955–1965. doi: 10.2147/CMAR.S294035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yangngam S, Prasopsiri J, Hatthakarnkul P, Thongchot S, Thuwajit P, Yenchitsomanus PT, Edwards J, Thuwajit C. Cellular localization of nucleolin determines the prognosis in cancers: A meta-analysis. J Mol Med (Berl) 2022;100:1145–1157. doi: 10.1007/s00109-022-02228-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger CM, Gaume X, Bouvet P. The roles of nucleolin subcellular localization in cancer. Biochimie. 2015;113:78–85. doi: 10.1016/j.biochi.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Yenchitsomanus PT, Vasuvattakul S, Kirdpon S, Wasanawatana S, Susaengrat W, Sreethiphayawan S, Chuawatana D, Mingkum S, Sawasdee N, Thuwajit P, et al. Autosomal recessive distal renal tubular acidosis caused by G701D mutation of anion exchanger 1 gene. Am J Kidney Dis. 2002;40:21–29. doi: 10.1053/ajkd.2002.33909. [DOI] [PubMed] [Google Scholar]
- 29.Xu JY, Lu S, Xu XY, Hu SL, Li B, Li WX, Chang JY. Prognostic significance of nuclear or cytoplasmic nucleolin expression in human non-small cell lung cancer and its relationship with DNA-PKcs. Tumour Biol. 2016;37:10349–10356. doi: 10.1007/s13277-016-4920-6. [DOI] [PubMed] [Google Scholar]
- 30.Willmer T, Damerell V, Smyly S, Sims D, Du Toit M, Ncube S, Sinkala M, Govender D, Sturrock E, Blackburn JM, Prince S. Targeting the oncogenic TBX3: Nucleolin complex to treat multiple sarcoma subtypes. Am J Cancer Res. 2021;11:5680–5700. [PMC free article] [PubMed] [Google Scholar]
- 31.Palmieri D, Richmond T, Piovan C, Sheetz T, Zanesi N, Troise F, James C, Wernicke D, Nyei F, Gordon TJ, et al. Human anti-nucleolin recombinant immunoagent for cancer therapy. Proc Natl Acad Sci USA. 2015;112:9418–9423. doi: 10.1073/pnas.1507087112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dzhumashev D, Timpanaro A, Ali S, De Micheli AJ, Mamchaoui K, Cascone I, Rössler J, Bernasconi M. Quantum Dot-based screening identifies F3 peptide and reveals cell surface nucleolin as a therapeutic target for rhabdomyosarcoma. Cancers (Basel) 2022;14:5048. doi: 10.3390/cancers14205048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiki H, Watanabe T, Suganuma M. Cell-surface nucleolin acts as a central mediator for carcinogenic, anti-carcinogenic, and disease-related ligands. J Cancer Res Clin Oncol. 2014;140:689–699. doi: 10.1007/s00432-014-1587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng GZ, Xiao SJ, Zeng SE, Li YQ. Downregulation of cell-surface-expressed nucleolin inhibits the growth of hepatocellular carcinoma cells in vitro. Zhonghua Zhong Liu Za Zhi. 2011;33:23–27. In Chinese. [PubMed] [Google Scholar]
- 35.D'Avino C, Palmieri D, Braddom A, Zanesi N, James C, Cole S, Salvatore F, Croce CM, De Lorenzo C. A novel fully human anti-NCL immunoRNase for triple-negative breast cancer therapy. Oncotarget. 2016;7:87016–87030. doi: 10.18632/oncotarget.13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawson MA, Kouzarides T. Cancer epigenetics: From mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Nebbioso A, Tambaro FP, Dell'Aversana C, Altucci L. Cancer epigenetics: Moving forward. PLoS Genet. 2018;14:e1007362. doi: 10.1371/journal.pgen.1007362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gougousis S, Petanidis S, Poutoglidis A, Tsetsos N, Vrochidis P, Skoumpas I, Argyriou N, Katopodi T, Domvri K. Epigenetic editing and tumor-dependent immunosuppressive signaling in head and neck malignancies. Oncol Lett. 2022;23:196. doi: 10.3892/ol.2022.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toh TB, Lim JJ, Chow EKH. Epigenetics in cancer stem cells. Mol Cancer. 2017;16:29. doi: 10.1186/s12943-017-0596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen N, Yan F, Pang J, Wu LC, Al-Kali A, Litzw MR, Liu S. A nucleolin-DNMT1 regulatory axis in acute myeloid leukemogenesis. Oncotarget. 2014;5:5494–5509. doi: 10.18632/oncotarget.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuteja R, Tuteja N. Nucleolin: A multifunctional major nucleolar phosphoprotein. Crit Rev Biochem Mol Biol. 1998;33:407–436. doi: 10.1080/10409239891204260. [DOI] [PubMed] [Google Scholar]
- 42.Mamrack MD, Olson MO, Busch H. Amino acid sequence and sites of phosphorylation in a highly acidic region of nucleolar nonhistone protein C23. Biochemistry. 1979;18:3381–3386. doi: 10.1021/bi00582a026. [DOI] [PubMed] [Google Scholar]
- 43.Ginisty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J Cell Sci. 1999;112:761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- 44.Peter M, Nakagawa J, Dorée M, Labbé JC, Nigg EA. Identification of major nucleolar proteins as candidate mitotic substrates of cdc2 kinase. Cell. 1990;60:791–801. doi: 10.1016/0092-8674(90)90093-T. [DOI] [PubMed] [Google Scholar]
- 45.Caizergues-Ferrer M, Belenguer P, Lapeyre B, Amalric F, Wallace MO, Olson MO. Phosphorylation of nucleolin by a nucleolar type NII protein kinase. Biochemistry. 1987;26:7876–7883. doi: 10.1021/bi00398a051. [DOI] [PubMed] [Google Scholar]
- 46.Belenguer P, Caizergues-Ferrer M, Labbé JC, Doree M, Amalric F. Mitosis-specific phosphorylation of nucleolin by p34cdc2 protein kinase. Mol Cell Biol. 1990;10:3607–3618. doi: 10.1128/mcb.10.7.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romano S, Fonseca N, Simões S, Gonçalves J, Moreira JN. Nucleolin-based targeting strategies for cancer therapy: From targeted drug delivery to cytotoxic ligands. Drug Discov Today. 2019;24:1985–2001. doi: 10.1016/j.drudis.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Ghisolfi-Nieto L, Joseph G, Puvion-Dutilleul F, Amalric F, Bouvet P. Nucleolin is a sequence-specific RNA-binding protein: Characterization of targets on pre-ribosomal RNA. J Mol Biol. 1996;260:34–53. doi: 10.1006/jmbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- 49.Cong R, Das S, Ugrinova I, Kumar S, Mongelard F, Wong J, Bouvet P. Interaction of nucleolin with ribosomal RNA genes and its role in RNA polymerase I transcription. Nucleic Acids Res. 2012;40:9441–9454. doi: 10.1093/nar/gks720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roger B, Moisand A, Amalric F, Bouvet P. Nucleolin provides a link between RNA polymerase I transcription and pre-ribosome assembly. Chromosoma. 2003;111:399–407. doi: 10.1007/s00412-002-0221-5. [DOI] [PubMed] [Google Scholar]
- 51.Serin G, Joseph G, Ghisolfi L, Bauzan M, Erard M, Amalric F, Bouvet P. Two RNA-binding domains determine the RNA-binding specificity of nucleolin. J Biol Chem. 1997;272:13109–13116. doi: 10.1074/jbc.272.20.13109. [DOI] [PubMed] [Google Scholar]
- 52.Allain FH, Bouvet P, Dieckmann T, Feigon J. Molecular basis of sequence-specific recognition of pre-ribosomal RNA by nucleolin. EMBO J. 2000;19:6870–6881. doi: 10.1093/emboj/19.24.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishikawa F, Matunis MJ, Dreyfuss G, Cech TR. Nuclear proteins that bind the pre-mRNA 3′ splice site sequence r(UUAG/G) and the human telomeric DNA sequence d(TTAGGG)n. Mol Cell Biol. 1993;13:4301–4310. doi: 10.1128/mcb.13.7.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lapeyre B, Amalric F, Ghaffari SH, Rao SV, Dumbar TS, Olson MO. Protein and cDNA sequence of a glycine-rich, dimethylarginine-containing region located near the carboxyl-terminal end of nucleolin (C23 and 100 kDa) J Biol Chem. 1986;261:9167–9173. doi: 10.1016/S0021-9258(18)67634-3. [DOI] [PubMed] [Google Scholar]
- 55.Ghisolfi L, Joseph G, Amalric F, Erard M. The glycine-rich domain of nucleolin has an unusual supersecondary structure responsible for its RNA-helix-destabilizing properties. J Biol Chem. 1992;267:2955–2959. doi: 10.1016/S0021-9258(19)50679-2. [DOI] [PubMed] [Google Scholar]
- 56.Gaume X, Tassin AM, Ugrinova I, Mongelard F, Monier K, Bouvet P. Centrosomal nucleolin is required for microtubule network organization. Cell Cycle. 2015;14:902–919. doi: 10.1080/15384101.2014.1000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scherl A, Couté Y, Déon C, Callé A, Kindbeiter K, Sanchez JC, Greco A, Hochstrasser D, Diaz JJ. Functional proteomic analysis of human nucleolus. Mol Biol Cell. 2002;13:4100–4109. doi: 10.1091/mbc.e02-05-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwab MS, Dreyer C. Protein phosphorylation sites regulate the function of the bipartite NLS of nucleolin. Eur J Cell Biol. 1997;73:287–297. [PubMed] [Google Scholar]
- 59.Shen EC, Henry MF, Weiss VH, Valentini SR, Silver PA, Lee MS. Arginine methylation facilitates the nuclear export of hnRNP proteins. Genes Dev. 1998;12:679–691. doi: 10.1101/gad.12.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hammoudi A, Song F, Reed KR, Jenkins RE, Meniel VS, Watson AJ, Pritchard DM, Clarke AR, Jenkins JR. Proteomic profiling of a mouse model of acute intestinal Apc deletion leads to identification of potential novel biomarkers of human colorectal cancer (CRC) Biochem Biophys Res Commun. 2013;440:364–370. doi: 10.1016/j.bbrc.2013.08.076. [DOI] [PubMed] [Google Scholar]
- 62.Pichiorri F, Palmieri D, De Luca L, Consiglio J, You J, Rocci A, Talabere T, Piovan C, Lagana A, Cascione L, et al. In vivo NCL targeting affects breast cancer aggressiveness through miRNA regulation. J Exp Med. 2013;210:951–968. doi: 10.1084/jem.20120950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56:379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- 64.Takagi M, Absalon MJ, McLure KG, Kastan MB. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 65.Chen J, Guo K, Kastan MB. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J Biol Chem. 2012;287:16467–16476. doi: 10.1074/jbc.M112.349274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Otake Y, Soundararajan S, Sengupta TK, Kio EA, Smith JC, Pineda-Roman M, Stuart RK, Spicer EK, Fernandes DJ. Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mRNA. Blood. 2007;109:3069–3075. doi: 10.1182/blood-2006-08-043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farin K, Di Segni A, Mor A, Pinkas-Kramarski R. Structure-function analysis of nucleolin and ErbB receptors interactions. PLoS One. 2009;4:e6128. doi: 10.1371/journal.pone.0006128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koutsioumpa M, Papadimitriou E. Cell surface nucleolin as a target for anti-cancer therapies. Recent Pat Anticancer Drug Discov. 2014;9:137–152. doi: 10.2174/1574892808666131119095953. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe T, Hirano K, Takahashi A, Yamaguchi K, Beppu M, Fujiki H, Suganuma M. Nucleolin on the cell surface as a new molecular target for gastric cancer treatment. Biol Pharm Bull. 2010;33:796–803. doi: 10.1248/bpb.33.796. [DOI] [PubMed] [Google Scholar]
- 70.Wise JF, Berkova Z, Mathur R, Zhu H, Braun FK, Tao RH, Sabichi AL, Ao X, Maeng H, Samaniego F. Nucleolin inhibits Fas ligand binding and suppresses Fas-mediated apoptosis in vivo via a surface nucleolin-Fas complex. Blood. 2013;121:4729–4739. doi: 10.1182/blood-2012-12-471094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schokoroy S, Juster D, Kloog Y, Pinkas-Kramarski R. Disrupting the oncogenic synergism between nucleolin and Ras results in cell growth inhibition and cell death. PLoS One. 2013;8:e75269. doi: 10.1371/journal.pone.0075269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang B, Wang H, Jiang B, Liang P, Liu M, Deng G, Xiao X. Nucleolin/C23 is a negative regulator of hydrogen peroxide-induced apoptosis in HUVECs. Cell Stress Chaperones. 2010;15:249–257. doi: 10.1007/s12192-009-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kirman DC, Renganathan B, Chui WK, Chen MW, Kaya NA, Ge R. Cell surface nucleolin is a novel ADAMTS5 receptor mediating endothelial cell apoptosis. Cell Death Dis. 2022;13:172. doi: 10.1038/s41419-022-04618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Semenkovich CF, Ostlund RE, Jr, Olson MO, Yang JW. A protein partially expressed on the surface of HepG2 cells that binds lipoproteins specifically is nucleolin. Biochemistry. 1990;29:9708–9713. doi: 10.1021/bi00493a028. [DOI] [PubMed] [Google Scholar]
- 75.Deng JS, Ballou B, Hofmeister JK. Internalization of anti-nucleolin antibody into viable HEp-2 cells. Mol Biol Rep. 1996;23:191–195. doi: 10.1007/BF00351168. [DOI] [PubMed] [Google Scholar]
- 76.Hovanessian AG, Puvion-Dutilleul F, Nisole S, Svab J, Perret E, Deng JS, Krust B. The cell-surface-expressed nucleolin is associated with the actin cytoskeleton. Exp Cell Res. 2000;261:312–328. doi: 10.1006/excr.2000.5071. [DOI] [PubMed] [Google Scholar]
- 77.Fonseca NA, Rodrigues AS, Rodrigues-Santos P, Alves V, Gregório AC, Valério-Fernandes Â, Gomes-da-Silva LC, Rosa MS, Moura V, Ramalho-Santos J, et al. Nucleolin overexpression in breast cancer cell sub-populations with different stem-like phenotype enables targeted intracellular delivery of synergistic drug combination. Biomaterials. 2015;69:76–88. doi: 10.1016/j.biomaterials.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 78.Chen C, Chen L, Yao Y, Qin Z, Chen H. Nucleolin overexpression is associated with an unfavorable outcome for ependymoma: A multifactorial analysis of 176 patients. J Neurooncol. 2016;127:43–52. doi: 10.1007/s11060-015-2007-7. [DOI] [PubMed] [Google Scholar]
- 79.Liu J, Wei T, Zhao J, Huang Y, Deng H, Kumar A, Wang C, Liang Z, Ma X, Liang XJ. Multifunctional aptamer-based nanoparticles for targeted drug delivery to circumvent cancer resistance. Biomaterials. 2016;91:44–56. doi: 10.1016/j.biomaterials.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 80.Mosafer J, Mokhtarzadeh A. Cell surface nucleolin as a promising receptor for effective AS1411 aptamer-mediated targeted drug delivery into cancer cells. Curr Drug Deliv. 2018;15:1323–1329. doi: 10.2174/1567201815666180724104451. [DOI] [PubMed] [Google Scholar]
- 81.Ke J, Gu C, Zhang H, Liu Y, Zhang W, Rao H, Li S, Wu F. Nucleolin promotes cisplatin resistance in cervical cancer by the YB1-MDR1 pathway. J Oncol. 2021;2021:9992218. doi: 10.1155/2021/9992218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fu Z, Fenselau C. Proteomic evidence for roles for nucleolin and poly[ADP-ribosyl] transferase in drug resistance. J Proteome Res. 2005;4:1583–1591. doi: 10.1021/pr0501158. [DOI] [PubMed] [Google Scholar]
- 83.Hu J, Chen Y, Wu Z, Wang L, Wen J, Jiang P, Zhang Y, Lin M. Targeting nucleolin for reversal of chemotherapy resistance in acute lymphoblastic leukemia. Blood. 2019;134:5058. doi: 10.1182/blood-2019-127073. [DOI] [Google Scholar]
- 84.Cornelissen B, Waller A, Target C, Kersemans V, Smart S, Vallis KA. 111In-BnDTPA-F3: An Auger electron-emitting radiotherapeutic agent that targets nucleolin. EJNMMI Res. 2012;2:9. doi: 10.1186/2191-219X-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Porkka K, Laakkonen P, Hoffman JA, Bernasconi M, Ruoslahti E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc Natl Acad Sci USA. 2002;99:7444–7449. doi: 10.1073/pnas.062189599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balça-Silva J, do Carmo A, Tão H, Rebelo O, Barbosa M, Moura-Neto V, Sarmento-Ribeiro AB, Lopes MC, Moreira JN. Nucleolin is expressed in patient-derived samples and glioblastoma cells, enabling improved intracellular drug delivery and cytotoxicity. Exp Cell Res. 2018;370:68–77. doi: 10.1016/j.yexcr.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 88.Moura V, Lacerda M, Figueiredo P, Corvo ML, Cruz MEM, Soares R, de Lima MCP, Simoes S, Moreira JN. Targeted and intracellular triggered delivery of therapeutics to cancer cells and the tumor microenvironment: Impact on the treatment of breast cancer. Breast Cancer Res Treat. 2012;133:61–73. doi: 10.1007/s10549-011-1688-7. [DOI] [PubMed] [Google Scholar]
- 89.Winer I, Wang S, Lee YEK, Fan W, Gong Y, Burgos-Ojeda D, Spahlinger G, Kopelman R, Buckanovich RJ. F3-targeted cisplatin-hydrogel nanoparticles as an effective therapeutic that targets both murine and human ovarian tumor endothelial cells in vivo. Cancer Res. 2010;70:8674–8683. doi: 10.1158/0008-5472.CAN-10-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fonseca NA, Gomes-da-Silva LC, Moura V, Simões S, Moreira JN. Simultaneous active intracellular delivery of doxorubicin and C6-ceramide shifts the additive/antagonistic drug interaction of non-encapsulated combination. J Control Release. 2014;196:122–131. doi: 10.1016/j.jconrel.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 91.Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo YEL, Woolliscroft MJ, Sugai JV, et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin Cancer Res. 2006;12:6677–6686. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 92.Drecoll E, Gaertner FC, Miederer M, Blechert B, Vallon M, Müller JM, Alke A, Seidl C, Bruchertseifer F, Morgenstern A, et al. Treatment of peritoneal carcinomatosis by targeted delivery of the radio-labeled tumor homing peptide 213Bi-DTPA-[F3]2 into the nucleus of tumor cells. PLoS One. 2009;4:e5715. doi: 10.1371/journal.pone.0005715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brignole C, Bensa V, Fonseca NA, Del Zotto G, Bruno S, Cruz AF, Malaguti F, Carlini B, Morandi F, Calarco E, et al. Cell surface nucleolin represents a novel cellular target for neuroblastoma therapy. J Exp Clin Cancer Res. 2021;40:180. doi: 10.1186/s13046-021-01993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai Y, Xu Z, Shuai Q, Zhu F, Xu J, Gao X, Sun X. Tumor-targeting peptide functionalized PEG-PLA micelles for efficient drug delivery. Biomater Sci. 2020;8:2274–2282. doi: 10.1039/C9BM02036E. [DOI] [PubMed] [Google Scholar]
- 95.Chariou PL, Wang L, Desai C, Park J, Robbins LK, von Recum HA, Ghiladi RA, Steinmetz NF. Let there be light: Targeted photodynamic therapy using high aspect ratio plant viral nanoparticles. Macromol Biosci. 2019;19:1800407. doi: 10.1002/mabi.201800407. [DOI] [PubMed] [Google Scholar]
- 96.Chen D, Yang D, Dougherty CA, Lu W, Wu H, He X, Cai T, Van Dort ME, Ross BD, Hong H. In vivo targeting and positron emission tomography imaging of tumor with intrinsically radioactive metal-organic frameworks nanomaterials. ACS Nano. 2017;11:4315–4327. doi: 10.1021/acsnano.7b01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cruz AF, Caleiras MB, Fonseca NA, Gonçalves N, Mendes VM, Sampaio SF, Moura V, Melo JB, Almeida RD, Manadas B, et al. The enhanced efficacy of intracellular delivery of doxorubicin/C6-ceramide combination mediated by the F3 peptide/nucleolin system is supported by the downregulation of the PI3K/Akt pathway. Cancers (Basel) 2021;13:3052. doi: 10.3390/cancers13123052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Essler M, Gärtner FC, Neff F, Blechert B, Senekowitsch-Schmidtke R, Bruchertseifer F, Morgenstern A, Seidl C. Therapeutic efficacy and toxicity of 225Ac-labelled vs. 213Bi-labelled tumour-homing peptides in a preclinical mouse model of peritoneal carcinomatosis. Eur J Nucl Med Mol Imaging. 2012;39:602–612. doi: 10.1007/s00259-011-2023-6. [DOI] [PubMed] [Google Scholar]
- 99.Ferrara B, Belbekhouche S, Habert D, Houppe C, Vallée B, Bourgoin-Voillard S, Cohen JL, Cascone I, Courty J. Cell surface nucleolin as active bait for nanomedicine in cancer therapy: A promising option. Nanotechnology. 2021;32:322001. doi: 10.1088/1361-6528/abfb30. [DOI] [PubMed] [Google Scholar]
- 100.Gomes-da-Silva LC, Santos AO, Bimbo LM, Moura V, Ramalho JS, Pedroso de Lima MC, Simões S, Moreira JN. Toward a siRNA-containing nanoparticle targeted to breast cancer cells and the tumor microenvironment. Int J Pharm. 2012;434:9–19. doi: 10.1016/j.ijpharm.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 101.Hu Q, Gu G, Liu Z, Jiang M, Kang T, Miao D, Tu Y, Pang Z, Song Q, Yao L, et al. F3 peptide-functionalized PEG-PLA nanoparticles co-administrated with tLyp-1 peptide for anti-glioma drug delivery. Biomaterials. 2013;34:1135–1145. doi: 10.1016/j.biomaterials.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 102.Karamchand L, Kim G, Wang S, Hah HJ, Ray A, Jiddou R, Koo Lee YE, Philbert MA, Kopelman R. Modulation of hydrogel nanoparticle intracellular trafficking by multivalent surface engineering with tumor targeting peptide. Nanoscale. 2013;5:10327–10344. doi: 10.1039/c3nr00908d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lam PYH, Hillyar CRT, Able S, Vallis KA. Synthesis and evaluation of an 18 F-labeled derivative of F3 for targeting surface-expressed nucleolin in cancer and tumor endothelial cells. J Labelled Comp Radiopharm. 2016;59:492–499. doi: 10.1002/jlcr.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lopes R, Shi K, Fonseca NA, Gama A, Ramalho JS, Almeida L, Moura V, Simões S, Tidor B, Moreira JN. Modelling the impact of nucleolin expression level on the activity of F3 peptide-targeted pH-sensitive pegylated liposomes containing doxorubicin. Drug Deliv Transl Res. 2022;12:629–646. doi: 10.1007/s13346-021-00972-z. [DOI] [PubMed] [Google Scholar]
- 105.Mäkelä AR, Närvänen A, Oker-Blom C. Peptide-mediated interference with baculovirus transduction. J Biotechnol. 2008;134:20–32. doi: 10.1016/j.jbiotec.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 106.Orringer DA, Koo YEL, Chen T, Kim G, Hah HJ, Xu H, Wang S, Keep R, Philbert MA, Kopelman R, Sagher O. In vitro characterization of a targeted, dye-loaded nanodevice for intraoperative tumor delineation. Neurosurgery. 2009;64:965–972. doi: 10.1227/01.NEU.0000344150.81021.AA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pesarrodona M, Sánchez-García L, Seras-Franzoso J, Sánchez-Chardi A, Baltá-Foix R, Cámara-Sánchez P, Gener P, Jara JJ, Pulido D, Serna N, et al. Engineering a nanostructured nucleolin-binding peptide for intracellular drug delivery in triple-negative breast cancer stem cells. ACS Appl Mater Interfaces. 2020;12:5381–5388. doi: 10.1021/acsami.9b15803. [DOI] [PubMed] [Google Scholar]
- 108.Pozdniakova NV, Ryabaya OV, Semkina AS, Skribitsky VA, Shevelev AB. Using ELP repeats as a scaffold for de novo construction of gadolinium-binding domains within multifunctional recombinant proteins for targeted delivery of gadolinium to tumour cells. Int J Mol Sci. 2022;23:3297. doi: 10.3390/ijms23063297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prickett WM, Van Rite BD, Resasco DE, Harrison RG. Vascular targeted single-walled carbon nanotubes for near-infrared light therapy of cancer. Nanotechnology. 2011;22:455101. doi: 10.1088/0957-4484/22/45/455101. [DOI] [PubMed] [Google Scholar]
- 110.Qin M, Zong H, Kopelman R. Click conjugation of peptide to hydrogel nanoparticles for tumor-targeted drug delivery. Biomacromolecules. 2014;15:3728–3734. doi: 10.1021/bm501028c. [DOI] [PubMed] [Google Scholar]
- 111.Romano S, Moura V, Simões S, Moreira JN, Gonçalves J. Anticancer activity and antibody-dependent cell-mediated cytotoxicity of novel anti-nucleolin antibodies. Sci Rep. 2018;8:7450. doi: 10.1038/s41598-018-25816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Valério-Fernandes Â, Fonseca NA, Gonçalves N, Cruz AF, Pereira MI, Gregório AC, Moura V, Ladeirinha AF, Alarcão A, Gonçalves J, et al. Nucleolin overexpression predicts patient prognosis while providing a framework for targeted therapeutic intervention in lung cancer. Cancers (Basel) 2022;14:2217. doi: 10.3390/cancers14092217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu G, Qin M, Mukundan A, Siddiqui J, Takada M, Vilar-Saavedra P, Tomlins SA, Kopelman R, Wang X. Prostate cancer characterization by optical contrast enhanced photoacoustics. Proc SPIE Int Soc Opt Eng. 2016;9708:97080I. doi: 10.1117/12.2213064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang J, Lu W, Xiao J, Zong Q, Xu H, Yin Y, Hong H, Xu W. A positron emission tomography image-guidable unimolecular micelle nanoplatform for cancer theranostic applications. Acta Biomater. 2018;79:306–316. doi: 10.1016/j.actbio.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 115.Zeng Y, Xiao J, Cong Y, Liu J, He Y, Ross BD, Xu H, Yin Y, Hong H, Xu W. PEGylated nanoscale metal-organic frameworks for targeted cancer imaging and drug delivery. Bioconjug Chem. 2021;32:2195–2204. doi: 10.1021/acs.bioconjchem.1c00368. [DOI] [PubMed] [Google Scholar]
- 116.Zhang H, Ingham ES, Gagnon MKJ, Mahakian LM, Liu J, Foiret JL, Willmann JK, Ferrara KW. In vitro characterization and in vivo ultrasound molecular imaging of nucleolin-targeted microbubbles. Biomaterials. 2017;118:63–73. doi: 10.1016/j.biomaterials.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Y, Yang M, Park JH, Singelyn J, Ma H, Sailor MJ, Ruoslahti E, Ozkan M, Ozkan C. A surface-charge study on cellular-uptake behavior of F3-peptide-conjugated iron oxide nanoparticles. Small. 2009;5:1990–1996. doi: 10.1002/smll.200900520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Henke E, Perk J, Vider J, De Candia P, Chin Y, Solit DB, Ponomarev V, Cartegni L, Manova K, Rosen N, Benezra R. Peptide-conjugated antisense oligonucleotides for targeted inhibition of a transcriptional regulator in vivo. Nat Biotechnol. 2008;26:91–100. doi: 10.1038/nbt1366. [DOI] [PubMed] [Google Scholar]
- 119.Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Mol Cancer Ther. 2006;5:2957–2962. doi: 10.1158/1535-7163.MCT-06-0172. [DOI] [PubMed] [Google Scholar]
- 120.Morita Y, Leslie M, Kameyama H, Volk DE, Tanaka T. Aptamer therapeutics in cancer: Current and future. Cancers (Basel) 2018;10:80. doi: 10.3390/cancers10030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gomes-da-Silva LC, Ramalho JS, Pedroso de Lima MC, Simões S, Moreira JN. Impact of anti-PLK1 siRNA-containing F3-targeted liposomes on the viability of both cancer and endothelial cells. Eur J Pharm Biopharm. 2013;85:356–364. doi: 10.1016/j.ejpb.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 122.Drecoll E, Gaertner FC, Miederer M, Blechert B, Vallon M, Müller JM, Alke A, Seidl C, Bruchertseifer F, Morgenstern A, et al. Treatment of peritoneal carcinomatosis by targeted delivery of the radio-labeled tumor homing peptide bi-DTPA-[F3]2 into the nucleus of tumor cells. PLoS One. 2009;4:e5715. doi: 10.1371/journal.pone.0005715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ayatollahi S, Salmasi Z, Hashemi M, Askarian S, Oskuee RK, Abnous K, Ramezani M. Aptamer-targeted delivery of Bcl-xL shRNA using alkyl modified PAMAM dendrimers into lung cancer cells. Int J Biochem Cell Biol. 2017;92:210–217. doi: 10.1016/j.biocel.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 124.Tong X, Ga L, Ai J, Wang Y. Progress in cancer drug delivery based on AS1411 oriented nanomaterials. J Nanobiotechnology. 2022;20:57. doi: 10.1186/s12951-022-01240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trinh TL, Zhu G, Xiao X, Puszyk W, Sefah K, Wu Q, Tan W, Liu C. A Synthetic aptamer-drug adduct for targeted liver cancer therapy. PLoS One. 2015;10:e0136673. doi: 10.1371/journal.pone.0136673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lohlamoh W, Soontornworajit B, Rotkrua P. Anti-proliferative effect of doxorubicin-loaded AS1411 aptamer on colorectal cancer cell. Asian Pac J Cancer Prev. 2021;22:2209–2219. doi: 10.31557/APJCP.2021.22.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheng Y, Zhao G, Zhang S, Nigim F, Zhou G, Yu Z, Song Y, Chen Y, Li Y. AS1411-induced growth inhibition of glioma cells by up-regulation of p53 and down-regulation of Bcl-2 and Akt1 via nucleolin. PLoS One. 2016;11:e0167094. doi: 10.1371/journal.pone.0167094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ishimaru D, Zuraw L, Ramalingam S, Sengupta TK, Bandyopadhyay S, Reuben A, Fernandes DJ, Spicer EK. Mechanism of regulation of bcl-2 mRNA by nucleolin and A+ U-rich element-binding factor 1 (AUF1) J Biol Chem. 2010;285:27182–27191. doi: 10.1074/jbc.M109.098830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu J, Song C, Jiang C, Shen X, Qiao Q, Hu Y. Nucleolin targeting AS1411 modified protein nanoparticle for antitumor drugs delivery. Mol Pharm. 2013;10:3555–3563. doi: 10.1021/mp300686g. [DOI] [PubMed] [Google Scholar]
- 130.Ai J, Xu Y, Lou B, Li D, Wang E. Multifunctional AS1411-functionalized fluorescent gold nanoparticles for targeted cancer cell imaging and efficient photodynamic therapy. Talanta. 2014;118:54–60. doi: 10.1016/j.talanta.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 131.Mongelard F, Bouvet P. AS-1411, a guanosine-rich oligonucleotide aptamer targeting nucleolin for the potential treatment of cancer, including acute myeloid leukemia. Curr Opin Mol Ther. 2010;12:107–114. [PubMed] [Google Scholar]
- 132.Soundararajan S, Wang L, Sridharan V, Chen W, Courtenay-Luck N, Jones D, Spicer EK, Fernandes DJ. Plasma membrane nucleolin is a receptor for the anticancer aptamer AS1411 in MV4-11 leukemia cells. Mol Pharmacol. 2009;76:984–991. doi: 10.1124/mol.109.055947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reyes-Reyes EM, Teng Y, Bates PJ. A new paradigm for aptamer therapeutic AS1411 action: Uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 2010;70:8617–8629. doi: 10.1158/0008-5472.CAN-10-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Laber DA, Sharma VR, Bhupalam L, Taft B, Hendler FJ, Barnhart KM. Update on the first phase I study of AGRO100 in advanced cancer. J Clin Oncol. 2005;23(Suppl):S3064. doi: 10.1200/jco.2005.23.16_suppl.3064. [DOI] [Google Scholar]
- 135.Storck S, Shukla M, Dimitrov S, Bouvet P. Functions of the histone chaperone nucleolin in diseases. Subcell Biochem. 2007;41:125–144. doi: 10.1007/1-4020-5466-1_7. [DOI] [PubMed] [Google Scholar]
- 136.Stuart RK, Acton G. Relapsed and refractory acute myeloid leukemia (AML) treated with AS1411 and cytarabine: A randomized phase II trial. Blood. 2008;112:1935. doi: 10.1182/blood.V112.11.1935.1935. [DOI] [Google Scholar]
- 137.Rizzieri D, Stockerl-Goldstein K, Wei A, Herzig RH, Erlandsson F, Stuart RK. Long-term outcomes of responders in a randomized, controlled phase II trial of aptamer AS1411 in AML. J Clin Oncol. 2010;28(Suppl):S6557. doi: 10.1200/jco.2010.28.15_suppl.6557. [DOI] [Google Scholar]
- 138.Stuart R, Stockerl-Goldstein K, Cooper M, Devetten M, Herzig R, Medeiros B, Schiller G, Wei A, Acton G, Rizzieri D. Randomized phase II trial of the nucleolin targeting aptamer AS1411 combined with high-dose cytarabine in relapsed/refractory acute myeloid leukemia (AML) J Clin Oncol. 2009;27(Suppl):S7019. doi: 10.1200/jco.2009.27.15_suppl.7019. [DOI] [Google Scholar]
- 139.Rosenberg JE, Bambury RM, Van Allen EM, Drabkin HA, Lara PN, Jr, Harzstark AL, Wagle N, Figlin RA, Smith GW, Garraway LA, et al. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Invest New Drugs. 2014;32:178–187. doi: 10.1007/s10637-013-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Medicine NLo: Phase I open label study of AS1411 in advanced solid tumours. [Accessed June 3, 2022]. https://clinicaltrials.gov/ct2/show/NCT00881244 .
- 141.Medicine NLo: An open label randomized controlled dose escalating phase II study of AS1411 combined with cytarabine in the treatment of patients with primary refractory or relapsed acute myeloid leukemia. [Accessed June 3, 2022]. https://clinicaltrials.gov/ct2/show/NCT00512083 .
- 142.Medicine NLo: A phase II, open label, single arm study of AS1411 in patients with metastatic renal cell carcinoma. [Accessed June 3, 2022]. https://clinicaltrials.gov/ct2/show/NCT00740441 .
- 143.Medicine NLo: An open-label randomized controlled phase II study of AS1411 combined with cytarabine in the treatment of patients with primary refractory or relapsed acute myeloid leukemia. [Accessed June 3, 2022]. https://clinicaltrials.gov/ct2/show/NCT01034410 .
- 144.Malik MT, O'Toole MG, Casson LK, Thomas SD, Bardi GT, Reyes-Reyes EM, Ng CK, Kang KA, Bates PJ. AS1411-conjugated gold nanospheres and their potential for breast cancer therapy. Oncotarget. 2015;6:22270–22281. doi: 10.18632/oncotarget.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alibolandi M, Ramezani M, Abnous K, Hadizadeh F. AS1411 aptamer-decorated biodegradable polyethylene glycol-poly(lactic-co-glycolic acid) nanopolymersomes for the targeted delivery of gemcitabine to non-small cell lung cancer in vitro. J Pharm Sci. 2016;105:1741–1750. doi: 10.1016/j.xphs.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 146.Lale SV, R G A, Aravind A, Kumar DS, Koul V. AS1411 aptamer and folic acid functionalized pH-responsive ATRP fabricated pPEGMA-PCL-pPEGMA polymeric nanoparticles for targeted drug delivery in cancer therapy. Biomacromolecules. 2014;15:1737–1752. doi: 10.1021/bm5001263. [DOI] [PubMed] [Google Scholar]
- 147.Teng Y, Girvan AC, Casson LK, Pierce WM, Jr, Qian M, Thomas SD, Bates PJ. AS1411 alters the localization of a complex containing protein arginine methyltransferase 5 and nucleolin. Cancer Res. 2007;67:10491–10500. doi: 10.1158/0008-5472.CAN-06-4206. [DOI] [PubMed] [Google Scholar]
- 148.Abdelmohsen K, Gorospe M. RNA-binding protein nucleolin in disease. RNA Biol. 2012;9:799–808. doi: 10.4161/rna.19718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vigneron N. Human tumor antigens and cancer immunotherapy. Biomed Res Int. 2015;2015:948501. doi: 10.1155/2015/948501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Decker WK, Safdar A. Bioimmunoadjuvants for the treatment of neoplastic and infectious disease: Coley's legacy revisited. Cytokine Growth Factor Rev. 2009;20:271–281. doi: 10.1016/j.cytogfr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 151.Wojtukiewicz MZ, Rek MM, Karpowicz K, Górska M, Polityńska B, Wojtukiewicz AM, Moniuszko M, Radziwon P, Tucker SC, Honn KV. Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021;40:949–982. doi: 10.1007/s10555-021-09976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang QT, Nie Y, Sun SN, Lin T, Han RJ, Jiang J, Li Z, Li JQ, Xiao YP, Fan YY, et al. Tumor-associated antigen-based personalized dendritic cell vaccine in solid tumor patients. Cancer Immunol Immunother. 2020;69:1375–1387. doi: 10.1007/s00262-020-02496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen CM, Chiang SY, Yeh NH. Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J Biol Chem. 1991;266:7754–7758. doi: 10.1016/S0021-9258(20)89514-3. [DOI] [PubMed] [Google Scholar]
- 154.Fogal V, Sugahara KN, Ruoslahti E, Christian S. Cell surface nucleolin antagonist causes endothelial cell apoptosis and normalization of tumor vasculature. Angiogenesis. 2009;12:91–100. doi: 10.1007/s10456-009-9137-5. [DOI] [PubMed] [Google Scholar]
- 155.Fernandes DJ, Tholanikunnel BG. Abstract 1488: Development of anti-nucleolin antibodies with broad spectrum anticancer activity and negligible toxicity to normal cells. Cancer Res. 2016;76(14 Suppl):S1488. doi: 10.1158/1538-7445.AM2016-1488. [DOI] [Google Scholar]
- 156.De Greve H, Virdi V, Bakshi S, Depicker A. Simplified monomeric VHH-Fc antibodies provide new opportunities for passive immunization. Curr Opin Biotechnol. 2020;61:96–101. doi: 10.1016/j.copbio.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 157.Callebaut C, Blanco J, Benkirane N, Krust B, Jacotot E, Guichard G, Seddiki N, Svab J, Dam E, Muller S, et al. Identification of V3 loop-binding proteins as potential receptors implicated in the binding of HIV particles to CD4(+) cells. J Biol Chem. 1998;273:21988–21997. doi: 10.1074/jbc.273.34.21988. [DOI] [PubMed] [Google Scholar]
- 158.Nisole S, Krust B, Callebaut C, Guichard G, Muller S, Briand JP, Hovanessian AG. The anti-HIV pseudopeptide HB-19 forms a complex with the cell-surface-expressed nucleolin independent of heparan sulfate proteoglycans. J Biol Chem. 1999;274:27875–27884. doi: 10.1074/jbc.274.39.27875. [DOI] [PubMed] [Google Scholar]
- 159.Nisole S, Krust B, Dam E, Bianco A, Seddiki N, Loaec S, Callebaut C, Guichard G, Muller S, Briand JP, Hovanessian AG. The HB-19 pseudopeptide 5[Kpsi(CH2N) PR]-TASP inhibits attachment of T lymophocyte- and macrophage-tropic HIV to permissive cells. AIDS Res Hum Retroviruses. 2000;16:237–249. doi: 10.1089/088922200309331. [DOI] [PubMed] [Google Scholar]
- 160.Nisole S, Said EA, Mische C, Prevost MC, Krust B, Bouvet P, Bianco A, Briand JP, Hovanessian AG. The anti-HIV pentameric pseudopeptide HB-19 binds the C-terminal end of nucleolin and prevents anchorage of virus particles in the plasma membrane of target cells. J Biol Chem. 2002;277:20877–20886. doi: 10.1074/jbc.M110024200. [DOI] [PubMed] [Google Scholar]
- 161.Said EA, Krust B, Nisole S, Svab J, Briand JP, Hovanessian AG. The anti-HIV cytokine midkine binds the cell surface-expressed nucleolin as a low affinity receptor. J Biol Chem. 2002;277:37492–37502. doi: 10.1074/jbc.M201194200. [DOI] [PubMed] [Google Scholar]
- 162.Legrand D, Vigié K, Said EA, Elass E, Masson M, Slomianny MC, Carpentier M, Briand JP, Mazurier J, Hovanessian AG. Surface nucleolin participates in both the binding and endocytosis of lactoferrin in target cells. Eur J Biochem. 2004;271:303–317. doi: 10.1046/j.1432-1033.2003.03929.x. [DOI] [PubMed] [Google Scholar]
- 163.Said EA, Courty J, Svab J, Delbé J, Krust B, Hovanessian AG. Pleiotrophin inhibits HIV infection by binding the cell surface-expressed nucleolin. FEBS J. 2005;272:4646–4659. doi: 10.1111/j.1742-4658.2005.04870.x. [DOI] [PubMed] [Google Scholar]
- 164.Hovanessian AG. Midkine, a cytokine that inhibits HIV infection by binding to the cell surface expressed nucleolin. Cell Res. 2006;16:174–181. doi: 10.1038/sj.cr.7310024. [DOI] [PubMed] [Google Scholar]
- 165.Barel M, Hovanessian AG, Meibom K, Briand JP, Dupuis M, Charbit A. A novel receptor-ligand pathway for entry of Francisella tularensis in monocyte-like THP-1 cells: Interaction between surface nucleolin and bacteria elongation factor Tu. BMC Microbiol. 2008;8:145. doi: 10.1186/1471-2180-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Losfeld ME, Khoury DE, Mariot P, Carpentier M, Krust B, Briand JP, Mazurier J, Hovanessian AG, Legrand D. The cell surface expressed nucleolin is a glycoprotein that triggers calcium entry into mammalian cells. Exp Cell Res. 2009;315:357–369. doi: 10.1016/j.yexcr.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 167.El Khoury D, Destouches D, Lengagne R, Krust B, Hamma-Kourbali Y, Garcette M, Niro S, Kato M, Briand JP, Courty J, et al. Targeting surface nucleolin with a multivalent pseudopeptide delays development of spontaneous melanoma in RET transgenic mice. BMC Cancer. 2010;10:325. doi: 10.1186/1471-2407-10-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Destouches D, Page N, Hamma-Kourbali Y, Machi V, Chaloin O, Frechault S, Birmpas C, Katsoris P, Beyrath J, Albanese P, et al. A simple approach to cancer therapy afforded by multivalent pseudopeptides that target cell-surface nucleoproteins. Cancer Res. 2011;71:3296–3305. doi: 10.1158/0008-5472.CAN-10-3459. [DOI] [PubMed] [Google Scholar]
- 169.Krust B, El Khoury D, Soundaramourty C, Nondier I, Hovanessian AG. Suppression of tumorigenicity of rhabdoid tumor derived G401 cells by the multivalent HB-19 pseudopeptide that targets surface nucleolin. Biochimie. 2011;93:426–433. doi: 10.1016/j.biochi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 170.Birmpas C, Briand JP, Courty J, Katsoris P. Nucleolin mediates the antiangiogenesis effect of the pseudopeptide N6L. BMC Cell Biol. 2012;13:32. doi: 10.1186/1471-2121-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Birmpas C, Briand JP, Courty J, Katsoris P. The pseudopeptide HB-19 binds to cell surface nucleolin and inhibits angiogenesis. Vasc Cell. 2012;4:21. doi: 10.1186/2045-824X-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Destouches D, Huet E, Sader M, Frechault S, Carpentier G, Ayoul F, Briand JP, Menashi S, Courty J. Multivalent pseudopeptides targeting cell surface nucleoproteins inhibit cancer cell invasion through tissue inhibitor of metalloproteinases 3 (TIMP-3) release. J Biol Chem. 2012;287:43685–43693. doi: 10.1074/jbc.M112.380402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Koutsioumpa M, Polytarchou C, Courty J, Zhang Y, Kieffer N, Mikelis C, Skandalis SS, Hellman U, Iliopoulos D, Papadimitriou E. Interplay between αvβ3 integrin and nucleolin regulates human endothelial and glioma cell migration. J Biol Chem. 2013;288:343–354. doi: 10.1074/jbc.M112.387076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Benedetti E, Antonosante A, d'Angelo M, Cristiano L, Galzio R, Destouches D, Florio TM, Dhez AC, Astarita C, Cinque B, et al. Nucleolin antagonist triggers autophagic cell death in human glioblastoma primary cells and decreased in vivo tumor growth in orthotopic brain tumor model. Oncotarget. 2015;6:42091. doi: 10.18632/oncotarget.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Diamantopoulou Z, Gilles ME, Sader M, Cossutta M, Vallée B, Houppe C, Habert D, Brissault B, Leroy E, Maione F, et al. Multivalent cationic pseudopeptide polyplexes as a tool for cancer therapy. Oncotarget. 2017;8:90108–90122. doi: 10.18632/oncotarget.21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.De Cola A, Franceschini M, Di Matteo A, Colotti G, Celani R, Clemente E, Ippoliti R, Cimini A, Dhez A, Vallée B, et al. N6L pseudopeptide interferes with nucleophosmin protein-protein interactions and sensitizes leukemic cells to chemotherapy. Cancer Lett. 2018;412:272–282. doi: 10.1016/j.canlet.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 177.Dhez AC, Benedetti E, Antonosante A, Panella G, Ranieri B, Florio TM, Cristiano L, Angelucci F, Giansanti F, Di Leandro L, et al. Targeted therapy of human glioblastoma via delivery of a toxin through a peptide directed to cell surface nucleolin. J Cell Physiol. 2018;233:4091–4105. doi: 10.1002/jcp.26205. [DOI] [PubMed] [Google Scholar]
- 178.Darche M, Cossutta M, Caruana L, Houppe C, Gilles ME, Habert D, Guilloneau X, Vignaud L, Paques M, Courty J, Cascone I. Antagonist of nucleolin, N6L, inhibits neovascularization in mouse models of retinopathies. FASEB J. 2020;34:5851–5862. doi: 10.1096/fj.201901876R. [DOI] [PubMed] [Google Scholar]
- 179.Chalabi-Dchar M, Cruz E, Mertani HC, Diaz JJ, Courty J, Cascone I, Bouvet P. Nucleolin aptamer N6L reprograms the translational machinery and acts synergistically with mTORi to inhibit pancreatic cancer proliferation. Cancers (Basel) 2021;13:4957. doi: 10.3390/cancers13194957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lamprou M, Koutsioumpa M, Kaspiris A, Zompra K, Tselios T, Papadimitriou E. Binding of pleiotrophin to cell surface nucleolin mediates prostate cancer cell adhesion to osteoblasts. Tissue Cell. 2022;76:101801. doi: 10.1016/j.tice.2022.101801. [DOI] [PubMed] [Google Scholar]
- 181.Thongchot S, Jirapongwattana N, Luangwattananun P, Chiraphapphaiboon W, Chuangchot N, Sa-Nguanraksa D, O-Charoenrat P, Thuwajit P, Yenchitsomanus PT, Thuwajit C. Adoptive transfer of anti-nucleolin T cells combined with PD-L1 inhibition against triple-negative breast cancer. Mol Cancer Ther. 2022;21:727–739. doi: 10.1158/1535-7163.MCT-21-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pincha M, Sundarasetty BS, Salguero G, Gutzmer R, Garritsen H, Macke L, Schneider A, Lenz D, Figueiredo C, Blasczyk R, et al. Identity, potency, in vivo viability, and scaling up production of lentiviral vector-induced dendritic cells for melanoma immunotherapy. Hum Gene Ther Methods. 2012;23:38–55. doi: 10.1089/hgtb.2011.170. [DOI] [PubMed] [Google Scholar]
- 183.Nakayama M. Antigen presentation by MHC-dressed cells. Front Immunol. 2015;5:672. doi: 10.3389/fimmu.2014.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mangare C, Tischer-Zimmermann S, Riese SB, Dragon AC, Prinz I, Blasczyk R, Maecker-Kolhoff B, Eiz-Vesper B. Robust identification of suitable T-cell subsets for personalized CMV-specific T-cell immunotherapy using CD45RA and CD62L microbeads. Int J Mol Sci. 2019;20:1415. doi: 10.3390/ijms20061415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li ZL, Zhang HL, Huang Y, Huang JH, Sun P, Zhou NN, Chen YH, Mai J, Wang Y, Yu Y, et al. Autophagy deficiency promotes triple-negative breast cancer resistance to T cell-mediated cytotoxicity by blocking tenascin-C degradation. Nat Commun. 2020;11:3806. doi: 10.1038/s41467-020-17395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gurung S, Khan F, Gunassekaran GR, Yoo JD, Poongkavithai Vadevoo SM, Permpoon U, Kim SH, Kim HJ, Kim IS, Han H, et al. Phage display-identified PD-L1-binding peptides reinvigorate T-cell activity and inhibit tumor progression. Biomaterials. 2020;247:119984. doi: 10.1016/j.biomaterials.2020.119984. [DOI] [PubMed] [Google Scholar]
- 187.Liao F, Liu L, Luo E, Hu J. Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Arch Oral Biol. 2018;92:32–37. doi: 10.1016/j.archoralbio.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 188.Zhang J, Dang F, Ren J, Wei W. Biochemical aspects of PD-L1 regulation in cancer immunotherapy. Trends Biochem Sci. 2018;43:1014–1032. doi: 10.1016/j.tibs.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 189.Wang X, Wu WKK, Gao J, Li Z, Dong B, Lin X, Li Y, Li Y, Gong J, Qi C, et al. Autophagy inhibition enhances PD-L1 expression in gastric cancer. J Exp Clin Cancer Res. 2019;38:140. doi: 10.1186/s13046-019-1148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Abreu TR, Godinho-Santos A, Moreira J, Gonçalves J. P06. 02 Anti-nucleolin CAR (chimeric antigen receptor)-T cell targeting triple-negative breast cancer-critical parameters in CAR-T cells generation. J Immunother Cancer. 2022;10(Suppl 1):A23. [Google Scholar]
- 191.Vigneron N, Stroobant V, Van den Eynde BJ, van der Bruggen P. Database of T cell-defined human tumor antigens: The 2013 update. Cancer Immun. 2013;13:15. [PMC free article] [PubMed] [Google Scholar]
- 192.Wu D, Gao Y, Qi Y, Chen L, Ma Y, Li Y. Peptide-based cancer therapy: opportunity and challenge. Cancer Lett. 2014;351:13–22. doi: 10.1016/j.canlet.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 193.Kim JH, Bae C, Kim MJ, Song IH, Ryu JH, Choi JH, Lee CJ, Nam JS, Kim JI. A novel nucleolin-binding peptide for cancer theranostics. Theranostics. 2020;10:9153–9171. doi: 10.7150/thno.43502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 195.Shadidi M, Sioud M. Selective targeting of cancer cells using synthetic peptides. Drug Resist Updat. 2003;6:363–371. doi: 10.1016/j.drup.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 196.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–484. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tobias J, Garner-Spitzer E, Drinić M, Wiedermann U. Vaccination against Her-2/neu, with focus on peptide-based vaccines. ESMO Open. 2022;7:100361. doi: 10.1016/j.esmoop.2021.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Carmichael MG, Benavides LC, Holmes JP, Gates JD, Mittendorf EA, Ponniah S, Peoples GE. Results of the first phase 1 clinical trial of the HER-2/neu peptide (GP2) vaccine in disease-free breast cancer patients: United States military cancer institute clinical trials group study I-04. Cancer. 2010;116:292–301. doi: 10.1002/cncr.24756. [DOI] [PubMed] [Google Scholar]
- 199.Abbaspour M, Akbari V. Cancer vaccines as a targeted immunotherapy approach for breast cancer: An update of clinical evidence. Expert Rev Vaccines. 2022;21:337–353. doi: 10.1080/14760584.2022.2021884. [DOI] [PubMed] [Google Scholar]
- 200.Mitchell MS, Lund TA, Sewell AK, Marincola FM, Paul E, Schroder K, Wilson DB, Kan-Mitchell J. The cytotoxic T cell response to peptide analogs of the HLA-A*0201-restricted MUC1 signal sequence epitope, M1.2. Cancer Immunol Immunother. 2007;56:287–301. doi: 10.1007/s00262-006-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Thueng-in K, Thanongsaksrikul J, Jittavisutthikul S, Seesuay W, Chulanetra M, Sakolvaree Y, Srimanote P, Chaicumpa W. Interference of HCV replication by cell penetrable human monoclonal scFv specific to NS5B polymerase. MAbs. 2014;6:1327–1339. doi: 10.4161/mabs.29978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Medicine NLo: A non-randomized, open-label, multi-centric dose-finding adaptive phase I/IIa study to assess safety, tolerability, pharmacokinetics and preliminary efficacy of repeated intravenous IPP-204106N administrations in adult patients with advanced solid tumors. [Accessed June 3, 2022]. Available online: https://clinicaltrials.gov/ct2/show/NCT01711398.
- 203.Ramos KS, Moore S, Runge I, Tavera-Garcia MA, Cascone I, Courty J, Rayes-Reyes EM. The nucleolin antagonist N6L inhibits LINE1 retrotransposon activity in non-small cell lung carcinoma cells. J Cancer. 2020;11:733–740. doi: 10.7150/jca.37776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hovanessian AG, Soundaramourty C, El Khoury D, Nondier I, Svab J, Krust B. Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization. PLoS One. 2010;5:e15787. doi: 10.1371/journal.pone.0015787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.