Abstract
BACKGROUND
Cerebral meningiomas and brain abscesses are common independently, but intrameningioma abscesses rarely occur, with only 15 cases in the literature. These abscesses most frequently develop in patients with a known source of bacteremia; only one case of intrameningioma abscess without a known source of infection has been reported previously.
OBSERVATIONS
This is the second reported case of an intrameningioma abscess without a clear source of infection, occurring in a 70-year-old female with a history of transsphenoidal craniopharyngioma resection and radiation many years prior. She presented with severe fatigue and altered mental status initially ascribed to adrenal insufficiency, and magnetic resonance imaging showed a new heterogeneously enhancing left temporal mass with surrounding edema. After urgent tumor resection, pathology demonstrated a World Health Organization grade II meningioma (radiation induced). After a course of steroids and intravenous nafcillin, the patient recovered without neurological deficits.
LESSONS
The natural history of intrameningioma abscesses is not fully understood. These uncommon lesions can form secondary to hematogenous spread facilitated by meningiomas’ robust vascularization, typically in patients with bacteremia. Even when no significant source of infection is identified, the differential diagnosis of intrameningioma abscess should be considered because this pathology can be rapidly progressive, even fatal, but is treatable if recognized promptly.
Keywords: abscess, intratumoral, meningioma, atypical, radiation induced
ABBREVIATIONS: CT = computed tomography, ICP = intracranial pressure, IV = intravenous, MRI = magnetic resonance imaging
Meningiomas are the most commonly diagnosed benign primary brain mass, accounting for more than 35% of all brain neoplasms diagnosed annually.1 In the Western world, brain abscesses occur at a rate of approximately 4 per 1 million annually and are most commonly due to infection by Staphylococcus or Streptococcus bacteria.2 Peritumoral abscesses are historically associated with intra- or parasellar tumors and have predominantly originated from direct contact with the prenasal sinuses.3 However, in most reported cases of meningioma-associated abscess, sources of infection such as urosepsis or recent dental work suggest hematogenous seeding. Patients with bacteremia with brain meningiomas appear to be at increased risk of peritumoral abscess formation, according to a growing body of evidence.
Brain meningiomas and abscesses are common independently, but intrameningioma abscesses are rare, with only 15 cases previously described in the literature.4–21 In all but one of these cases, either a known source of infection or a likely cause of infection was identified.20
One prior case describes a “sterile” abscess within a meningioma, or an abscess without microbial growth from its samples. This abscess was attributed to spontaneous necrosis related to hormone therapy in the treatment of prostate cancer.20 In the present case, a 70-year-old female who had presented with altered mental status in the setting of a previously resected craniopharyngioma complicated by adrenal insufficiency, panhypopituitarism, and diabetes insipidus was found to have an intrameningioma abscess despite lacking infectious stigmata, including leukocytosis and fever. Thus, we present the second case of intrameningioma abscess without a significant preceding infectious source.
Illustrative Case
A 70-year-old female had a history of hypertension and craniopharyngioma, which had been resected transsphenoidally in 1994 with subsequent radiation therapy, complicated by panhypopituitarism with diabetes insipidus. Further history included an episode of left orbital cellulitis with epidural abscess in 2016, requiring evacuation, and prior cholecystectomy and hysterectomy. The patient presented to an outside hospital emergency department in April 2021 because of increasingly severe fatigue and progressive altered mental status. Upon arrival, she was found to be hypotensive, tachycardic, and tachypneic, though afebrile and without leukocytosis. She was admitted to the intensive care unit, given concerns for adrenal insufficiency. Noncontrast head computed tomography (CT) revealed a lobulated mass in the left temporal lobe measuring 4.1 × 3.8 × 3.7 cm with surrounding vasogenic edema, consistent with meningioma (Fig. 1). Brain magnetic resonance imaging (MRI) also showed a lobulated left temporal mass, which was isointense to cortex on T1- and T2-weighted sequences, which did not restrict diffusion, suggestive of atypical meningioma. The patient was started on steroids and intravenous (IV) fluids. Blood cultures at this time were positive for Staphylococcus hominis growth in two of four anaerobic culture bottles, and the patient was started on vancomycin for infection of unknown origin. Subsequent blood cultures revealed no growth, and the original culture growing S. hominis was presumed a contaminant. Following resolution of her symptoms, the patient was discharged on hospital day 4 with a steroid taper and plans for elective meningioma resection in the near future.
FIG. 1.
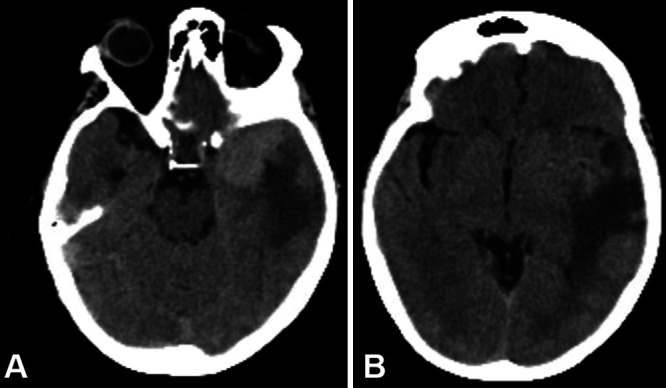
A: Axial noncontrast CT revealing a left temporal lobe lobulated mass measuring 4.1 × 3.8 × 3.7 cm with surrounding vasogenic edema, consistent with meningioma. B: Same CT scan demonstrating extent of vasogenic edema caused by the mass lesion and extending into the left parietal lobe.
The patient presented again 4 days later with altered mental status beginning as lethargy and malaise that progressed rapidly to somnolence. Physical examination revealed a newly proptotic and injected left eye, at which time empirical treatment for meningitis with vancomycin and ceftriaxone was initiated. Repeat cranial CT studies revealed a significant increase in the size of the left temporal lesion (now measuring 3.6 × 4.0 × 3.5 cm) with central necrosis and associated edema (Fig. 2). Brain MRI revealed a heterogeneously enhancing lobulated mass with new central diffusion restriction in the left anterior temporal lobe, thought to represent acute tumor necrosis or possible abscess (Fig. 3). This pattern of central diffusion restriction corresponded with the hypoenhancing center of the mass demonstrated on T1 sequencing. Peritumoral inflammation involving the left lateral rectus muscle was also noted. Last, MRI showed an interval increase in ventricular caliber, raising concern for evolving hydrocephalus. Because of the patient’s poor neurological status and imaging findings suggestive of peritumoral abscess, she was taken urgently to the operating room in May 2021 for left frontotemporal craniotomy for resection of her temporal fossa meningioma.
FIG. 2.

Axial noncontrast CT scans (A, B) revealing a significant interval increase in the size of the left temporal lesion with central necrosis and associated edema.
FIG. 3.

Brain MRI revealing new central restricted diffusion in the left anterior temporal lobe, thought to represent acute tumor necrosis or possible abscess, and interval increase in ventricular caliber. A: Axial postcontrast T1-weighted image demonstrating a maximum tumor diameter of 33.1 mm. B: Axial postcontrast T1-weighted image demonstrating heterogeneous lesion enhancement. C: Diffusion-weighted imaging demonstrating left temporal diffusion restriction. D: Apparent diffusion coefficient imaging correlating to the left temporal diffusion restriction seen in panel C.
Intraoperatively, the meningioma appeared to originate from the left anterior temporal fossa medial wall. The meningioma was debulked and found to contain central abscess or necrosis, a sample of which was sent for pathology. The anterior temporal lobe appeared red and hyperemic. Bipolar cautery was used to coagulate any fragments adherent to the medial wall.
Preliminary pathology reports indicated the mass was a meningioma with necrosis and white blood cells. Because of this, intraoperative specimens of the center of the mass were sent for microbiology, which grew S. hominis in broth only. The final pathology report was consistent with World Health Organization grade II atypical meningioma with abundant necrosis and neutrophilic inflammation (Fig. 4). Other histological features included elevated mitotic activity, prominent nucleoli, and necrosis. Significant inflammation was present within areas of necrosis.
FIG. 4.

Histology of meningioma, central nervous system (CNS) World Health Organization (WHO) grade II, with evidence of abscess on hematoxylin and eosin stains. A: Low-power image of the mass showing a distinct zonal pattern, with abundant geographic necrosis and neutrophils present in the upper left, and CNS WHO grade II meningioma histology in the lower right. B: High-power examination of the meningothelial component of the mass shows round to oval meningothelial cells with occasional mitoses present (arrow). C: In addition to elevated mitotic activity (up to 4 per high-power field), atypical features including necrosis and prominent nucleoli (not pictured) were present. D: High-power examination of the area in the upper left of panel A shows abundant neutrophilic inflammation consistent with abscess. No definitive organisms were identified by histological examination.
The patient’s course was followed closely by the infectious disease team throughout the admission. The finding for serum Aspergillus antigen was positive from the May 2021 sample, and the patient was started on voriconazole therapy to address this finding. Intraoperative brain tissue culture results were positive for S. hominis. The patient was discharged home with 6 weeks of IV nafcillin therapy, and she initially recovered well. Postoperative imaging at 2 months showed gross-total resection with expected changes and no tumor or abscess recurrence. At the patient’s last follow-up with neuro-oncology in December 2021, she opted against further radiation treatment because of ongoing fatigue. She otherwise remained at her neurological baseline and continues to be seen in follow-up every 3 months.
Patient Informed Consent
Informed consent was not obtained in this case, as surgery was performed urgently because of increasing intracranial pressure (ICP).
Discussion
Observations
According to the literature, there are 18 reported cases of abscesses associated with cerebral meningioma (either peri- or intratumoral) and 15 cases of intrameningioma abcess4–21 (Table 1). Typically, these abscessed tumors are close to avenues of entry into the cranial vault—that is, near the skull base, sellar region, or sinuses.12 In all but one reported case, bacteria in the bloodstream was thought to seed the abscess. In the one case of abscess formation where there was no bacteremia, it was thought that hormone therapy related to prostate cancer treatment caused aberrant meningioma growth leading to spontaneous intratumoral necrosis and reactive inflammation.20 In the present case, the first set of blood culture results positive for S. hominis was initially attributed to a contaminant, because the patient had no leukocytosis or fever, no clear infective source, and the bacteria grew in only two of four culture bottles. For this reason, the patient was thus deemed appropriate for surgery by the infectious disease team; ultimately, however, intraoperative cultures of the abscess also isolated S. hominis. Thus, it is most likely that the abscess in this case was seeded hematogenously by a remote S. hominis bacteremia and that the initial positive culture results were not attributable to a contaminant. Regardless, assuming the initial S. hominis cultures were due to contaminant delayed the administration of antibiotics. Even such an uncommon bacteria can seed a cerebral abscess and should prompt initiation of broad-spectrum antibiotic treatment and repeat cultures.
TABLE 1.
Prior cases of peri- or intrameningioma abscess
| Authors & Year | Age (yrs)/Sex | Tumor Location | Organism | Source of Infection |
|---|---|---|---|---|
| Shimomura et al., 19944 |
64/F |
Rt frontal |
Bacteroides oralis
|
GU surgery |
| Nassar et al., 19975 |
78/F |
Rt occipital |
Escherichia coli
|
UTI |
| Eisenberg et al., 19986 |
78/F |
Lt frontal |
E. coli
|
Sinus, GU surgery |
| Onopchenko et al., 19997 |
63/F |
Lt convexity |
Staphylococcus
|
Recent nephrectomy |
| Yeates et al., 20038 |
38/F |
Lt frontal |
Bacteroides fragilis
|
Recent vaginal hysterectomy |
| Lind et al., 20059 |
74/F |
Rt frontal |
Citrobacter koseri
|
HO AF |
| Young et al., 200510 |
38/M |
Rt temporal |
B Streptococcus, Peptostreptococcus |
Dental surgery |
| Krishnan et al., 201411 |
55/F |
Lt frontal convexity |
E. coli
|
HO DJ stent |
| Lo et al., 201412 |
70/F |
Rt parietal & lt frontal |
E. coli
|
Recent ureteroscopy/lithotripsy |
| Molière et al., 201513 |
65/F |
Lt occipital |
Nocardia
|
Unknown |
| Patibandla et al., 201714 |
35/M |
Rt lat ventricle |
Proteus mirabilis
|
UTI |
| Sannareddy et al., 201715 |
56/M |
Lt occipital |
E. coli
|
Unknown |
| Chandra et al., 201816 |
70/M |
Lt posterior frontal/parietal lobe |
Streptococcus constellatus
|
Unknown |
| Ponce-Ayala et al., 201817 |
63/M |
Lt hemispheric |
None identified |
NA |
| Sosa-Najera et al., 201818 |
56/M |
Rt parietal |
E. coli
|
Unknown |
| Christopher et al., 202019 |
75/M |
Known lt frontal & parietal meningiomas |
E. coli
|
UTI |
| Fabbri et al., 202020 |
76/M |
Rt convexity |
None identified |
NA |
| Rashed et al., 202121 |
52/F |
Rt occipital |
Staphylococcus aureus (MSSA) |
Unknown |
| Present case, 2021 | 70/F | Lt frontal | None identified | NA |
AF = atrial fibrillation; DJ = double J; GU = genitourinary; HO = history of; MSSA = methicillin-sensitive Staphylococcus aureus; NA = not applicable; UTI = urinary tract infection.
This initial assumption delayed treatment for the patient, a problem that could have been avoided by earlier recognition of the intratumoral abscess. The patient’s initial MRI did not raise concern for abscess, because the mass did not appear to restrict diffusion, nor were there other suggestive imaging features. However, the rapid progression of symptoms in this case might have prompted additional imaging sooner than at the time of the patient’s re-presentation to the hospital (8 days following her initial MRI). Earlier repeat interval imaging would likely have shown some diffusion restriction within the mass, which may have raised earlier concern for a possible developing abscess, especially in a febrile patient. When repeat imaging was performed 8 days after the first MRI, the new central diffusion restriction was interpreted by radiology as “favored to represent acute tumor necrosis” because it was mild, instead of the avid diffusion restriction, which would be more typical of a cerebral abscess. This underscores the importance of considering imaging findings in the greater context of a patient’s clinical presentation. The patient’s mental status (progressing quickly from malaise to somnolence) should have signaled the presence of a more rapidly expanding mass lesion than would have been expected from a meningioma.
The patient had several risk factors predisposing her to intrameningioma abscess formation. She was at increased risk for meningioma development due to prior radiation and increased risk for craniofacial infection, given her prior episode of orbital cellulitis and epidural abscess (ipsilateral to the proptotic and injected left eye described in this case). Although her precise risk factors for abscess formation remain uncertain, this previous history of infection, previous brain surgery, and steroid-induced chronic immunosuppression likely all contributed to her clinical presentation. Her presentation was similar to that of a simple (not peritumoral) brain abscess: largely due to increased ICP, cerebral abscesses tend to present with headache, nausea and vomiting, lethargy, and confusion.22 Their presentation is similar to that of any intracranial mass lesion but typically with more rapidly developing symptoms, and there are no findings specific only to cerebral abscess.22 Cerebral abscesses associated with meningiomas appear to have a somewhat more characteristic presentation, which, though variable and nonspecific, can include neurological deficits, headaches, dizziness, nausea, and vomiting.12 One review of the literature on meningioma-associated cerebral abscesses notes that focal neurological deficits and signs of increased ICP are often less prevalent than those in nontumoral cerebral abscesses.12 Other signs associated more specifically with intrameningioma abscess can include fever, migraine, altered sensation, and multiple seizures.18
Prior studies have postulated that the increased vascularity, lack of protection from the blood–brain barrier due to increased fenestration, and blood flow pattern within the neoplasm increase the risk of peritumoral abscess development specific to meningioma.4 Because of their robust blood supply, meningiomas are more susceptible than other tumors to hematogenous seeding, especially by metastatic cancer cells in the bloodstream.23 Bacteria and other microbes may be just as likely to be retained by the highly vascular channels of meningioma, which are often nonbranching until they reach the meningioma’s center.14 The lipid- and glycoprotein-rich tissue in the tumor center provides fertile ground for abscess formation; this paired with the lack of immune response intratumorally provides a highly favorable environment for abscess development.14,18 In our patient, without a clearer inciting event or infective source, we believe that the long-term use of steroids likely contributed to a relatively immunocompromised state, which facilitated abscess formation secondary to S. hominis bacteremia. Long-term steroid use has been linked to abscess development in glioblastoma multiforme in several cases, and, certainly, immunosuppression is a risk factor for cerebral abscess formation in general.24,25
Lessons
Peritumoral brain abscesses are uncommon, but the number of reported cases of intrameningioma abscesses is growing. These lesions likely form as a result of meningiomas’ robust vascularization facilitating hematogenous spread, most often in patients with bacteremia with a known source of infection. However, even when an infective source is not clearly identified in a patient, the differential of perimeningioma abscess should be considered.
This is the second reported case of intrameningioma abscess without a clear cause for infection. The patient in this case had several risk factors for infection, including chronic use of steroids, prior craniofacial infection, and prior craniofacial surgery. In patients with meningiomas and clinical signs of infection, rapidly progressive symptoms should prompt suspicion for perimeningioma abscess. The diagnosis can be fatal but is easily treatable with timely and proper intervention (surgical excision and long-term antibiotics).
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Callahan, Tranmer. Acquisition of data: Beach, Casale, DeWitt. Analysis and interpretation of data: Beach, Callahan, DeWitt. Drafting the article: Beach, Callahan, Casale, DeWitt. Critically revising the article: Beach, Callahan, Casale, Tranmer. Reviewed submitted version of manuscript: Beach, Casale, Tranmer. Approved the final version of the manuscript on behalf of all authors: Beach. Administrative/technical/material support: Beach, Tranmer. MRI and CT image preparation: Beach.
References
- 1.Alruwaili AA, De Jesus O. StatPearls. StatPearls Publishing; Nov 30, 2022. Meningioma. [PubMed] [Google Scholar]
- 2.Bokhari MR, Mesfin FB. StatPearls. StatPearls Publishing; May 11, 2022. Brain abscess. [PubMed] [Google Scholar]
- 3. Campennì A, Caruso G, Barresi V, et al. Gliomas with intratumoral abscess formation: description of new cases, review of the literature, and the role of 99mTC-Leukoscan. Kaohsiung J Med Sci. 2015;31(7):377–383. doi: 10.1016/j.kjms.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shimomura T, Hori S, Kasai N, Tsuruta K, Okada H. Meningioma associated with intratumoral abscess formation – case report. Neurol Med Chir (Tokyo) 1994;34(7):440–443. doi: 10.2176/nmc.34.440. [DOI] [PubMed] [Google Scholar]
- 5. Nassar SI, Haddad FS, Hanbali FS, Kanaan NV. Abscess superimposed on brain tumor: two case reports and review of the literature. Surg Neurol. 1997;47(5):484–488. doi: 10.1016/s0090-3019(96)00146-2. [DOI] [PubMed] [Google Scholar]
- 6. Eisenberg MB, Lopez R, Stanek AE. Abscess formation within a parasagittal meningioma. Case report. J Neurosurg. 1998;88(5):895–897. doi: 10.3171/jns.1998.88.5.0895. [DOI] [PubMed] [Google Scholar]
- 7. Onopchenko EV, Grigorian IA. Meningioma with a peritumoral abscess. Vopr Neirokhir. 1999;(1):28–30. Article in Russian. [PubMed] [Google Scholar]
- 8. Yeates KE, Halliday W, Miyasaki J, Vellend H, Straus S. A case of ‘circling seizures’ and an intratumoral abscess. Clin Neurol Neurosurg. 2003;105(2):128–131. doi: 10.1016/s0303-8467(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 9. Lind CR, Muthiah K, Bok AP. Peritumoral Citrobacter koseri abscess associated with parasagittal meningioma. Neurosurgery. 2005;57(4):E814. doi: 10.1093/neurosurgery/57.4.e814. [DOI] [PubMed] [Google Scholar]
- 10. Young JP, Young PH. Meningioma associated with abscess formation – a case report. Surg Neurol. 2005;63(6):584–585. doi: 10.1016/j.surneu.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 11. Krishnan SS, Panigrahi M, Pattanagare SG, Varma RD, Rao SI. Abscess within a meningioma: pathogenesis and rare case report. Neurol India. 2014;62(2):196–198. doi: 10.4103/0028-3886.132398. [DOI] [PubMed] [Google Scholar]
- 12. Lo WB, Cahill J, Carey M, Mehta H, Shad A. Infected intracranial meningiomas. World Neurosurg. 2014;81(3–4):651.e9–651.e13. doi: 10.1016/j.wneu.2013.07.081. [DOI] [PubMed] [Google Scholar]
- 13. Molière S, Krémer S. When meningioma becomes an emergency: nocardial brain abscesses superimposed on meningioma. J Neuroradiol. 2015;42(4):249–251. doi: 10.1016/j.neurad.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 14. Patibandla MR, Addagada DC, Addagada GC. Meningioma with intratumoral abscess: review of literature. J Surg. 2017;2017:159. [Google Scholar]
- 15. Sannareddy R, Lath R, Padua R, et al. Meningioma with intra- and peritumoral abscess. Indian J Neurosurg. 2018;7:220–222. [Google Scholar]
- 16. Chandra V, Agarwal N, Zenonos GA, Zhang X, Hamilton RL, Gardner PA. Concomitant parasagittal meningioma and adjacent intracranial abscess of occult etiology. J Clin Neurosci. 2020;72:474–480. doi: 10.1016/j.jocn.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 17. Ponce-Ayala A, Carrizales-Rodríguez J, Ramírez-Loera C, et al. Anaplastic meningioma with intratumoral abscess; case report and literature review. Interdiscip Neurosurg. 2021;23:101007. [Google Scholar]
- 18. Sosa-Najera A, Solorio-Pineda S, Tafur-Grandett A, et al. Atypical abscessed parasagittal meningioma. Arch Neurocienc (Mex) 2018;23:35–41. [Google Scholar]
- 19. Christopher E, Moreton FC, Torgersen A, Foley P. Seeding of infection in previously asymptomatic meningioma. Pract Neurol. 2020;20(3):247–248. doi: 10.1136/practneurol-2019-002418. [DOI] [PubMed] [Google Scholar]
- 20. Fabbri VP, Asioli S, Palandri G. An unusual case of “sterile” abscess within low-grade meningioma during anti androgenic therapy and LH-releasing hormone agonist treatment for prostate cancer. Clin Neurol Neurosurg. 2020;196:105993. doi: 10.1016/j.clineuro.2020.105993. [DOI] [PubMed] [Google Scholar]
- 21. Rashed S, Vassiliou A, Ogborne R, McKenna G. Meningioma-associated abscess: an unusual case report and review of the literature. J Surg Case Rep. 2022;2022(1):rjab582. doi: 10.1093/jscr/rjab582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg MS. Handbook of Neurosurgery. 9th ed. Thieme; 2019. [Google Scholar]
- 23. Bucciero A, del Basso de Caro M, Vizioli L, Carraturo S, Cerillo A, Tedeschi G. Metastasis of breast carcinoma to intracranial meningioma. Case report and review of the literature. J Neurosurg Sci. 1992;36(3):169–172. [PubMed] [Google Scholar]
- 24. Noguerado A, Cabanyes J, Vivancos J, et al. Abscess caused by Salmonella enteritidis within a glioblastoma multiforme. J Infect. 1987;15(1):61–63. doi: 10.1016/s0163-4453(87)91476-9. [DOI] [PubMed] [Google Scholar]
- 25. Damek DM, Lillehei KO, Kleinschmidt-DeMasters BK. Aspergillus terreus brain abscess mimicking tumor progression in a patient with treated glioblastoma multiforme. Clin Neuropathol. 2008;27(6):400–407. doi: 10.5414/npp27400. [DOI] [PubMed] [Google Scholar]


