Abstract
The increasing emergence of penicillin-resistant and multidrug-resistant strains of Streptococcus pneumoniae will create a serious therapeutic problem in coming years. Trovafloxacin is a novel naphthyridone quinolone with promising activity against S. pneumoniae, including penicillin-resistant strains (MIC for 90% of the isolates tested, 0.25 μg/ml). We compared its in vivo efficacy with that of other fluoroquinolones (ciprofloxacin, temafloxacin, and sparfloxacin) and a reference beta-lactam (amoxicillin) in a model of acute experimental pneumonia. Immunocompetent Swiss mice were infected by peroral tracheal delivery of a virulent, penicillin-susceptible strain (MIC, 0.03 μg/ml); leukopenic Swiss mice were infected with three poorly virulent, penicillin-resistant strains (MICs, 4 to 8 μg/ml) and a ciprofloxacin-resistant strain (MIC, 32 μg/ml). Treatments were started 6 h (immunocompetent mice) or 3 h (leukopenic mice) after infection. Doses ranging from 12.5 to 300 mg/kg were given at 12- or 8-h intervals for 3 days. Trovafloxacin (25 mg/kg) was the most effective agent in vivo against penicillin-susceptible and -resistant strains. Corresponding survival rates were 2- to 4-fold higher than with 50-mg/kg sparfloxacin or temafloxacin and 8- to 16-fold higher than with 100-mg/kg ciprofloxacin. The ratios of the area under the concentration-time curve to the MIC in serum and lung tissue were more favorable with trovafloxacin than with the other quinolones. Efficacy in vivo correlated with pharmacokinetic parameters. Trovafloxacin shows potential for the treatment of infections due to penicillin-susceptible and -resistant S. pneumoniae but appears to be ineffective against a ciprofloxacin-resistant strain.
Streptococcus pneumoniae remains the leading cause of community-acquired pneumonia and continues to be a significant cause of mortality (27). The worldwide prevalence of infections caused by pneumococci resistant to penicillin, macrolides, and other antimicrobials has increased at an alarming rate during the past 2 decades (1, 2). In some countries, the incidence of resistant pneumococci isolated from clinical specimens has reached extremely high levels (50 to 70% in Spain and Hungary) (23). There is an urgent need for oral compounds active against penicillin-resistant pneumococci in patients with pneumonia, bronchitis, sinusitis, and otitis media (17).
As a class, the fluoroquinolones possess good in vitro activity against most gram-negative bacteria but only poor or moderate activity against most gram-positive bacteria (10, 12, 13). The available fluoroquinolones, such as ciprofloxacin, ofloxacin, and lomefloxacin, have relatively high MICs that limit their therapeutic value against pneumococcal strains for which the MICs are around or above the breakpoint (17).
Trovafloxacin (CP 99,219) is a novel trifluoronaphthyridone quinolone with improved activity against gram-positive bacteria, including some strains resistant to ciprofloxacin (15, 17, 19). Studies of single-dose pharmacokinetics in humans have shown a long (11.5-h) elimination half-life (t1/2) and good systemic diffusion, allowing once-daily dosing (30). Several of the newer quinolones with increased antipneumococcal activity (temafloxacin and sparfloxacin) have potent activity in murine models of pneumococcal pneumonia (4, 5), as well as in the human disease (3, 11). Oral trovafloxacin controls systemic gram-positive and gram-negative infections in mice and is more potent than temafloxacin, ciprofloxacin, and ofloxacin in protecting mice against lethal infections with penicillin-susceptible strains of S. pneumoniae or S. pyogenes (18).
We compared the efficacy of trovafloxacin with that of ciprofloxacin, sparfloxacin, and temafloxacin in a mouse model of acute S. pneumoniae pneumonia induced by a penicillin-susceptible strain, three penicillin-resistant or multidrug-resistant strains, and a quinolone-resistant strain. The latter strain was chosen because of the emergence of sparfloxacin resistance in France and elsewhere (28). Survival, clearance of bacteria from the serum and lungs, and pharmacokinetic data on both uninfected and infected mice were used to evaluate the efficacy of trovafloxacin.
(This work was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 17 to 21 September 1996 [abstract B-43].)
MATERIALS AND METHODS
Animals.
Female Swiss mice (body weight, 20 to 22 g) were obtained from Iffa-Credo Laboratories.
Challenge organisms.
Pneumococcal pneumonia was induced in immunocompetent mice with a virulent serotype 3 strain (P-4241; penicillin MIC, 0.03 μg/ml) originally isolated from a blood culture and provided by the Centre de Référence du Pneumocoque (P. Geslin, Créteil, France). All strains belonging to serotypes 6, 9, 14, 19, and 23 are naturally avirulent in Swiss mice, independently of their site of isolation in humans (7, 9). Thus, leukopenia was first induced in mice infected with these strains. Two serotype 23 penicillin-resistant strains (MIC, 4 μg/ml) were used. P-12698 was isolated from a tracheal aspirate, and P-54986 was isolated from a patient with sinusitis; both were kindly provided by the Laboratoire de Microbiologie, Hôpital Bichat, Paris, France. P-15986, a serotype 19 highly penicillin-resistant (MIC, 8 μg/ml) and erythromycin-resistant (MIC, >32 μg/ml) strain isolated from middle ear fluid, and P-18316, a serotype 23 ciprofloxacin-resistant strain (MIC, 32 μg/ml) isolated from cerebrospinal fluid of a patient with meningitis (P. Geslin), were also tested. The intrinsic characteristics of these strains are different. Both P-12698 and P-15986 are tolerant to penicillin, as they are lysed by about 1 log10 CFU at 50 times the relevant MIC, whereas P-54988 is not tolerant as it is lysed by about 3 log10 CFU under the same conditions; moreover, P-15986 is a nonautolytic strain (6). It was also reported that after exposure to 20 times the penicillin MIC for 6 h, a nontolerant strain loses 4 to 5 log units of its viable count, whereas a tolerant one loses only 1 log unit (24).
In vitro studies.
MICs and MBCs were determined in Mueller-Hinton infusion broth (Diagnostic Pasteur, Marnes-la-Coquette, France) supplemented with 5% sheep blood by the tube dilution method (25). The tubes contained twofold dilutions of antibiotics and a final bacterial density of 106 CFU/ml. The tubes were incubated for 18 h at 37°C in 10% CO2–air. The MIC was defined as the lowest concentration of antibiotic at which no turbidity was visible to the naked eye. For MBC determination, 0.01-ml aliquots from tubes with no visible growth were plated onto Columbia agar with 5% sheep blood (Bio-Mérieux, Lyon, France) and incubated overnight at 37°C in 10% CO2–air. The MBC was defined as the lowest concentration of antibiotic killing ≥99.9% of the original inoculum.
Leukocyte depletion in mice.
We induced sustained leukopenia in Swiss mice by three daily intraperitoneal injections (150 mg/kg) of cyclophosphamide (Endoxan; Sarget Laboratories, Mérignac, France) starting 4 days before infection. Counts of circulating leukocytes were reduced from about 7,000 to 1,000/mm3 of blood on the day of infection. These mice are more susceptible to poorly virulent strains.
Experimental pneumococcal pneumonia.
Pneumococcal pneumonia was induced in mice as described in detail elsewhere (4). Briefly, animals were anesthetized by intraperitoneal injection of sodium pentobarbital and then infected with approximately 105 or 107 log-phase CFU of penicillin-susceptible or -resistant strains, respectively. Under these conditions, mice develop acute pneumonia and die within 3 to 4 days. The animals quickly become bacteremic. Death occurs when the bacterial population exceeds 108 CFU/lung.
Antibiotics.
The study drugs included the fluoroquinolones trovafloxacin (Pfizer Laboratories, Groton, Conn.), ciprofloxacin (Ciflox, Bayer Laboratories, Sens, France), sparfloxacin (Rhône-Poulenc, Antony, France), and temafloxacin (Abbott Laboratories, North Chicago, Ill.). Amoxicillin sodium salt (Beecham) was used as the reference antibiotic. Each antibiotic was made up as directed on the package insert and diluted in sterile water to the desired concentration. Although temafloxacin is no longer used in human medicine, it was included in these studies as it was reported to be effective against S. pneumoniae in this model (4).
Survival studies.
Treatments were started 3 or 6 h after bacterial challenge and were given in six or nine subcutaneous injections at 8- or 12-h intervals. Treatment regimens are presented with the results. Fifteen animals were used per treatment group, and all of the animals in the same experiment were infected simultaneously. Experiments were repeated at least twice. When results were similar, no further experiments were performed. Only the results of one representative experiment are given. The observation period was 10 days. Death rates were recorded daily, and cumulative survival rates were compared.
Bactericidal activity in vivo.
The study drugs were assessed for the ability to eradicate bacteria in the lungs. Single drug injections were given 3 h after infection. At 1, 3, 6, and 9 h later, mice were killed by intraperitoneal injection of sodium pentobarbital and exsanguinated by cardiac puncture; blood was cultured. The lungs were removed and homogenized in 1 ml of saline. The total numbers of CFU recovered from whole-lung homogenates were determined by serial 10-fold dilutions plated onto Columbia agar. Results are expressed as the mean number (log10) of CFU per lung (± the standard deviation) for groups of three mice.
Pharmacokinetic studies.
The pharmacokinetic profiles of trovafloxacin were examined in parallel in immunocompetent mice infected with P-4241, in leukopenic mice infected with P-54988, and in uninfected controls. Concentrations in lung tissue and serum were determined after administration of a single subcutaneous (s.c.) dose of 25 mg/kg given 6 h (P-4241) or 3 h (P-54988) after infection. Serum samples and lungs were collected from groups of six mice 0.25, 0.5, 1, 2, 4, 6, 8, 12, and 24 h postdosing. Animals were killed by intraperitoneal injection of sodium pentobarbital and exsanguinated by intracardiac puncture. Blood samples were centrifuged, and serum was collected and stored at −80°C until assay. Lungs were harvested from exsanguinated mice, washed in sterile sodium chloride, weighed, and frozen.
The concentrations of trovafloxacin were determined by reverse-phase high-performance liquid chromatography with UV detection (280 nm) and solid-phase extraction as reported by Teng et al. (31), with slight modifications. Chromatographic separation was accomplished by using a C8 Hypersil BDS column (Shandon UK) and an acetate mobile phase. (80% CH3COO− [0.002 M], 19.6% acetonitrile [ACN; Merck], 0.4% triethylamine [Merck]; pH 4). For serum preparation, a 200-μl aliquot of the serum calibration standard or an unknown sample containing 0.4 μg of an analytic internal standard (a methyl derivative of trovafloxacin) was applied to a Baker C2 (50-mg) cartridge which had been previously prepared with 2 ml of ACN and 2 ml of phosphate solution (pH 3). The sample was eluted with 700 μl of a solution containing 70% buffer, pH 9 (Carlo Erba) and 30% ACN. A 200-μl aliquot of eluate was then injected onto the high-performance liquid chromatography column. Lungs were homogenized with 3 ml of buffer, pH 3 (Merck). Supernatants of lung samples were processed in the same way as serum. The calibration curves were linear within a concentration range of 0.125 to 64 μg/ml. The average recoveries were greater than 75% for both compounds. The within-day and between-days coefficients of variation in both serum and lungs were less than 5%.
Pharmacokinetic analyses.
Concentration-time data were modeled, and pharmacokinetic parameters (peak level, t1/2, and AUC0–24 [area under the concentration-time curve from 0 to 24 h]) were calculated by using nonlinear least-squares regression analysis (nonlinear Apis pharmacokinetic software) (21). One- and two-compartment models and F ratios were evaluated. Comparison of the two models by analysis of variance (serum or lung, control or infected animals, both strains) showed no significant F ratio between one and two compartments. The best fit of experimental data was obtained by using a one-compartment open model with zero-order absorption and first-order elimination. Optimization was accomplished by employing the maximum-likehood estimation criterion. Cmax is the highest calculated concentration; Tmax is the earliest time at which Cmax occurred; the terminal t1/2 was calculated as ln 2/kel, where kel is the elimination rate constant derived from the slope obtained by least-squares regression analysis for apparently linear portions of the log concentration-time curve; and AUC0–24 was calculated by the trapezoid method.
Statistical analysis.
Survival rates were compared between treatment groups by using the Mantel-Haenzel method. Data on bacterial clearance were compared between groups by analysis of variance followed by Bonferroni-Dunn tests for multiple comparisons. Pharmacokinetic analyses were performed by analysis of variance, and the F ratios between one- and two-compartment models were evaluated in the sera and lungs of control and infected animals and with both test strains. Differences were considered significant when P was ≤0.05.
RESULTS
In vitro data.
Overall, trovafloxacin was 8 to 16 times more active than ciprofloxacin, 4 to 16 times more active than temafloxacin, and 2 to 8 times more active than sparfloxacin (Table 1).
TABLE 1.
Susceptibility of S. pneumoniae challenge strains to study drugs
| Drug | MIC/MBC (μg/ml) for strain:
|
||||
|---|---|---|---|---|---|
| P-4241 | P-54988 | P-12698 | P-15986 | P-18316 | |
| Penicillin | 0.03/0.03 | 4/8 | 4/8 | 8/16 | 1/2 |
| Amoxicillin | 0.03/0.03 | 2/4 | 2/4 | 4/8 | 2/4 |
| Trovafloxacin | 0.06/0.25 | 0.125/0.25 | 0.125/0.25 | 0.125/0.25 | 4/8 |
| Ciprofloxacin | 1/2 | 1/4 | 1/4 | 1/4 | 32/64 |
| Sparfloxacin | 0.25/0.5 | 0.5/2 | 0.5/2 | 0.5/2 | 8/16 |
| Temafloxacin | 1/1 | 1/2 | 1/2 | 1/2 | 32/64 |
Therapeutic efficacy in experimental pneumonia.
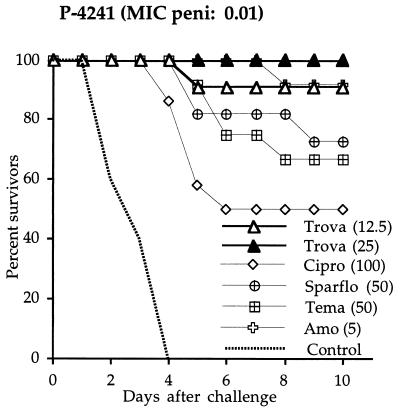
Immunocompetent mice infected with penicillin-susceptible strain P-4241 (Fig. 1) received six antibiotic injections (twice a day for 3 days). Trovafloxacin at 12.5 and 25 mg/kg protected 91 and 100% of the animals, respectively. Sparfloxacin at 50 mg/kg yielded 73% survival (not significantly different from that produced by trovafloxacin at 25 mg/kg), and temafloxacin at the same dose protected 67% of the animals (P < 0.05 versus trovafloxacin at 25 mg/kg). Ciprofloxacin protected only 50% of the animals, despite a high dose of 100 mg/kg (P < 0.05 versus trovafloxacin at 25 mg/kg). Amoxicillin at 5 mg/kg was as effective as trovafloxacin at 12.5 mg/kg.
FIG. 1.
Survival of immunocompetent mice challenged with a penicillin-susceptible pneumococcal strain (P-4241) and treated with four different quinolones (trovafloxacin [Trova], ciprofloxacin [Cipro], sparfloxacin [Sparflo], and temafloxacin [Tema]) and amoxicillin (Amo). Antibiotics were injected s.c. twice a day for 3 days. The values in parentheses are doses in milligrams per kilogram.
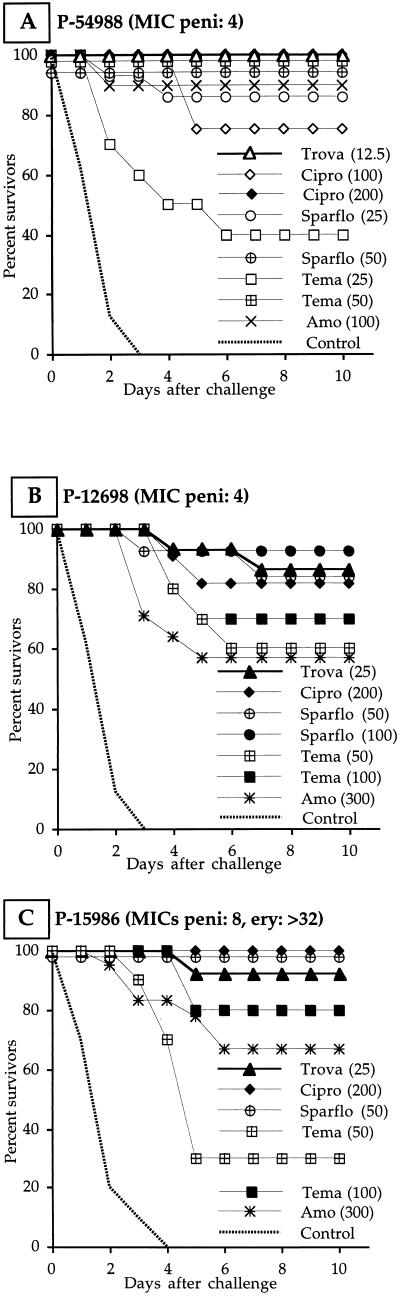
Leukopenic mice infected with the penicillin-resistant strains also received six antibiotic injections (twice a day for 3 days). When infection was induced by nontolerant strain P-54988 (Fig. 2A), trovafloxacin at 12.5 mg/kg protected 100% of the mice. Sparfloxacin at 25 mg/kg also gave a high survival rate (86%). Temafloxacin at the same dose was poorly effective (40% survivors), but 50 mg/kg gave 100% survival. Ciprofloxacin at 100 and 200 mg/kg protected 75 and 100% of the mice, respectively. Amoxicillin at 100 mg/kg gave 90% survival. When infection was induced by tolerant strain P-12698 (Fig. 2B), trovafloxacin at 25 mg/kg protected 87% of the mice. To achieve a similar survival rate, sparfloxacin had to be given at twice, temafloxacin at four times, and ciprofloxacin at eight times the dose of trovafloxacin. Amoxicillin at 300 mg/kg protected only 57% of the animals. Trovafloxacin at 25 mg/kg protected 93% of the mice from tolerant, nonautolytic strain P-15986 (Fig. 2C). Sparfloxacin at twice, temafloxacin at four times, and ciprofloxacin at eight times the dose of trovafloxacin were similar in efficacy. Amoxicillin at 100 mg/kg was poorly effective (23% survivors), but the survival rate increased to 67% with 300 mg/kg.
FIG. 2.
Survival of leukopenic mice challenged with penicillin-resistant strains P-54988 (A; penicillin MIC, 4 μg/ml), P-12698 (B; penicillin MIC, 4 μg/ml), and P-15986 (C; penicillin MIC, 8 μg/ml; erythromycin MIC, >32 μg/ml) and treated with four different quinolones (trovafloxacin [Trova], ciprofloxacin [Cipro], sparfloxacin [Sparflo], and temafloxacin [Tema]) and amoxicillin (Amo). Antibiotics were injected s.c. twice a day for 3 days. The values in parentheses are doses in milligrams per kilogram.
Leukopenic mice infected with quinolone-resistant strain P-18316 received a total of nine antibiotic injections (three times a day for 3 days). Only amoxicillin at 50 mg/kg was effective, giving 75% survival. Trovafloxacin and sparfloxacin at 100 mg/kg gave 15 and 8% survival, respectively. Temafloxacin at 200 mg/kg and ciprofloxacin at 300 mg/kg were inactive against this strain in vivo (no survivors). However, no mutants with higher resistance to trovafloxacin emerged during or after treatment.
Bacterial clearance from the lungs.
Bacterial counts in the lungs of mice infected with penicillin-resistant strain P-15986 (Table 2) and treated with a single injection of antibiotic, regardless the compound, were significantly lower than those in the lungs of untreated controls. At 9 h, bacterial counts fell markedly in the lungs of quinolone-treated mice. No significant differences were found between trovafloxacin at 25 mg/kg and the other quinolones given at higher doses. Blood samples from quinolone-treated mice were sterile as early as 1 h after treatment. Similar bacterial clearance results were found in mice infected with penicillin-susceptible strain P-4241 (data not shown).
TABLE 2.
Time course of bacterial clearance from lungs and blood of mice infected with penicillin-resistant S. pneumoniae P-15986a
| Drug (dose [mg/kg]) | Result for lungs (blood) at postdosing time (h)b:
|
|||
|---|---|---|---|---|
| 1 | 3 | 6 | 9 | |
| None (control) | 6.1 ± 0.3c (1/3) | 5.4 ± 0.6c (2/3) | 6.1 ± 0.3c (2/3) | 6.2 ± 0.2c (2/3) |
| Trovafloxacin (25) | 4.6 ± 0.2 (0/3) | 4.1 ± 0.5 (0/3) | 3.3 ± 0.2 (0/3) | 2.8 ± 0.3 (0/3) |
| Ciprofloxacin (100) | 4.7 ± 0.2 (0/3) | 3.8 ± 0.5 (0/3) | 3.1 ± 0.5 (0/3) | 3.5 ± 0.5 (0/3) |
| Sparfloxacin (50) | 4.9 ± 0.1 (0/3) | 3.7 ± 0.2 (0/3) | 3.8 ± 0.2 (0/3) | 3.0 ± 0.9 (0/3) |
| Temafloxacin (100) | 4.4 ± 0.2 (0/3) | 3.0 ± 0.3 (0/3) | 1.7 ± 0.5 (0/3) | 1.3 ± 1.4d (0/3) |
Single s.c. doses were given 3 h after a bacterial challenge.
Values are means (log10 CFU per milliliter of whole lung homogenate) ± the standard deviation (n = 3). The detection limit was 1 log10 CFU/ml. The number of animals with positive blood cultures and the total number of animals are in parentheses.
Greater than all quinolone-treated groups (P < 0.001).
Lower than the value for ciprofloxacin (P < 0.05).
Serum and lung pharmacokinetics.
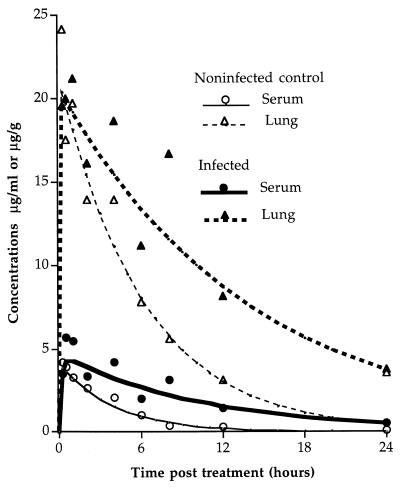
The best fit of the experimental data was obtained by using a one-compartment model. The serum and lung pharmacokinetic parameters of trovafloxacin following administration of a single s.c. injection of 25 mg/kg to mice infected with strains P-4241 (immunocompetent mice) and P-54988 (leukopenic mice) and to uninfected controls are given in Tables 3 and 4. For the other quinolones, these parameters are given in Table 5. When immunocompetent mice were infected with virulent strain P-4241, serum and lung tissue trovafloxacin concentrations were higher in infected animals than in controls; the peak concentrations were, however, not influenced by the infection status. The t1/2 and AUCs were twice or three times as large as those of control animals (Fig. 3 and Table 3). In contrast, no differences in trovafloxacin concentrations in the serum and lungs or the AUCs were found between leukopenic uninfected mice and those infected with penicillin-resistant strain P-54988; only the t1/2 was higher in infected than in uninfected mice in both the serum and the lungs (Table 4). In immunocompetent mice, lung pharmacokinetic parameters were significantly higher than in immunosuppressed animals, suggesting accumulation of trovafloxacin in phagocytes during inflammation of infected lung tissue.
TABLE 3.
PK/PD parameters of trovafloxacin in the sera and lungs of immunocompetent control mice and mice infected with penicillin-susceptible strain P-4241 following s.c. administration of a single dose of 25 mg/kga
| Mouse group and sample | Cmax (μg/ml or g) | C24h (μg/ml or g) | Tmax (h) | t1/2 (h) | AUC0–24 (μg · h/ml or g) | Lung/serum AUC ratio | AUC/MIC ratio |
|---|---|---|---|---|---|---|---|
| Uninfected control | |||||||
| Serum | 3.8 | 0.01 | 0.22 | 2.8 | 16.6 | 277 | |
| Lung tissue | 20.4 | 0.43 | 0.24 | 4.2 | 131 | 7.9 | 2,183 |
| Infected | |||||||
| Serum | 4.4 | 0.51 | 0.66 | 7.5 | 49.5 | 825 | |
| Lung tissue | 19.9 | 3.7 | 0.40 | 9.7 | 287 | 5.8 | 4,783 |
Values are calculated data for 2 × 3 pooled serum and lung samples taken at 0.25, 0.5, 1, 2, 4, 6, 8, 12, and 24 h postdosing. The trovafloxacin MIC and MBC for the challenge strain are 0.06 and 0.25 μg/ml, respectively.
TABLE 4.
PK/PD parameters of trovafloxacin in the sera and lungs of leukopenic control mice and mice infected with penicillin-resistant strain P-54988 following s.c. administration of a single dose of 25 mg/kga
| Mouse group and sample | Cmax (μg/ml or g) | C24h (μg/ml or g) | Tmax (h) | t1/2 (h) | AUC0–24 (μg · h/ml or g) | Lung/serum AUC ratio | AUC/MIC ratio |
|---|---|---|---|---|---|---|---|
| Uninfected control | |||||||
| Serum | 3.7 | 0.1 | 0.70 | 4.8 | 29 | 232 | |
| Lung tissue | 3.6 | 0.2 | 0.97 | 5.3 | 33 | 1.1 | 264 |
| Infected | |||||||
| Serum | 2.9 | 0.3 | 0.53 | 7.4 | 33 | 264 | |
| Lung tissue | 2.7 | 0.3 | 0.86 | 6.7 | 29 | 0.9 | 232 |
Values are calculated data for 2 × 3 pooled serum and lung samples taken 0.25, 0.5, 1, 2, 4, 6, 8, 12, and 24 h postdosing. The trovafloxacin MIC and MBC for the challenge strain are 0.125 and 0.25 μg/ml, respectively.
TABLE 5.
PK/PD parameters of ciprofloxacin, sparfloxacin, and temafloxacin in the sera and lungs of immunocompetent mice infected with penicillin-susceptible strain P-4241 following s.c. administration of a single dosea
| Drug (dose [mg/kg]) and sample | Cmax (μg/ml or g) | t1/2 (h) | AUC0–24 (μg · h/ml or g) | Lung/serum AUC ratio | AUC/MIC ratio |
|---|---|---|---|---|---|
| Ciprofloxacin (100) | |||||
| Serum | 12.6 | 1.9 | 15 | 15 | |
| Lung tissue | 62.0 | 1.1 | 47 | 3.1 | 47 |
| Sparfloxacin (50) | |||||
| Serum | 9.4 | 4.2 | 25 | 100 | |
| Lung tissue | 27.0 | 5.7 | 64 | 2.6 | 256 |
| Temafloxacin (100) | |||||
| Serum | 14.7 | 3.8 | 142 | 142 | |
| Lung tissue | 124 | 2.8 | 490 | 3.4 | 490 |
Values are for six pooled serum and lung samples taken 0.5, 1, 3, 6, 8, and 24 h postdosing. The MICs and MBCs, respectively, for the challenge strain are as follows: ciprofloxacin, 1 and 2 μg/ml; sparfloxacin, 0.25 and 0.5 μg/ml; temafloxacin, 1 and 1 μg/ml.
FIG. 3.
Concentration-time curves fitted to a one-compartment model with plotted experimental data for trovafloxacin in the sera and lungs of mice given a single s.c. injection of 25 mg/kg. A healthy control group and a group challenged with strain P-4241 6 h before drug administration are compared.
In P-4241-infected animals, comparison of the pharmacokinetic parameters of trovafloxacin at 25 mg/kg (Table 3) and the other quinolones at 50 mg/kg (sparfloxacin) and 100 mg/kg (temafloxacin) (Table 5) showed that trovafloxacin had the longest t1/2 in the serum and lungs and the largest AUC/MIC ratio. Trovafloxacin was still detectable in lung tissue 24 h after treatment.
DISCUSSION
Trovafloxacin showed better in vitro activity against penicillin-susceptible and penicillin-resistant S. pneumoniae strains than did the commonly used quinolones ciprofloxacin, sparfloxacin, and temafloxacin. The latter two quinolones also had better antipneumococcal activity than did ciprofloxacin.
The in vitro activity of trovafloxacin has been extensively studied. Trovafloxacin has a broad spectrum of activity against gram-positive and gram-negative aerobic and anaerobic bacteria, including some strains resistant to ciprofloxacin (15, 26, 29, 33). Our data are in agreement with those of Gootz et al. (19), who found that the MIC of trovafloxacin for 90% of the isolates tested (MIC90) was 4-, 8- and 32-fold lower than those of sparfloxacin, ciprofloxacin, and ofloxacin, respectively, against penicillin-susceptible strains. Moreover, trovafloxacin had the same MIC50s and MIC90s against strains with reduced susceptibility to beta-lactams and macrolides.
Only one in vivo study (18) of the efficacy of trovafloxacin has been reported, and only one penicillin-susceptible S. pneumoniae strain was used. Our data show that trovafloxacin is more effective than other quinolones against a penicillin-susceptible S. pneumoniae strain in the acute-pneumonia model and that it is as active as amoxicillin. These results are in agreement with those of Girard et al. (18).
Against the penicillin-resistant strains, trovafloxacin was the most active quinolone. As regards the highly penicillin-resistant and -tolerant strains, trovafloxacin yielded more than 90% survival at 25 mg/kg, whereas amoxicillin at 300 mg/kg protected only 70% of the animals. The other quinolones were significantly less active than trovafloxacin, even at higher concentrations. However, none of the quinolones tested was effective against the quinolone-resistant strain. This agrees with recent data from Gootz et al. (19), who studied the activity of trovafloxacin against DNA gyrase and topoisomerase IV mutants of S. pneumoniae selected in vitro in stepwise fashion on agar containing ciprofloxacin at 2 to 10 times the MIC. Ciprofloxacin MICs for first-step mutants ranged from 4 to 8 μg/ml, whereas trovafloxacin MICs were 0.25 to 0.5 μg/ml. MICs for second-step mutants were 32 to 256 μg of ciprofloxacin per ml and 4 to 16 μg of trovafloxacin per ml. Those authors demonstrated that trovafloxacin protected mice whose lungs were inoculated with a lethal dose of the parent strain or the first-step mutant. While the MIC of trovafloxacin for the mutant was fourfold higher than for the parent strain, the 50% protective dose increased only 1.9-fold, from 6.0 to 11.1 mg/kg.
The excellent in vivo activity of trovafloxacin is partly due to the fact that its in vitro activity against penicillin-susceptible and -resistant S. pneumoniae strains is generally higher than that of the older quinolones. However, other factors are involved in the in vivo efficacy of quinolones, particularly pharmacokinetic-pharmacodynamic (PK/PD) parameters (22, 34). Recently, Forrest et al. (16) and Hyatt et al. (20) reported that the AUC/MIC ratio of ciprofloxacin was the main parameter associated with bacterial eradication and clinical cure in nosocomial pneumonia. Those authors found that the minimal clinically effective AUC/MIC ratio was 125. We recently studied the in vivo efficacy of ciprofloxacin and sparfloxacin in an immunocompetent-mouse model of severe S. pneumoniae pneumonia (8) to probe the lower limits of the AUC/MIC ratio and found that an AUC/MIC ratio of 160 or more was associated with a 100% clinical cure rate. The favorable PK/PD parameters of trovafloxacin with an AUC/MIC ratio far above 160 explain its efficacy. Its longer t1/2 and its better in vitro activity yield the highest serum AUC/MIC ratio in both immunocompetent and leukopenic mice. The other quinolones had lower AUC/MIC ratios. In particular, the ciprofloxacin AUC/MIC ratio was only 15 at 100 mg/kg, explaining its poor efficacy. Our results are in agreement with those of Girard et al. (18), who found that trovafloxacin had far higher AUCs and AUC/MIC ratios in the serum and lungs than did sparfloxacin and temafloxacin in controls and mice infected with a susceptible strain. Moreover, in terms of 50% effective doses, trovafloxacin was more efficacious than the other quinolones.
It must be emphasized that immunosuppressed mice had significantly lower PK/PD parameters than did immunocompetent animals, mainly in the lungs. This could be explained by the intracellular accumulation of trovafloxacin in phagocytes. It has been shown with Legionella isolates that trovafloxacin achieves intracellular levels that are up to 28 times the extracellular levels in guinea pig alveolar macrophages (14). Intracellular levels of this antibiotic were similar to those of erythromycin. It is already known that inflammatory cells serve as a reservoir for cellular uptake and transport of macrolides. The role of leukocytes in the transport and release of azithromycin at the site of infection has been confirmed (32). Histology of the lungs of infected immunocompetent animals shows that interstitial tissue is invaded by inflammatory cells (4).
It has been shown (31) that the binding of trovafloxacin to serum protein is 89 to 96, 75, and 65 to 67% in rats, dogs, and monkeys, respectively. Although we did not investigate the extent of trovafloxacin binding to serum protein in our mouse model, it is probably also very high. Our pharmacokinetic data match very well the survival response, and protein binding thus probably has little influence on therapeutic outcome, due to a lack of tight binding.
Favorable kinetic characteristics of trovafloxacin have also been found in healthy male volunteers (30). Doses of 300 mg or less were well tolerated and yielded a Cmax (4.4 μg/ml), an AUC (41.4 μg · h/ml), and a t1/2 (11.5 h) of the same order of magnitude as those found in the serum of mice after administration of an s.c. injection of 25 mg/kg. The pharmacokinetic profile in our mouse model differs from that in humans in that the t1/2 in serum is shorter in mice (2.8 and 7.5 h, respectively, in control and infected mice versus 10 h in humans). As a result, the best parameter for comparison is the serum AUC. Despite differences in the doses used, the serum AUCs are of the same order of magnitude: 49.5 μg/ml · h in infected mice (our results) versus 41.4 μg/ml · h in humans after administration of 300 mg.
In conclusion, to be sufficiently active against S. pneumoniae and useful for the empirical treatment of community-acquired pneumonia, a fluoroquinolone would have to exhibit a higher AUC/MIC ratio (i.e., lower MICs and/or higher AUCs via a higher Cmax in serum and/or a longer t1/2 in serum) than currently available fluoroquinolones. Trovafloxacin exhibits all of these characteristics and is effective in an experimental model of pneumonia induced not only by a penicillin-susceptible strain but also by penicillin-resistant and multidrug-resistant strains of S. pneumoniae. However, the possible spread of quinolone-resistant pneumococci may compromise the clinical efficacy of this compound. Controlled use of quinolones at optimal doses remains strongly recommended.
REFERENCES
- 1.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum, P. C. 1996. Emergence of resistance to antimicrobial agents in gram-positive bacteria—pneumococci. Drugs 51(Suppl. 1):1–5. [DOI] [PubMed]
- 3.Aubier, M., H. Lode, G. Gialdroni-Grassi, G. Huchon, J. Hosie, N. Legakis, C. Regamey, S. Segev, R. Vester, W. J. Wijnands, and N. Tolstuchow. 1996. Sparfloxacin for the treatment of community acquired pneumonia: a pooled data analysis of two studies. J. Antimicrob. Chemother. 37(Suppl. A):73–82. [DOI] [PubMed]
- 4.Azoulay-Dupuis E, Bédos J P, Vallée E, Hardy D J, Swanson R N, Pocidalo J J. Antipneumococcal activity of ciprofloxacin, ofloxacin, and temafloxacin in an experimental mouse pneumonia model at various stages of the disease. J Infect Dis. 1991;163:319–324. doi: 10.1093/infdis/163.2.319. [DOI] [PubMed] [Google Scholar]
- 5.Azoulay-Dupuis E, Vallée E, Veber B, Bédos J P, Bauchet J, Pocidalo J J. In vivo efficacy of a new fluoroquinolone, sparfloxacin, against penicillin-susceptible and -resistant and multiresistant strains of Streptococcus pneumoniae in a mouse model of pneumonia. Antimicrob Agents Chemother. 1992;36:2698–2703. doi: 10.1128/aac.36.12.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azoulay-Dupuis E, Moine P, Bédos J P, Rieux V, Vallée E. Amoxicillin dose-effect relationship with Streptococcus pneumoniae in a mouse pneumonia model and roles of in vitro penicillin susceptibilities, autolysis, and tolerance properties of the strains. Antimicrob Agents Chemother. 1996;40:941–946. doi: 10.1128/aac.40.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bédos J P, Rollin O, Bouanchaud D H, Pocidalo J J. Relation entre virulence et résistance aux antibiotiques des pneumocoques. Pathol Biol. 1991;39:984–990. [PubMed] [Google Scholar]
- 8.Bédos, J. P., E. Azoulay-Dupuis, P. Moine, M. Muffat-Joly, B. Veber, J. J. Pocidalo, and E. Vallée. Pharmacodynamic activities of ciprofloxacin and sparfloxacin in a murine pneumococcal pneumonia model. Relevance for drug efficacy. J. Pharm. Exp. Ther., in press. [PubMed]
- 9.Briles D E, Crain M J, Gray B M, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canton E, Peman J, Jimenez M T, Ramon M S, Gobernado M. In vitro activity of sparfloxacin compared with those of five other quinolones. Antimicrob Agents Chemother. 1992;36:558–565. doi: 10.1128/aac.36.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbon C, Leophonte P, Petitpretz P, Chauvin J P, Hazebrouck J. Efficacy and safety of temafloxacin versus those of amoxicillin in hospitalized adults with community-acquired pneumonia. Antimicrob Agents Chemother. 1992;36:833–839. doi: 10.1128/aac.36.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Child J, Andrews J, Boswell F, Brenwald N, Wise R. The in vitro activity of CP-99,219, a new naphthyridone antimicrobial agent: a comparison with fluoroquinolone agents. Antimicrob Agents Chemother. 1995;35:869–876. doi: 10.1093/jac/35.6.869. [DOI] [PubMed] [Google Scholar]
- 13.Davis R, Markham A, Balfour J A. Ciprofloxacin. An updated review of its pharmacology, therapeutic efficacy and tolerability. Drugs. 1996;51:1019–1074. doi: 10.2165/00003495-199651060-00010. [DOI] [PubMed] [Google Scholar]
- 14.Edelstein P H, Edelstein M A C, Ren J, Polzer R, Gladue R P. Activity of trovafloxacin (CP-99,219) against Legionella isolates: in vitro activity, intracellular accumulation and killing in macrophages, and pharmacokinetics and treatment of guinea pigs with L. pneumophila pneumonia. Antimicrob Agents Chemother. 1996;40:314–319. doi: 10.1128/aac.40.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eliopoulos G M, Klimm K, Eliopoulos C T, Ferraro M J, Moellering R C. In vitro activity of CP-99,219, a new fluoroquinolone, against clinical isolates of gram-positive bacteria. Antimicrob Agents Chemother. 1993;37:941–946. doi: 10.1128/aac.37.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedland I R, McCracken G H. Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 18.Girard A E, Girard D, Gootz T D, Faiella J A, Cimochowski C R. In vivo efficacy of trovafloxacin (CP-99,219), a new quinolone with extended activities against gram-positive pathogens, Streptococcus pneumoniae, and Bacteroides fragilis. Antimicrob Agents Chemother. 1995;39:2210–2216. doi: 10.1128/aac.39.10.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gootz T D, Zaniewski R, Haskell S, Schmieder B, Tankovic J, Girard D, Courvalin P, Polzer R J. Activity of the new fluoroquinolone trovafloxacin (CP-99,219) against DNA gyrase and topoisomerase IV mutants of Streptococcus pneumoniae selected in vitro. Antimicrob Agents Chemother. 1996;40:2691–2697. doi: 10.1128/aac.40.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyatt J M, Nix D E, Schentag J J. Pharmacokinetic and pharmacodynamic activities of ciprofloxacin against strains of Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa for which MICs are similar. Antimicrob Agents Chemother. 1994;38:2730–2737. doi: 10.1128/aac.38.12.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iliadis A, Brown C, Huggins M L. Apis: a software model for identification, simulation and dosage regimen calculation in clinical and experimental pharmacokinetics. Comput Methods Programs Biomed. 1992;38:227–239. doi: 10.1016/0169-2607(92)90103-e. [DOI] [PubMed] [Google Scholar]
- 22.Leggett, J. E., S. Ebert, B. Fantin, and W. A. Craig. 1991. Comparative dose-effect relations at several dosing intervals for β-lactam, amino-glycoside and quinolone antibiotics against gram-negative bacilli in murine thigh-infection and pneumonitis models. Scand. J. Infect. Dis. 74(Suppl.):179–184. [PubMed]
- 23.Marton A, Gulyas M, Munoz R, Tomasz A. Extremely high incidence of antibiotic resistance in clinical isolates of Streptococcus pneumoniae in Hungary. J Infect Dis. 1991;163:542–548. doi: 10.1093/infdis/163.3.542. [DOI] [PubMed] [Google Scholar]
- 24.Moreillon P, Marckiewicz Z, Nachman S, Tomasz A. Two bactericidal targets for penicillin in pneumococci: autolysis-dependent and autolysis-independent killing mechanisms. Antimicrob Agents Chemother. 1990;34:33–39. doi: 10.1128/aac.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1987. [Google Scholar]
- 26.Neu H C, Chin N X. In vitro activity of the new fluoroquinolone CP-99,219. Antimicrob Agents Chemother. 1994;38:2615–2622. doi: 10.1128/aac.38.11.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallarés R, Linarés J, Vadillo M, Cabellos C, Manresa F, Viladrich P F, Martin R, Gudiol F. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–480. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 28.Roussel Delvallez M, Wallet F, Caillaux M, Cattoen C, Verhaege A, Bonte M, Samaille S, Duhamel M, Fievet P, Tiry F, Reiter N, Descamp D, Marcolin M, Templier F, Noël A M, Varlet A, Morin C, Courcol R J. 16e Réunion Interdisciplinaire de Chimiothérapie Anti-Infectieuse. 1996. Observatoire Nord Pas de Calais: Enquête sur la résistance aux antibiotiques de 1087 souches de Streptococcus pneumoniae (S. P.) isolées dans la région, abstr. 80, p 6. [Google Scholar]
- 29.Spangler S K, Jacobs M R, Appelbaum P C. Activity of CP 99,219 compared with those of ciprofloxacin, grepafloxacin, metronidazole, cefoxitin, piperacillin, and piperacillin-tazobactam against 489 anaerobes. Antimicrob Agents Chemother. 1994;38:2471–2476. doi: 10.1128/aac.38.10.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng R, Harris S C, Nix D E, Schentag J J, Foulds G, Liston T E. Pharmacokinetics and safety of trovafloxacin (CP-99,219), a new quinolone antibiotic, following administration of single oral doses to healthy male volunteers. Antimicrob Agents Chemother. 1995;36:385–394. doi: 10.1093/jac/36.2.385. [DOI] [PubMed] [Google Scholar]
- 31.Teng R, Girard D, Gootz T D, Foulds G, Liston T E. Pharmacokinetics of trovafloxacin (CP-99,219), a new quinolone, in rats, dogs, and monkeys. Antimicrob Agents Chemother. 1996;40:561–566. doi: 10.1128/aac.40.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veber B, Pocidalo J J. Le cas particulier des azalides: l’antibiodiapédèse. Données expérimentales à partir d’un modèle murin de pneumonie à pneumocoque. Pathol Biol. 1995;43:524–528. [PubMed] [Google Scholar]
- 33.Vissalli M A, Jacobs M R, Appelbaum P C. Activity of CP 99,219 (trovafloxacin) compared with ciprofloxacin, sparfloxacin, clinafloxacin, lomefloxacin, and cefuroxime against ten penicillin-susceptible and penicillin-resistant pneumococci by time-kill methodology. J Antimicrob Chemother. 1996;37:77–84. doi: 10.1093/jac/37.1.77. [DOI] [PubMed] [Google Scholar]
- 34.Vogelman B, Gudmunsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]