Abstract
BACKGROUND
Atypical teratoid/rhabdoid tumor (ATRT) is a rare, highly aggressive central nervous system tumor predominantly found in children. Limited information exists on ATRT in adults, posing challenges in diagnosis and treatment. This study presents the case of an adult patient with ATRT in the sellar region and explores the impact of different treatment regimens on patient survival.
OBSERVATIONS
A 60-year-old female with an ATRT underwent resection of the tumor, followed by adjuvant chemoradiotherapy. Molecular genetic analysis revealed compound heterozygous SMARCB1 point mutations. Survival analysis was performed on previously published adult ATRT cases, comparing treatment approaches. The cohort’s overall median survival was 6 months, with patients receiving combined chemoradiotherapy showing the longest median survival of 23.5 months. Statistical analysis demonstrated a significant difference in survival between patients treated with surgery alone and those receiving surgery followed by chemoradiotherapy (p < 0.001). However, no significant difference was observed between patients treated with surgery alone and those with postoperative radiotherapy (p = 0.105).
LESSONS
Early initiation of adjuvant chemoradiotherapy following surgery improves survival outcomes in adult patients with ATRT. Because of limited data on standardized treatment protocols for adults with ATRT, further research and larger-scale studies are needed to establish effective treatment guidelines for this population.
Keywords: adult, sellar atypical teratoid/rhabdoid tumor, ATRT, sellar ATRT
ABBREVIATIONS: ATRT = atypical teratoid/rhabdoid tumor, CN = cranial nerve, CT = computed tomography, MRI = magnetic resonance imaging
Atypical teratoid/rhabdoid tumor (ATRT) is a rare and highly aggressive embryonal tumor of the central nervous system, primarily affecting children.1 The incidence of adult ATRT cases is relatively low, with fewer than 100 cases reported in the medical literature.2,3 These tumors predominantly occur in females and are usually located in the supratentorial region, particularly affecting midline structures such as the sellar region,2,4 whereas nonmidline tumors are more commonly found in males between the ages of 18 and 40 years.4 The median overall survival for adults with ATRT is approximately 20 months; in the pediatric population, survival ranges from 6 to 17 months.1,5
Given its rarity and aggressive nature, ATRT presents significant challenges for clinicians in terms of diagnosis and treatment. Surgery is typically the initial approach, but the role of radiotherapy and chemotherapy has also been explored.3,6 Chemotherapy protocols for ATRT in adults are mainly adapted from those used in pediatric patients. However, the optimal treatment strategy for adult ATRT remains uncertain, highlighting the need for further research to determine the most effective approach.
In this study, we present the case of a 60-year-old female patient diagnosed with an ATRT in the sellar region. The patient underwent resection followed by combined chemoradiotherapy. Additionally, we conducted a survival analysis of previously published cases of adult ATRT to evaluate the impact of different treatment strategies on patient survival. The aim of this study was to provide valuable insights into the efficacy of various treatment regimens and support clinical decision making for the management of ATRT.
Illustrative Case
A 60-year-old female presented with a 2-month history of right eye pain and binocular oblique diplopia. Clinical examination revealed that she had a right cranial nerve (CN) III palsy, which spared the pupil, and a right CN IV palsy. The patient denied any constitutional symptoms or any family history of pituitary tumors. Magnetic resonance imaging (MRI) revealed a 1.3-cm mass located in the sellar region. The mass involved the right cavernous sinus but did not displace the optic apparatus (Fig. 1). The patient did not exhibit any manifestations of endocrinal dysfunction, and hormone profiles, including morning cortisol, thyroid-stimulating hormone, free thyroxine, follicle-stimulating hormone, luteinizing hormone, growth hormone, and prolactin, were all within the normal range.
FIG. 1.
Sagittal (A) and coronal (B) T1-weighted magnetic resonance (MR) images at clinical presentation revealed a 1.3-cm mass in the sella with involvement of the right cavernous sinus.
After receiving dexamethasone, the patient underwent resection of the tumor via an endoscopic endonasal approach. Pathology results indicated that the tumor was a malignant neoplasm with an embryonal morphology. The tumor consisted of sheets of cells exhibiting open chromatin, varying prominent nucleoli, and limited eosinophilic cytoplasm. The lesion displayed high vascularity and areas of hemorrhage (Fig. 2A and B). Immunohistochemistry results showed positive staining for CAM5.2, synaptophysin, and EMA, while there was no INI1 expression (Fig. 2C, B, E). Molecular genetic testing revealed 2 variants of clinical significance: SMARCB1 L135fs c.403delC and SMARCB1 Q260* c.778C > T.
FIG. 2.
Histological examination of the tumor. Hematoxylin-and-eosin staining, original magnification ×20 (A) and ×40 (B), showed sheets of cells with open chromatin, variably prominent nucleoli, and scant eosinophilic cytoplasm. Immunohistochemistry, original magnification ×40, with immunoperoxidase staining: CAM5.2-positive staining (C) and synaptophysin-positive staining (D). INI-1 immunostaining shows loss of nuclear staining in the tumor nuclei and positive in the endothelial cells and residual pituitary tissue (E, original magnification ×40).
Further diagnostic workup, including chest computed tomography (CT) and spinal MRI, did not reveal any evidence of metastatic disease. Additionally, lumbar puncture was performed to stage the disease, and it showed no signs of metastasis.
Despite initial symptom improvement following surgery, the patient developed right-sided CN III palsy and numbness along the distribution of the CN V1 3 weeks later. Follow-up brain and spine MRI indicated tumor regrowth in the sellar region with extension into the right cavernous sinus (Fig. 3). The tumor was deemed Knosp grade 4 on the right side and grade 1 on the left. There was no indication of spinal metastasis. Subsequently, the patient received radiotherapy with a total dose of 54 Gy administered over 30 fractions. She is currently undergoing chemotherapy with cyclophosphamide, doxorubicin, vincristine, and etoposide.
FIG. 3.
Sagittal (A) and coronal (B) T1-weighted MR images obtained 3 weeks postoperatively, showing a 2.2 × 2.0 × 3.0–cm enhancing mass extending to the right cavernous sinus and encasing the right internal carotid artery, indicating tumor regrowth.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
ATRT is an uncommon and highly aggressive tumor that is predominantly diagnosed in children under the age of 3.7 In infants, ATRTs typically arise in the posterior fossa; in adults, they more commonly occur in the supratentorial region.2,3,8,9 Among adults, approximately 60 cases of ATRT have been reported in the sellar region.
Molecular genetic analysis of the ATRT case presented in this study revealed compound heterozygous SMARCB1 point mutations, specifically the SMARCB1 L135fs c.403delC and SMARCB1 Q260* c.778C > T variants. These mutations, affecting different alleles, are considered to have strong clinical significance. Interestingly, these specific mutations are rare in pediatric SMARCB1-deficient tumors, with only 1 tumor of 50 ATRTs and none of 150 ATRTs examined exhibiting these mutations.10,11 However, they have been previously reported in sellar region ATRTs in adults.12 This rarity of mutations in pediatric ATRT suggests the existence of different underlying mechanisms contributing to the development of ATRTs in adults and children.
The divergence in the genetic characteristics between adult and pediatric ATRTs raises the question of whether adapting pediatric treatment regimens is the most effective approach for treating adult ATRT. The typical treatment for sellar ATRT in adults involves resection, followed by chemotherapy, radiotherapy, or a combination of the 2. However, there is currently no consensus regarding the optimal adjuvant therapy for these patients.
In the reported case, the patient initially underwent resection of the tumor, followed by a decision to initiate adjuvant therapy with chemoradiotherapy. The optimal chemotherapeutic regimen for ATRT in adults is still unknown, and there is a lack of standardized chemotherapy protocols specifically tailored for this population. However, commonly used chemotherapeutic agents for ATRT include ifosfamide, cisplatin, etoposide, doxorubicin, cyclophosphamide, vincristine, methotrexate, and temozolomide, administered in various combinations.13,14
To evaluate the impact of different treatments on adult ATRT patient survival, we conducted a survival analysis of previously reported adult ATRT cases (Table 1, Fig. 4). The overall median patient survival was 6 months, with an interquartile range (IQR) of 2–25.5 months. Patients who underwent resection alone had a median survival of 2.5 months (IQR: 1–4.75 mos), whereas those who received postoperative radiotherapy had a median survival of 7.5 months (IQR: 1.75–24.25 mos). Patients who underwent both radiotherapy and chemotherapy after surgery had a median survival of 22.5 months (IQR: 8.25–29.75 mos). Only 3 patients received chemotherapy alone after surgery.
TABLE 1.
Previously published adult ATRT cases
| Authors & Year | Age (yrs) | Gender | MRI Findings | Treatment* | Outcome |
|---|---|---|---|---|---|
| Kuge et al., 200015 |
32 |
F |
Contrast-enhancing sellar lesion |
Resection; RT; chemo: cisplatin, etoposide, interferon-gamma, methotrexate |
Death at 28 mos |
| Raisanen et al., 200516 |
20 |
F |
2.0 × 1.9–cm, partially cystic, heterogeneously enhancing mass in sellar region |
Resection; RT; chemo |
Alive at 28 mos |
| 31 |
F |
1.6-cm enhancing mass |
Resection; RT |
Death at 9 mos |
|
| Arita et al., 200817 |
56 |
F |
Heterogeneously enhancing mass invading rt CS & displacing pituitary gland |
Resection; RT: 17 Gy to periphery & 34 Gy to center of residual tumor |
Death at 23 mos |
| Las Heras et al., 201018 |
46 |
F |
NR |
NR |
NR |
| Schneiderhan et al., 201119 |
61 |
F |
Heterogeneously enhancing sellar/suprasellar mass w/ parasellar expansion; edema of adjacent brain parenchyma & bilat optic tracts |
Resection |
Death at 3 mos |
| 57 |
F |
Heterogeneously enhancing sellar lesion w/ rt-sided parasellar expansion |
Resection; RT; chemo: 3 cycles of doxorubicin & cisplatin |
Alive at 6 mos |
|
| Chou et al., 201320 |
43 |
F |
Isointense/hypointense sellar lesion w/ heterogeneous enhancement; invasion into lt CS |
Resection; RT |
Alive at 0.5 mo |
| Moretti et al., 201321 |
60 |
F |
Heterogeneously enhancing sellar lesion w/ extrasellar; lt CS invasion; encasing ICA |
Resection; chemo: doxorubicin & vinorelbine |
Death at 30 mos |
| Park et al., 201422 |
42 |
F |
Heterogeneously enhancing solid & cystic sellar/suprasellar mass |
Resection; RT; chemo: 10 mos of vincristine, cisplatin, adriamycin, etoposide, & cyclophosphamide alternating w/ vincristine, etoposide, ifosfamide, & carboplatin |
Alive at 27 mos |
| Shitara et al., 201423 |
44 |
F |
Heterogeneously enhancing mass |
Resection; RT; chemo: ifosfamide, cisplatine & etoposide |
Death at 17 mos |
| Lev et al., 201524 |
36 |
F |
3.3 × 3.2 × 2.3–cm heterogeneously enhancing sellar lesion; compression of optic chiasm, lt CS invasion |
Multiple resections; RT; chemo: cytoxan, adriamycin, vincristine, & cisplatin |
Death at 29 mos |
| Biswas et al., 201525 |
48 |
F |
Sellar lesion w/ “malignant characteristics” |
Neoadjuvant chemo: vincristine, doxorubicin; resection; chemo: cyclophosphamide alternating w/ ifosfamide, carboplatin, etoposide; RT |
Death at 2 mos |
| Regan et al., 201526 |
45 |
F |
Hypointense sellar lesion w/ extension into lt CS |
Resection; stereotactic RT to CS & parasellar region |
Death at 6 mos |
| Nobusawa et al., 201627 |
69 |
F |
2.8 x 1.6–cm isointense sellar lesion, extension into lt CS surrounding ICA |
Resection; chemo: temozolomide; focal RT |
Alive at 24 mos |
| Almalki et al., 201628 |
36 |
F |
Heterogeneously enhancing sellar/suprasellar lesion; bilat invasion of CS & clivus w/ posterior destruction of clinoid |
Resection; chemo: vincristine; fractionated RT: total 60 Gy; chemo: ifosfamide, cisplatin, & etoposide |
Alive at 37 mos |
| Larran-Escandon et al., 201629 |
43 |
F |
2.0 x 2.3–cm sellar/suprasellar lesion w/ subacute hemorrhage |
Resection |
Death at 1 mo |
| Elsayad et al., 201630 |
66 |
M |
2.2 × 1.6 × 1.4–cm heterogeneously enhancing sellar lesion |
Resection; RT: 59.4 Gy |
Alive at 48 mos |
| Nakata et al., 201712 |
31 |
F |
NR |
Resection; chemo: cisplatin & etoposide, followed by methotrexate (intrathecal); local & posterior fossa RT |
Death at 28 mos |
| 56 |
F |
NR |
Resection; stereotactic radiosurgery; craniospinal RT |
Death at 23 mos |
|
| 44 |
F |
NR |
Resection; chemo: ifosfamide, cisplatin, & etoposide; RT |
Death at 17 mos |
|
| 26 |
F |
NR |
Resection; chemo: methotrexate (intrathecal), ifosfamide, cisplatin, & etoposide; local spine RT |
Death at 33 mos |
|
| 21 |
F |
NR |
Resection; chemo: ifosfamide, cisplatin, etoposide; local RT |
Death at 35 mos |
|
| 69 |
F |
NR |
Resection; chemo: temozolomide; local RT |
Alive at 37 mos |
|
| Dardis et al., 201731 |
35 |
M |
Mixed cystic/solid heterogeneously enhancing suprasellar & interpeduncular lesion |
2 resections; fractioned craniospinal RT w/ localized boosts w/ cisplatin sensitizer (total 66 Gy); chemo: high-dose cyclophosphamide & vincristine, followed by autologous stem cell transplant |
Alive at 30 mos |
| Pratt et al., 201732 |
47 |
F |
2.6 x 3.9 x 3.2–cm heterogeneously enhancing sellar mass; erosion of surrounding bone & extension into bilat CSs, complete encasement of lt carotid artery |
Resection; RT |
NR |
| Johann et al., 201833 |
20 |
F |
NR |
Resection; chemo: ifosfamide, cisplatin, & etoposide, followed by autologous stem cell rescue |
Death at 120 mos |
| Nishikawa et al., 201834 |
42 |
F |
1.9 x 2.0 x 0.5–cm intrasellar mass, lt CS invasion, optic chiasm compression |
Resection; chemo: temozolomide; stereotactic RT x2 (total 30 Gy); after recurrence: resection; chemo: paclitaxel; RT |
Death at 11 mos |
| Paolini et al., 201835 |
31 |
F |
Heterogeneously enhancing sellar/suprasellar lesion |
Resection |
Death at 2 mos |
| 36 |
F |
NR |
Resection; unspecified chemo; RT |
Alive at 22 mos |
|
| 46 |
F |
NR |
2nd resection |
Death (postop) |
|
| 47 |
F |
NR |
Resection; chemo: 3 agents (NR); fractionated RT: total 20 Gy |
Alive at 62 mos |
|
| 65 |
F |
NR |
Resection; chemo: vincristine, cisplatin, doxorubicin, & cyclophosphamide; fractionated RT w/ cisplatin sensitizer (total 54 Gy) |
Death at 23 mos |
|
| Barresi et al., 201836 |
59 |
F |
2.3 x 1.2–cm heterogeneously enhancing sellar lesion; invasion into lt CS |
Resection; RT |
Death at 2 mos |
| Su et al., 201837 |
37 |
F |
Heterogeneously enhancing, 2.57 x 1.96 x 3.63–cm sellar/suprasellar lesion |
Resection |
Death at 3 mos |
| Barsky et al., 201838 |
54 |
F |
1.6 x 1.1 x 2.4–cm sellar/suprasellar lesion w/ edema |
Resection |
NR |
| Asmaro et al., 201939 |
62 |
F |
Heterogeneously enhancing sellar/suprasellar hemorrhagic lesion, w/ SAH & IVH |
Resection |
Death at 2 mos |
| Voisin et al., 201940 |
51 |
F |
Preop CT: lobulated heterogeneously enhancing suprasellar cystic lesion |
Resection; chemo: temozolomide; fractionated craniospinal RT: total 54 Gy; chemo: 1 dose ifosfamide, carboplatin, & etoposide |
Alive at 9 mos |
| Siddiqui et al., 201941 |
55 |
F |
Hemorrhagic sellar mass w/ SAH & IVH |
Resection |
Death at 1.5 mos |
| Lawler et al., 201942 |
27 |
F |
Enlarged pituitary fossa & gland w/ ill-defined lesion at floor of pituitary fossa |
Resection |
NR |
| Bokhari et al., 202043 |
40 |
F |
2.9 × 1.7 × 2.3–cm, sellar, enhancing cystic lesion |
Resection; chemo: NR; RT |
Death at 1 mo |
| Peng et al., 20212 |
43 |
F |
NR |
Resection; stereotactic RT: total 60 Gy |
Death at 4 mos |
| 52 |
F |
NR |
Resection |
Death at 2 mos |
|
| 50 |
F |
Cystic tumor |
Resection |
Death at 1 mo |
|
| 29 |
F |
NR |
Resection; chemo: cisplatin & dacabazide; RT: total 52 Gy |
Death at 8 mos |
|
| 70 |
F |
Tumor, hemorrhage |
Resection |
Death at 1 mo |
|
| Major et al., 20223 |
70 |
F |
1.8 x 2.2 x 1.7-cm heterogeneously enhancing sellar/suprasellar mass w/ invasion into rt CS encasing rt ICA |
Resection; chemo: carboplatin & etoposide; alternating intrathecal etoposide plus topotecan & intrathecal methotrexate, & thiotepa (3 cycles); fractionated external beam focal RT: total 30 Gy |
Death at 5.5 mos |
| Duan et al., 202244 |
47 |
M |
NR |
Resection; RT: Gamma Knife |
Death at 1 mo |
| 52 |
F |
NR |
2 resections |
Death at 2.5 mos |
|
| 47 |
F |
NR |
2 resections |
Death at 6 mos |
|
| 46 |
F |
NR |
Resection |
Death at 6 mos |
|
| 45 |
F |
NR |
Resection |
Death at 1 mos |
|
| 73 |
F |
NR |
Resection |
Death at 3 mos |
|
| 26 |
M |
NR |
2 resections |
Death at 3 mos |
|
| 41 |
F |
NR |
Resection |
Alive at 12 mos |
|
| Baiano et al., 20228 |
32 |
F |
Large sellar mass |
Resection; chemo: bleomycin (3 cycles) |
Death at 2 mos |
| 40 |
F |
Intra- & lt parasellar lesion |
Resection; chemo: bleomycin (3 cycles) |
Death at 2 mos |
|
| 41 |
F |
Intra-, infra-, supra-, & lt parasellar lesion w/ heterogeneous contrast enhancement, lt carotid artery encasement w/ extension to 3rd ventricle & lt tentorium of cerebellum infiltration |
Resection |
Death at 1 mo |
|
| 50 |
F |
Intra-, supra-, & lt parasellar lesion endowed w/ heterogeneous contrast enhancement & lt carotid artery encasement |
Resection; chemo: 6 cycles, 3 w/ vincristine, doxorubicin, cyclophosphamide alternating w/ 3 cycles of ifosfamide & etoposide |
Alive at 18 mos |
|
| Present case | 60 | F | 1.3-cm mass in sella w/ involvement of rt CS, no displacement of optic apparatus, Knosp grade 4 on rt & grade 1 on lt w/ no evidence of spinal metastasis | Resection; RT: total 54 Gy; chemo: cyclophosphamide, doxorubicin, vincristine, & etoposide | Alive at 6 mos |
Chemo = chemotherapy; CS = cavernous sinus; ICA = internal carotid artery; IVH = intraventricular hemorrhage; NR = not reported; RT = radiotherapy; SAH = subarachnoid hemorrhage.
Treatments administered to the patients are mentioned in the order they were given.
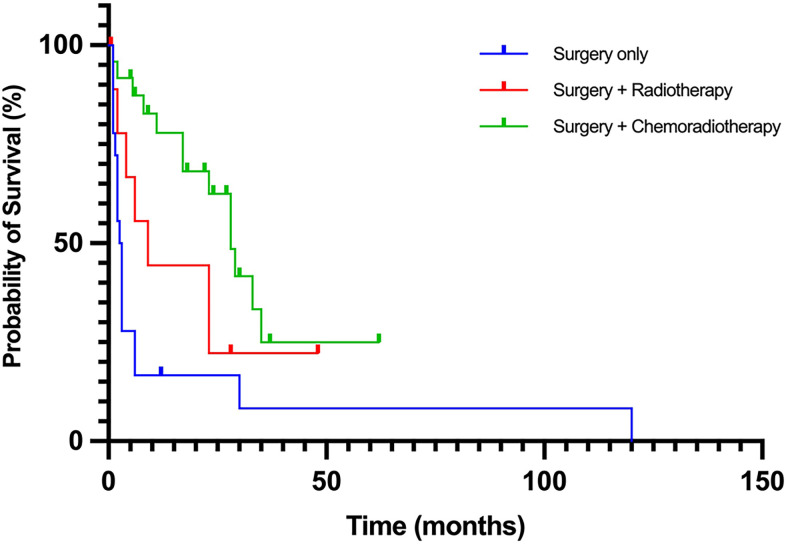
FIG. 4.
Kaplan-Meier survival curves comparing different treatment regimens in adult patients with atypical teratoid/rhabdoid tumor (ATRT). Log-rank test revealed a significant difference in survival time between patients treated with surgery alone and those treated with surgery followed by chemoradiotherapy (p < 0.001), but no significant difference between patients treated with surgery alone and those treated with surgery plus postoperative radiotherapy (p = 0.105). Cox proportional hazards analysis showed that patients undergoing surgery followed by chemoradiotherapy had a hazard ratio of 0.26 (95% confidence interval: 0.12–0.55, p < 0.001) compared to those who underwent only surgical intervention.
Statistical analysis using the log-rank test revealed a significant difference in survival time between patients treated with surgery alone and those treated with surgery followed by chemoradiotherapy (p < 0.001). However, no significant difference was observed between patients treated with only surgery and those treated with surgery plus postoperative radiotherapy (p = 0.105). Cox proportional hazards analysis was then used to calculate the hazard ratio (HR). The HR for patients undergoing surgery followed by chemoradiotherapy versus surgical intervention alone was 0.26 (95% confidence interval: 0.12–0.55; p < 0.001), indicating a significantly lower risk of death in patients who received combination therapy after surgery compared with those who only underwent surgical intervention. These findings suggest that the addition of postoperative chemoradiotherapy may improve survival outcomes in adult patients with ATRT. It should be noted that the choice of treatment, such as surgery with or without adjuvant therapy, is frequently determined based on the severity of the disease. However, in the cases reviewed, there was no clear indication of disease severity or the rationale behind treatment selection. This limitation is important to acknowledge when considering the survival analysis conducted in this study.
Patients who received chemoradiotherapy postoperatively demonstrated the longest median survival time of 22.5 months. Additionally, the results of the log-rank test revealed a significant difference in survival time between patients treated with only surgery and those treated with chemoradiotherapy, indicating that combination therapy may be the most effective treatment strategy for ATRT. These findings are consistent with previous reports in both children and adults, which have consistently shown that a combined radio-chemotherapy adjuvant approach is associated with better prognosis and longer survival.3,4
Lessons
In conclusion, our study indicates that early initiation of adjuvant chemoradiotherapy following surgery in adult patients with ATRT improves survival outcomes. However, because of the limited data on standardized treatment protocols for adults with ATRT, further research and larger-scale studies are necessary to validate these findings and establish effective treatment guidelines for this patient population.
Author Contributions
Conception and design: Garzon-Muvdi, Zamudio-Coronado, Zohdy. Acquisition of data: Garzon-Muvdi, Zamudio-Coronado. Analysis and interpretation of data: Zamudio-Coronado, Zohdy. Drafting of the article: Garzon-Muvdi, Zamudio-Coronado. Critically revising the article: Garzon-Muvdi, Zamudio-Coronado, Zohdy, Pradilla. Reviewed submitted version of the manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Garzon-Muvdi. Statistical analysis: Zohdy. Administrative/technical/material support: Zamudio-Coronado, Pradilla. Study supervision: Zamudio-Coronado, Pradilla.
References
- 1. Quinn TJ, Almahariq MF, Siddiqui ZA, et al. Trimodality therapy for atypical teratoid/rhabdoid tumor is associated with improved overall survival: a surveillance, epidemiology, and end results analysis. Pediatr Blood Cancer. 2019;66(12):e27969. doi: 10.1002/pbc.27969. [DOI] [PubMed] [Google Scholar]
- 2. Peng AJ, Fan SC, Chen YX, et al. Atypical teratoid/rhabdoid tumor in adult: case series and an integrated survival analysis. Br J Neurosurg. 2021:1–16. doi: 10.1080/02688697.2021.1885620. [DOI] [PubMed] [Google Scholar]
- 3. Major K, Daggubati LC, Mau C, Zacharia B, Glantz M, Pu C. Sellar atypical teratoid/rhabdoid tumors (AT/RT): a systematic review and case illustration. Cureus. 2022;14(7):e26838. doi: 10.7759/cureus.26838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Broggi G, Gianno F, Shemy DT, et al. Atypical teratoid/rhabdoid tumor in adults: a systematic review of the literature with meta-analysis and additional reports of 4 cases. J Neurooncol. 2022;157(1):1–14. doi: 10.1007/s11060-022-03959-z. [DOI] [PubMed] [Google Scholar]
- 5. Chan V, Marro A, Findlay JM, Schmitt LM, Das S. A systematic review of atypical teratoid rhabdoid tumor in adults. Front Oncol. 2018;8:567. doi: 10.3389/fonc.2018.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baliga S, Gandola L, Timmermann B, et al. Brain tumors: medulloblastoma, ATRT, ependymoma. Pediatr Blood Cancer. 2021;68(suppl 2):e28395. doi: 10.1002/pbc.28395. [DOI] [PubMed] [Google Scholar]
- 7. Lau CSM, Mahendraraj K, Chamberlain RS. Atypical teratoid rhabdoid tumors: a population-based clinical outcomes study involving 174 patients from the Surveillance, Epidemiology, and End Results database (1973-2010) Cancer Manag Res. 2015;7:301–309. doi: 10.2147/CMAR.S88561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baiano C, Della Monica R, Franca RA, et al. Atypical teratoid rhabdoid tumor: a possible oriented female pathology? Front Oncol. 2022;12:854437. doi: 10.3389/fonc.2022.854437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–1077. e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han ZY, Richer W, Fréneaux P, et al. The occurrence of intracranial rhabdoid tumours in mice depends on temporal control of Smarcb1 inactivation. Nat Commun. 2016;7:10421. doi: 10.1038/ncomms10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johann PD, Erkek S, Zapatka M, et al. Atypical teratoid/rhabdoid tumors are comprised of three epigenetic subgroups with distinct enhancer landscapes. Cancer Cell. 2016;29(3):379–393. doi: 10.1016/j.ccell.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 12. Nakata S, Nobusawa S, Hirose T, et al. Sellar atypical teratoid/rhabdoid tumor (AT/RT): a clinicopathologically and genetically distinct variant of AT/RT. Am J Surg Pathol. 2017;41(7):932–940. doi: 10.1097/PAS.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 13. Sredni ST, Tomita T. Rhabdoid tumor predisposition syndrome. Pediatr Dev Pathol. 2015;18(1):49–58. doi: 10.2350/14-07-1531-MISC.1. [DOI] [PubMed] [Google Scholar]
- 14. Slavc I, Chocholous M, Leiss U, et al. Atypical teratoid rhabdoid tumor: improved long-term survival with an intensive multimodal therapy and delayed radiotherapy. The Medical University of Vienna Experience 1992-2012. Cancer Med. 2014;3(1):91–100. doi: 10.1002/cam4.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuge A, Kayama T, Tsuchiya D, et al. Suprasellar primary malignant rhabdoid tumor in an adult: a case report. Article in Japanese. No Shinkei Geka. 2000;28(4):351–358. [PubMed] [Google Scholar]
- 16. Raisanen J, Biegel JA, Hatanpaa KJ, Judkins A, White CL, Perry A. Chromosome 22q deletions in atypical teratoid/rhabdoid tumors in adults. Brain Pathol. 2005;15(1):23–28. doi: 10.1111/j.1750-3639.2005.tb00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arita K, Sugiyama K, Sano T, Oka H. Atypical teratoid/rhabdoid tumour in sella turcica in an adult. Acta Neurochir (Wien) 2008;150(5):491–495. doi: 10.1007/s00701-008-1500-y. discussion 496. [DOI] [PubMed] [Google Scholar]
- 18. Las Heras F, Pritzker KP. Adult variant of atypical teratoid/rhabdoid tumor: immunohistochemical and ultrastructural confirmation of a rare tumor in the sella tursica. Pathol Res Pract. 2010;206(11):788–791. doi: 10.1016/j.prp.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 19. Schneiderhan TM, Beseoglu K, Bergmann M, et al. Sellar atypical teratoid/rhabdoid tumours in adults. Neuropathol Appl Neurobiol. 2011;37(3):326–329. doi: 10.1111/j.1365-2990.2010.01111.x. [DOI] [PubMed] [Google Scholar]
- 20. Chou S, Lo S, Wong H, et al. Atypical teratoid/rhabdoid tumour in the sella turcica of a female adult. Hong Kong J Radiol. 2013;16:65–68. [Google Scholar]
- 21. Moretti C, Lupoi D, Spasaro F, et al. Sella turcica atypical teratoid/rhabdoid tumor complicated with lung metastasis in an adult female. Clin Med Insights Case Rep. 2013;6:177–182. doi: 10.4137/CCRep.S12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park HG, Yoon JH, Kim SH, et al. Adult-onset sellar and suprasellar atypical teratoid rhabdoid tumor treated with a multimodal approach: a case report. Brain Tumor Res Treat. 2014;2(2):108–113. doi: 10.14791/btrt.2014.2.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shitara S, Akiyama Y. Atypical teratoid/rhabdoid tumor in sellar turcica in an adult: a case report and review of the literature. Surg Neurol Int. 2014;5:75. doi: 10.4103/2152-7806.133105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lev I, Fan X, Yu R. Sellar atypical teratoid/rhabdoid tumor: any preoperative diagnostic clues? AACE Clin Case Rep. 2015;1(1):e2–e7. [Google Scholar]
- 25. Biswas S, Wood M, Joshi A, et al. Exome sequencing of an adult pituitary atypical teratoid rhabdoid tumor. Front Oncol. 2015;5:236. doi: 10.3389/fonc.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Regan J, Tehrani M, Rodriguez F, Watson J. Adult At/Rt: predilection for the pituitary. J Neurol Neurosurg. 2015;2(1):110. [Google Scholar]
- 27. Nobusawa S, Nakata S, Hirato J, et al. Atypical teratoid/rhabdoid tumor in the sella turcica of an elderly female with a distinct vascular pattern and genetic alterations. Virchows Arch. 2016;469(6):711–715. doi: 10.1007/s00428-016-2017-7. [DOI] [PubMed] [Google Scholar]
- 28. Almalki MH, Alrogi A, Al-Rabie A, Al-Dandan S, Altwairgi A, Orz Y. Atypical teratoid/rhabdoid tumor of the sellar region in an adult with long survival: case report and review of the literature. J Clin Med Res. 2017;9(3):216–220. doi: 10.14740/jocmr2922w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larrán-Escandón L, Mateo-Gavira I, Vilchez-López FJ, Gómez Cárdenas E, Aguilar Diosdado M. Pituitary apoplexy as presentation of atypical teratoid/rhabdoid tumor in an adult. Article in English and Spanish. Endocrinol Nutr. 2016;63(7):364–365. doi: 10.1016/j.endonu.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 30. Elsayad K, Kriz J, Samhouri L, et al. Long-term survival following additive radiotherapy in patients with atypical teratoid rhabdoid tumors. Article in English and German. Strahlenther Onkol. 2016;192(8):569–581. doi: 10.1007/s00066-016-0978-8. [DOI] [PubMed] [Google Scholar]
- 31. Dardis C, Yeo J, Milton K, et al. Atypical teratoid rhabdoid tumor: two case reports and an analysis of adult cases with implications for pathophysiology and treatment. Front Neurol. 2017;8:247. doi: 10.3389/fneur.2017.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pratt D, Mehta GU, Wang HW, Chittiboina P, Quezado M. A 47-year old female with a destructive sellar mass. Brain Pathol. 2017;27(2):241–242. doi: 10.1111/bpa.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johann PD, Bens S, Oyen F, et al. Sellar region atypical teratoid/rhabdoid tumors (ATRT) in adults display DNA methylation profiles of the ATRT-MYC subgroup. Am J Surg Pathol. 2018;42(4):506–511. doi: 10.1097/PAS.0000000000001023. [DOI] [PubMed] [Google Scholar]
- 34. Nishikawa A, Ogiwara T, Nagm A, et al. Atypical teratoid/rhabdoid tumor of the sellar region in adult women: is it a sex-related disease? J Clin Neurosci. 2018;49:16–21. doi: 10.1016/j.jocn.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 35. Paolini MA, Kipp BR, Sukov WR, et al. Sellar region atypical teratoid/rhabdoid tumors in adults: clinicopathological characterization of five cases and review of the literature. J Neuropathol Exp Neurol. 2018;77(12):1115–1121. doi: 10.1093/jnen/nly091. [DOI] [PubMed] [Google Scholar]
- 36. Barresi V, Lionti S, Raso A, Esposito F, Cannavò S, Angileri FF. Pituitary atypical teratoid rhabdoid tumor in a patient with prolactinoma: a unique description. Neuropathology. 2018;38(3):260–267. doi: 10.1111/neup.12440. [DOI] [PubMed] [Google Scholar]
- 37. Su HY, Su YFA. A 37-year-old woman with progressive right side ptosis for one month. Brain Pathol. 2018;28(3):441–442. doi: 10.1111/bpa.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barsky D, Hadelsberg U, Gonen L, Margalit N. P05.91 Sellar atypical teratoid rhabdoid tumor (ATRT) in an adult: a case report and review of the literature. Neuro Oncol. 2018;20(3) suppl:iii324–iii325. [Google Scholar]
- 39. Asmaro K, Arshad M, Massie L, Griffith B, Lee I. Sellar atypical teratoid/rhabdoid tumor presenting with subarachnoid and intraventricular hemorrhage. World Neurosurg. 2019;123:e31–e38. doi: 10.1016/j.wneu.2018.10.198. [DOI] [PubMed] [Google Scholar]
- 40. Voisin MR, Ovenden C, Tsang DS, et al. Atypical teratoid/rhabdoid sellar tumor in an adult with a familial history of a germline SMARCB1 mutation: case report and review of the literature. World Neurosurg. 2019;127:336–345. doi: 10.1016/j.wneu.2019.04.083. [DOI] [PubMed] [Google Scholar]
- 41. Siddiqui M, Thoms D, Samples D, Caron J. Atypical teratoid/rhabdoid tumor presenting with subarachnoid and intraventricular hemorrhage. Surg Neurol Int. 2019;10:139. doi: 10.25259/SNI-59-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lawler K, Robertson T. A rare case of sellar region atypical teratoid/rhabdoid tumour in an adult female. Pathology. 2019;51:S89. [Google Scholar]
- 43. Bokhari RA, Bafaqeeh M, Al-Obaysi S, Al-Aman A, Alshakweer W. Atypical teratoid/rhabdoid tumor of the sellar region: a case report and review of the literature. J Neurol Res. 2020;10(1):13–16. [Google Scholar]
- 44. Duan Z, Yao K, Yang S, et al. Primary adult sellar SMARCB1/INI1-deficient tumor represents a subtype of atypical teratoid/rhabdoid tumor. Mod Pathol. 2022;35(12):1910–1920. doi: 10.1038/s41379-022-01127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]