Abstract
BACKGROUND
Syringomyelia is a neurological disorder that is caused by abnormal cerebrospinal fluid flow or circulation. It is an incidental finding in most cases, predominantly presenting with sensory symptoms of insensitivity to pain and temperature. Spinal ossified ligamentum flavum (OLF) leading to syringomyelia is one of the rare causes. The authors report an unusual case of syringomyelia due to a thoracic OLF.
OBSERVATIONS
A 54-year-old female presented with backache, difficulty walking, spasticity in the bilateral lower limbs, tingling sensation in the bilateral lower limbs, and paraparesis for 5 years. Her radiological investigations were suggestive of an OLF causing a syrinx. She underwent laminectomy, and her syrinx resolved on subsequent follow-up.
LESSONS
A syrinx due to a single-level OLF is rare, and this uncommon cause should be kept in mind while formulating treatment plans.
Keywords: syringomyelia, syrinx, OLF, ossified ligamentum flavum
ABBREVIATIONS: CSF = cerebrospinal fluid, MRI = magnetic resonance imaging, OLF = ossified ligamentum flavum
A syrinx is a fluid-filled cavity. Anatomically, it lies within the central canal or the spinal cord parenchyma.1 Syringomyelia is a neurological disorder that is caused by abnormal cerebrospinal fluid (CSF) flow or circulation. Syringomyelia is most commonly seen in conjunction with Chiari malformation type 1.2 Syringomyelia can also be caused by extradural compressive lesions, ossified ligamentum flavum (OLF), the aftereffects of trauma, spinal cord tumor, and adhesive infectious arachnoiditis.3,4 Syringomyelia is an incidental finding in most cases, predominantly presenting with sensory symptoms of insensitivity to pain and temperature.1,5 Spinal OLF leading to syringomyelia is one of the rare causes. Cases of cervical spondylolysis leading to syringomyelia have been reported.6–12 We describe an unusual case of syringomyelia due to a thoracic OLF.
Illustrative Case
History and Examination
A 54-year-old female presented with backache, difficulty walking, spasticity in the bilateral lower limbs, tingling sensation in the bilateral lower limbs, and paraparesis for 5 years. She was a homemaker and farmer by profession. She had no history of trauma or meningitis. She had not undergone any spine surgery. The power in her lower limbs progressively deteriorated from grade 5 to grade 4 over the past year. Pain sensation, temperature sensation, sense of vibration, pain, and joint position sense were diminished below the T12 level bilaterally. The patient did not have any bladder or bowel disturbances, and the reflexes were exaggerated in the bilateral lower limbs.
Imaging
Magnetic resonance imaging (MRI) of the spine (first imaging) revealed a syrinx extending from the C3 vertebral level up to the conus causing its expansion and thinning of the parenchyma. Severe impingement of the dorsal cord at the T11–12 level was noted (Fig. 1).
FIG. 1.
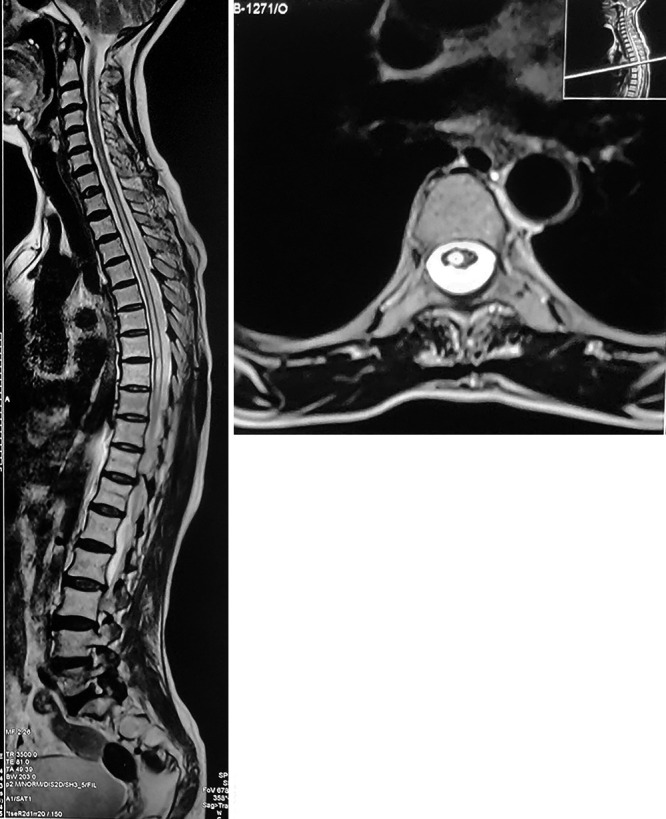
Sagittal (left) and axial (right) MRI showing a syrinx from the C3 vertebral level up to the conus and cord compression at T11–12 level.
The patient declined surgery and was lost to follow-up because of the coronavirus disease 2019 pandemic, and she presented 6 months later with aggravated signs and symptoms. She had severe back pain, flickering movements in the bilateral lower limbs, loss of sensation below the T12 level, bladder and bowel incontinence, and severe spasticity (grade 4). MRI of the spine (second imaging) revealed an increase in the syrinx dimension with almost complete central canal dilation extending from the C3 vertebral level up to the conus and papery thin parenchyma (Fig. 2). Computed tomography (CT) of the spine was suggestive of severe canal stenosis at the T11–12 level due to ligamentum flavum hypertrophy and ossification (Fig. 3). It was also suggestive of ossification of the interspinous ligaments.
FIG. 2.
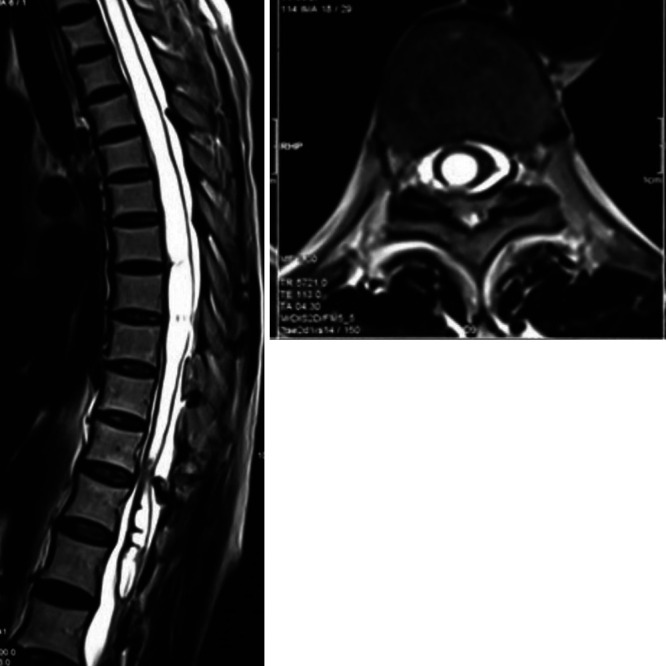
Sagittal (left) and axial (right) T2-weighted MRI on follow-up, with complete dilation of the central canal and thinned out parenchyma.
FIG. 3.
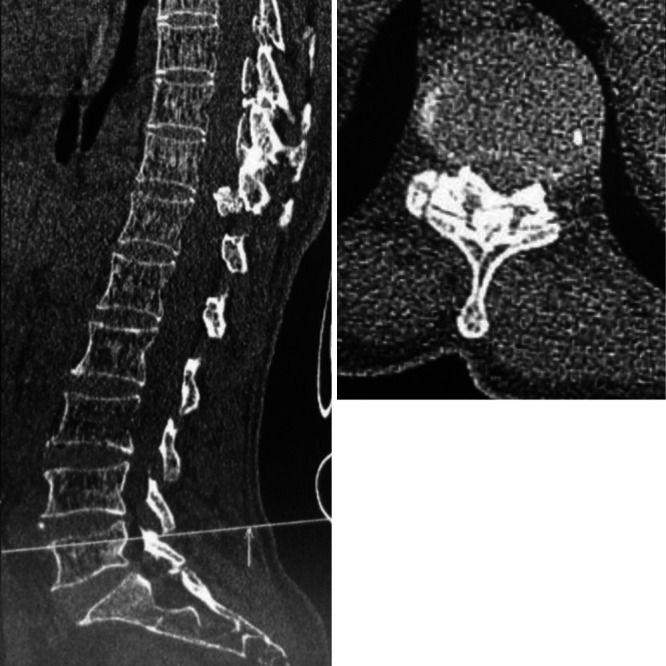
Sagittal (left) thoracic spine and axial (right) CT images at the T11–12 level, showing ligamentum flavum ossification compromising the canal with the fusion of bilateral ossified masses at the midline.
Treatment
The patient underwent T11–12 decompressive laminectomy. The interspinous ligaments were calcified. An ossified and hypertrophied ligamentum flavum was identified, which was compressing the dura, and the cord was excised to achieve sufficient complete decompression of the spinal canal. No identifiable plane or dura was seen deep to the ossified and hypertrophied ligamentum flavum; hence, the cord was exposed while excising the diseased ligamentum flavum (Fig. 4). The arachnoid membrane over the posterior aspect of the cord was partially adherent to the dura.
FIG. 4.
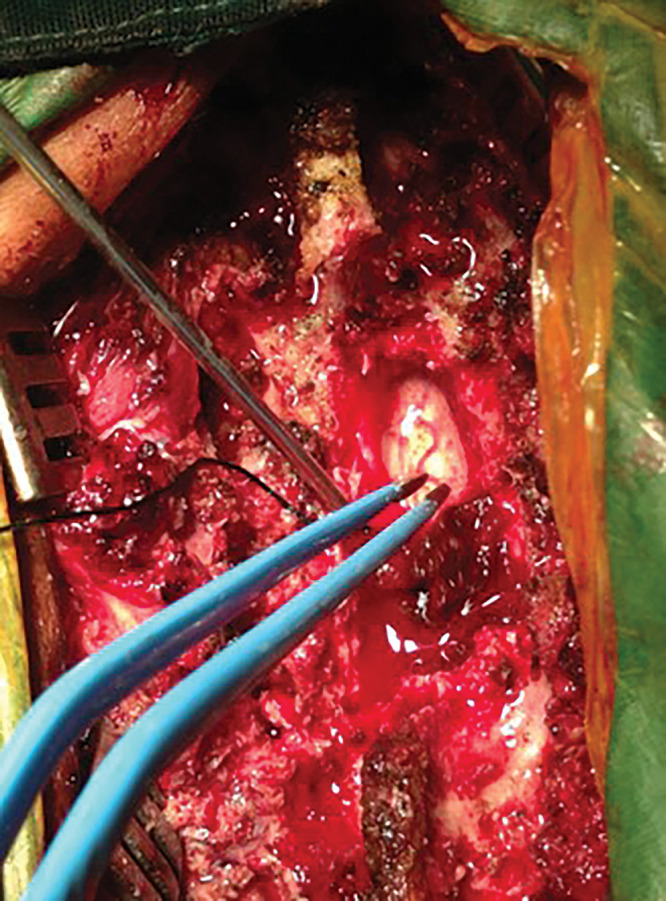
Intraoperative photograph showing complete excision of OLF with exposed spinal cord.
In the postoperative period, the patient had CSF leakage (Fig. 5) from the wound, which was managed conservatively. The patient showed improvement in the postoperative period, and her symptoms improved. Paresthesia decreased in both lower extremities. Power increased to grade 3/5, and there was a significant decrease in spasticity by 1 month after surgery and to grade 4/5 by 6 months postsurgery. The histopathological examination showed ligamentum flavum ossification, significant fibrosis, and inflamed thickened arachnoid.
FIG. 5.
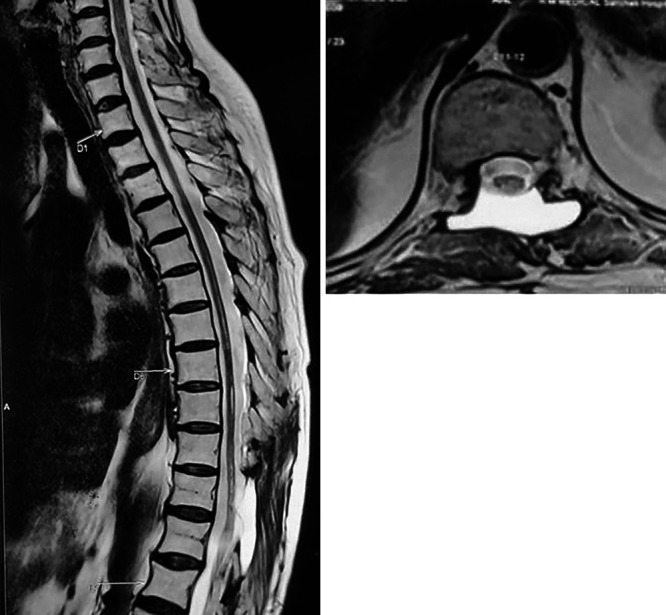
Postoperative sagittal (left) and axial (right) T2-weighted MRI showing a resolving syrinx with a CSF pocket at the operative site.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Primary syringomyelia due to spinal spondylolysis is rare, and only a few cases of cervical spondylolysis leading to syringomyelia have been reported.6–12 Only one case of cervical OLF resulting in syringomyelia has been reported to cause a syrinx,8 and not a single case has been reported to cause a holocord syrinx, as was noted in our current case. We have described a rare case of dorsal ligamentum ossification leading to the formation of a holocord syrinx. Ligamentum flavum ossification can lead to myelopathy, more commonl in cases of lower thoracic spine involvement.13 OLF and hypertrophy leading to long-standing compression of the spinal cord leads to an alteration in CSF dynamics in the parenchyma of the spinal cord, thinning of the parenchyma, and enlargement of cavities due to pulsatile CSF pressure.12
Mechanical stress as an extrinsic factor is an important element in the process of the formation of OLF. Under tension stress, some fibroblastic cells can transform into chondrocytes and the expression of BMP-2, TGFb, and SOX9 increases. Relative movement between OLF and compressed dura mater, due to flexion and extension of the spine, causes local inflammation, leading to dural adhesion. The adhesion tissue acts as a tunnel to transport osteogenic cytokines from the OLF to the compressed dura mater, which causes the dura mater directly adhered to the OLF to ossify.
Sato’s classification according to the progression of the ligament ossification is a valid tool for severity assessment and has 5 grades. Our case was Sato type D, the fused type, i.e., fusion of the bilateral ossified masses at the midline.
Resection aims at excision of the diseased segmental level and the OLF, exposing the dura for successful spinal canal decompression. No aggravated neural dysfunction or serious complications associated with this technique have been noted. Symptom duration and severity are the only factors found to be potentially predictive of surgical success.
In the present case, the patient did not have any traumatic injury or history of infection (meningitis) and had not undergone any previous spine surgery. The patient was a farmer and had had long-standing complaints. Despite all the symptoms, the patient was working until the symptoms were severely aggravated. Hence, ossified and hypertrophied ligamentum flavum at the level of spinal canal stenosis at the T11–12 level may have exposed the spinal cord at the same level to repeated minor mechanical injuries due to daily mechanical stress and exercise, thus inducing arachnoiditis, further leading to intramedullary cystic degeneration as a result of ischemia. Intramedullary cystic cavities are formed as a result of CSF pathway obstruction due to OLF.14 The on-and-off changes in the CSF pressure gradient within the spinal cord and outside the cord results in the formation of a syrinx.15
In previous reported cases of cervical spondylolysis due to OLF leading to syringomyelia, extradural decompression without any intradural surgical intervention showed significant improvement in symptoms and a decrease in the size of the syrinx.6,8–12,16,17 In this present case of dorsal ligamentum flavum ossification, we performed laminectomy and excision of the OLF (extradural decompression), but because of the long-standing spinal canal compression, the dura was adherent to the overlying ossified flavum and the arachnoid underneath the dura was adherent to the dura because of the long-standing arachnoiditis and hence was excised as part of surgery. The patient showed significant improvement in the postoperative period. Decompression of the spinal subarachnoid space along with decompression laminectomy and excision of the OLF has added beneficial effects in cases of extensive arachnoiditis, as it helps in the restoration of CSF dynamics.
Lessons
The resection of lamina and OLF is a safe and effective surgical technique in the treatment of thoracic OLF. Decompression of the spinal subarachnoid space along with decompression laminectomy and excision of the OLF has added benefit. We believe that durotomy is often part of the decompression surgery.
Author Contributions
Conception and design: Punia, Chugh. Acquisition of data: Lachake, Gotecha. Analysis and interpretation of data: Lachake, Chugh. Drafting the article: Punia, Chugh, Gotecha. Critically revising the article: Punia, Chugh, Gotecha. Reviewed submitted version of manuscript: Gotecha. Approved the final version of the manuscript on behalf of all authors: Lachake. Administrative/technical/material support: Lachake. Study supervision: Chugh.
References
- 1. Greitz D. Unraveling the riddle of syringomyelia. Neurosurg Rev. 2006;29(4):251–264. doi: 10.1007/s10143-006-0029-5. [DOI] [PubMed] [Google Scholar]
- 2. Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44(5):1005–1017. doi: 10.1097/00006123-199905000-00042. [DOI] [PubMed] [Google Scholar]
- 3. Roy AK, Slimack NP, Ganju A. Idiopathic syringomyelia: retrospective case series, comprehensive review, and update on management. Neurosurg Focus. 2011;31(6):E15. doi: 10.3171/2011.9.FOCUS11198. [DOI] [PubMed] [Google Scholar]
- 4. Klekamp J, Batzdorf U, Samii M, Bothe HW. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997;86(2):233–240. doi: 10.3171/jns.1997.86.2.0233. [DOI] [PubMed] [Google Scholar]
- 5. Roser F, Ebner FH, Sixt C, Hagen JM, Tatagiba MS. Defining the line between hydromyelia and syringomyelia. A differentiation is possible based on electrophysiological and magnetic resonance imaging studies. Acta Neurochir (Wien) 2010;152(2):213–219. doi: 10.1007/s00701-009-0427-x. discussion 219. [DOI] [PubMed] [Google Scholar]
- 6. Ball JR, Little NS. Chiari malformation, cervical disc prolapse and syringomyelia--always think twice. J Clin Neurosci. 2008;15(4):474–476. doi: 10.1016/j.jocn.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 7. Butteriss DJ, Birchall D. A case of syringomyelia associated with cervical spondylosis. Br J Radiol. 2006;79(946):e123–e125. doi: 10.1259/bjr/27982692. [DOI] [PubMed] [Google Scholar]
- 8. Fukuzumi Y, Tani S, Isoshima A, Nagashima H, Okuda Y, Abe T. A case of syringomyelia associated with ossification of the yellow ligament: assessment of surgical outcomes using PVI and Ro. Article in Japanese. Sekizui Geka. 2006;20(2):187–191. [Google Scholar]
- 9. Kaar GF, N’Dow JM, Bashir SH. Cervical spondylotic myelopathy with syringomyelia. Br J Neurosurg. 1996;10(4):413–415. doi: 10.1080/02688699647384. [DOI] [PubMed] [Google Scholar]
- 10. Kameyama T, Ando T, Fukatsu H, Mizuno T, Takahashi A. Syringomyelic syndrome secondary to cervical canal stenosis and cervical spondylosis. Article in Japanese. Rinsho Shinkeigaku. 1993;33(11):1179–1183. [PubMed] [Google Scholar]
- 11. Kato N, Tanaka T, Nagashima H, et al. Syrinx disappearance following laminoplasty in cervical canal stenosis associated with Chiari malformation—case report. Neurol Med Chir (Tokyo) 2010;50(2):172–174. doi: 10.2176/nmc.50.172. [DOI] [PubMed] [Google Scholar]
- 12. Kimura R, Park YS, Nakase H, Sakaki T. Syringomyelia caused by cervical spondylosis. Acta Neurochir (Wien) 2004;146(2):175–178. doi: 10.1007/s00701-003-0172-5. [DOI] [PubMed] [Google Scholar]
- 13. Ben Hamouda K, Jemel H, Haouet S, Khaldi M. Thoracic myelopathy caused by ossification of the ligamentum flavum: a report of 18 cases. J Neurosurg. 2003;99(2) suppl:157–161. doi: 10.3171/spi.2003.99.2.0157. [DOI] [PubMed] [Google Scholar]
- 14. Koyanagi I, Iwasaki Y, Hida K, Houkin K. Clinical features and pathomechanisms of syringomyelia associated with spinal arachnoiditis. Surg Neurol. 2005;63(4):350–355. doi: 10.1016/j.surneu.2004.05.038. discussion 355-356. [DOI] [PubMed] [Google Scholar]
- 15. Chang HS, Joko M, Matsuo N, Kim SD, Nakagawa H. Subarachnoid pressure-dependent change in syrinx size in a patient with syringomyelia associated with adhesive arachnoiditis. Case report. J Neurosurg Spine. 2005;2(2):209–214. doi: 10.3171/spi.2005.2.2.0209. [DOI] [PubMed] [Google Scholar]
- 16. Lucci B, Reverberi S, Greco G. Syringomyelia and syringomielic syndrome by cervical spondylosis. Report on three cases presenting with neurogenic osteoarthropathies. J Neurosurg Sci. 1981;25(3-4):169–172. [PubMed] [Google Scholar]
- 17. Rebai R, Boudawara MZ, Ben Yahia M, Mhiri C, Ben Mansour H. Syringomyelobulbia associated with cervical spondylosis. Pathophysiology and therapeutic implications. Article in French. Neurochirurgie. 2002;48(2-3 Pt 1):120–123. [PubMed] [Google Scholar]


