Abstract
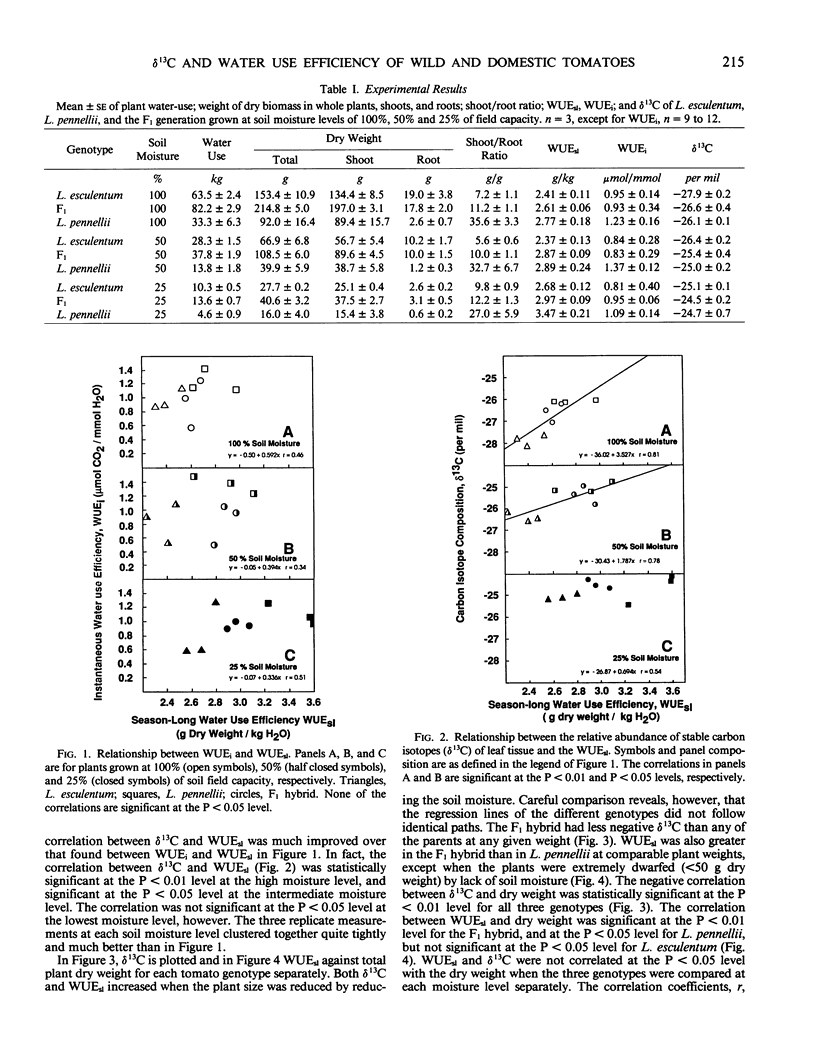
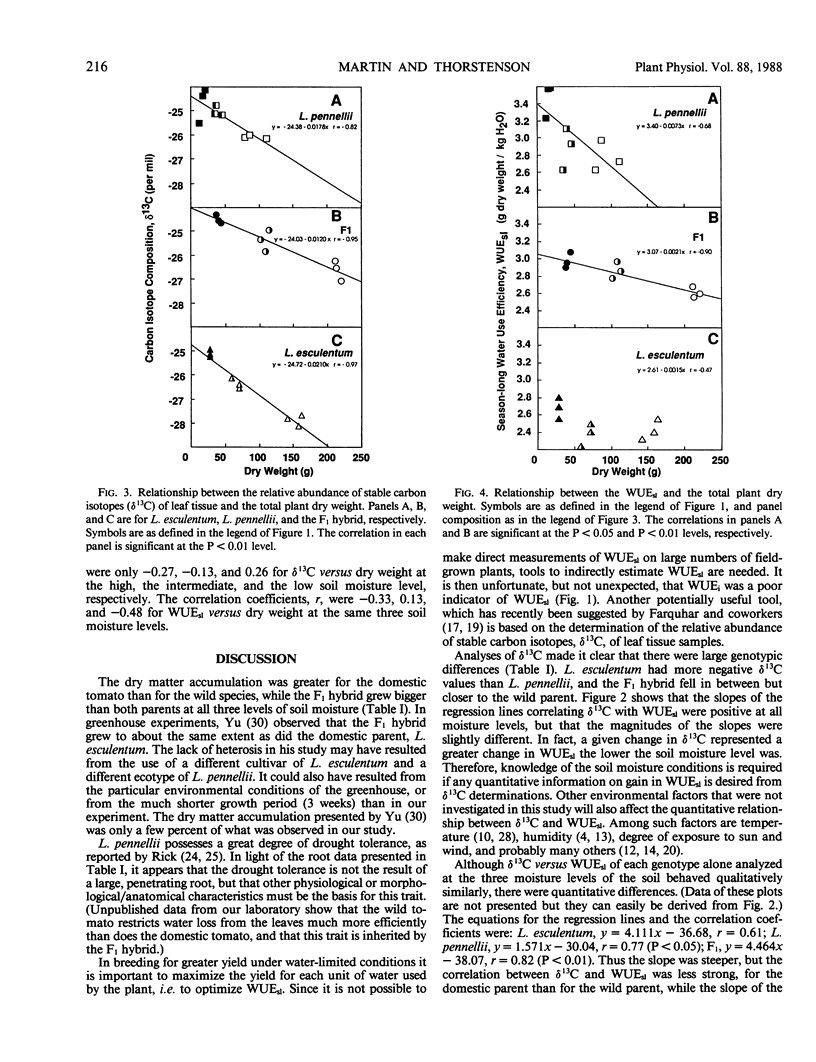
Three tomatoes, Lycopersicon esculentum Mill. cv UC82B, a droughttolerant wild related species, Lycopersicon pennellii (Cor.) D'Arcy, and their F1 hybrid, were grown in containers maintained at three levels of soil moisture. Season-long water use was obtained by summing over the season daily weight losses of each container corrected for soil evaporation. Plant biomass was determined by harvesting and weighing entire dried plants. Season-long water use efficiency (gram dry weight/kilogram H2O) was calculated by dividing the dry biomass by the season-long water use. The season-long water use efficiency was greatest in the wild parent, poorest in the domestic parent, and intermediate (but closer to the wild parent) in the F1 hybrid. Instantaneous water-use efficiency (micromole CO2/millimole H2O) determined by gas exchange measurements on individual leaves was poorly correlated with season-long water use efficiency. However, the relative abundance of stable carbon isotopes of leaf tissue samples was strongly correlated with the season-long water use efficiency. Also, the isotopic composition and the season-long water use efficiency of each genotype alone were strongly negatively correlated with plant dry weight when the dry weight varied as a function of soil moisture.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford K. J., Sharkey T. D., Farquhar G. D. Gas Exchange, Stomatal Behavior, and deltaC Values of the flacca Tomato Mutant in Relation to Abscisic Acid. Plant Physiol. 1983 May;72(1):245–250. doi: 10.1104/pp.72.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleens E., Treichel I., O'leary M. H. Temperature Dependence of Carbon Isotope Fractionation in CAM Plants. Plant Physiol. 1985 Sep;79(1):202–206. doi: 10.1104/pp.79.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park R., Epstein S. Metabolic fractionation of C & C in plants. Plant Physiol. 1961 Mar;36(2):133–138. doi: 10.1104/pp.36.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick C. M. HYBRIDIZATION BETWEEN LYCOPERSICON ESCULENTUM AND SOLANUM PENNELLII: PHYLOGENETIC AND CYTOGENETIC SIGNIFICANCE. Proc Natl Acad Sci U S A. 1960 Jan;46(1):78–82. doi: 10.1073/pnas.46.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick C. M. Potential genetic resources in tomato species: clues from observations in native habitats. Basic Life Sci. 1973;2:255–269. doi: 10.1007/978-1-4684-2880-3_17. [DOI] [PubMed] [Google Scholar]
- Wong W. W., Benedict C. R., Kohel R. J. Enzymic Fractionation of the Stable Carbon Isotopes of Carbon Dioxide by Ribulose-1,5-bisphosphate Carboxylase. Plant Physiol. 1979 May;63(5):852–856. doi: 10.1104/pp.63.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]


