Abstract
Background
Genetic factors influence the risk of fatty liver disease (FLD) in adults. The aim of this study was to test if, and when, genetic risk factors known to affect FLD in adults begin to exert their deleterious effects during childhood, adolescence and early adulthood.
Methods
We included up to 4018 British children and adolescents from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort. Three genetic variants known to associate robustly with FLD in adults (PNPLA3 rs738409, TM6SF2 rs58542926 and HSD17B13 rs72613567) were tested for association with plasma levels of alanine transaminase (ALT) and aspartate transaminase (AST) during childhood (mean age: 9.9 years), early adolescence (15.5 years), late adolescence (17.8 years), and early adulthood (24.5 years). We also tested the associations of a 17-variant score and whole-genome polygenic risk scores (PRS) derived from associations in adults with plasma ALT and AST at the same four time points. Associations with elastography-derived liver steatosis and fibrosis were tested in early adulthood.
Results
Genetic risk factors for FLD (individually, combined into a 3-variant score, a 17-variant score and as a genome-wide PRS), were associated with higher liver enzymes, beginning in childhood and throughout adolescence and early adulthood. The ALT-increasing effects of the genetic risk variants became larger with increasing age. The ALT-PRS was associated with liver steatosis in early adulthood. No genetic associations with fibrosis were observed.
Conclusions
Genetic factors that promote FLD in adults associate with elevated liver enzymes already during childhood, and their effects get amplified with increasing age.
Keywords: Genetic risk scores, polygenic prediction, liver enzymes, NAFLD, ALSPAC
Key Messages.
Genetic risk factors for fatty liver disease begin to exert their effects already during early childhood.
The effects get larger with increasing age throughout childhood and adolescence.
Fatty liver disease in adulthood could partly be due to an ‘unmasking’ of genetic risk by factors that emerge during childhood and adolescence.
Introduction
Fatty liver disease (FLD) encompasses a spectrum of hepatic pathologies stemming from accumulation of fat inside the liver cells (steatosis). Hepatic steatosis can lead to inflammation of the liver tissue (steatohepatitis) that in turn can progress to fibrosis and cirrhosis of the liver: serious, irreversible diseases that are associated with high morbidity and mortality. Previously an uncommon disease mainly caused by habitual excessive alcohol consumption, FLD due to non-alcoholic causes has become very common in recent decades due to the rising rates of obesity. Approximately 25% to 45% of adults have steatosis, the first stage of the FLD spectrum, and between 3% and 5% have non-alcoholic steatohepatitis.1–3
Mirroring the obesity pandemic in adults, the prevalence of paediatric obesity is rapidly growing throughout the world.4,5 In parallel with this, FLD has become the most common liver disease affecting children. A worrying aspect of paediatric FLD is that the disease is likely to persist into adulthood, conferring a substantial cumulative risk of progressing to chronic liver disease at a young age. Recent data from a study of young British adults (mean age 24 years) found that among those with obesity (body mass index >30 kg/m2), half had severe hepatic steatosis, and among those with severe steatosis, 5% had hepatic fibrosis.6
Approximately half of the interindividual variation in propensity to develop FLD in adults is due to genetic factors. So far, genome-wide association studies (GWAS) have identified eight different genetic variants that associate robustly with liver fat content7–11, 15 associated with chronic liver disease12–15 and 172 that associate with elevated plasma levels of alanine transaminase (ALT), a biochemical marker of liver cell damage.16–18 The most widely replicated of these variants are in the genes PNPLA3, TM6SF2 and HSD17B13. Homozygous carriers of the risk-increasing variants at each of these three loci have an approximately 2–3-fold higher risk of FLD and/or chronic liver disease as compared with non-carriers.19
It remains unclear if the above-mentioned genetic risk variants exert their effects from birth or from some later time point during childhood or adolescence. This is important, because the timing of these effects may shed light on the underlying biological pathways and provide clues to potentially permissive non-genetic factors (e.g. obesity, sex hormones or alcohol intake). Recent studies of the genetics of obesity during childhood have revealed a striking heterogeneity in onset and duration of the effects of the individual obesogenic variants. Some were associated with body mass index (BMI) at birth, others during infancy and early childhood (but not in adulthood) and some first began to exert their effects at 8 years of age.20
The aim of this study was to test if genetic susceptibility factors for FLD display similar age-varying association patterns. The study cohort included up to 4018 British children and adolescents from the Avon Longitudinal Study of Parents and Children (ALSPAC) study. We examined the associations of the three variants known to associate robustly with FLD in adults (in PNPLA3, TM6SF2 and HSD17B13) with liver enzymes during childhood (mean age: 9.9 years), early adolescence (15.5 years), late adolescence (17.8 years) and early adulthood (24.5 years). Furthermore, we tested the associations of a 17-variant score and polygenic risk scores (derived from associations in adults) with liver enzymes at the same four time points.
Methods
The Avon Longitudinal Study of Parents and Children
ALSPAC is a population-based cohort investigating genetic and environmental factors that affect the health and development of children. The study methods are described in detail elsewhere.21,22 In brief, 14 541 pregnant women resident in the former region of Avon, UK, with an expected delivery date between 1 April 1991 and 31 December 1992, were eligible to take part in ALSPAC. Of these initial pregnancies there was a total of 14 676 foetuses, resulting in 14 062 live births and 13 988 children who were alive at 1 year of age. Please note that the study website contains details of all the data that are available through a fully searchable data dictionary and variable search tool [http://www.bris.ac.uk/alspac/researchers/our-data/]. Written informed consent was obtained for all study participants. Ethical approval for this study was obtained from the ALSPAC Ethics and Law Committee and the local research ethics committees. All research was conducted in accordance with both the Declarations of Helsinki and of Istanbul.
In ALSPAC, there were data available on liver biomarkers at four time points over the life course, measured during clinic visits: ‘childhood’—mean age = 9.9 years clinic (range = 8.9 to 11.5 years); ‘mid-adolescence’—mean age = 15.5 years clinic (range = 14.3 to 17.7 years); ‘late adolescence’—mean age = 17.8 years clinic (range = 16.3 to 20 years); and ‘early adulthood’— mean age = 24.5 years clinic (range = 22.4 to 26.5 years). Measures for ALT and aspartate transaminase (AST) were obtained at each time point. Biomarker levels from the childhood time point at mean age 9.9 years were derived using non-fasting blood samples, whereas all other time points were based on samples after participants had not eaten or drunk anything apart from water for at least 6 h. Lipoprotein lipid levels [high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides] were also available as part of these biomarker panels. Measures of body mass index (BMI) and dual-energy X-ray absorptiometry (DXA)-assessed fat mass were also derived at each time point. BMI was calculated using height measured to the nearest 0.1 cm using a Harpenden stadiometer (Holtain Crosswell), and weight was measured to the nearest 0.1 kg on Tanita electronic scales. Whole-body DXA scans were conducted using a Lunar Prodigy fan-beam densitometer using paediatric software as described previously.23 Additionally, a subset of the participants examined at mean age = 24.5 years had hepatic fibrosis and steatosis assessed by transient elastography (FibroScan 502 Touch; Echosens, Paris, France).
Genome-wide association studies in the UK Biobank
Genome-wide association studies (GWAS) were undertaken in the UK Biobank (UKB) on ALT (field #30620) and AST (field #30650) to identify weights for genetic and whole-genome polygenic risk scores (PRS) (Supplementary Table S1, available as Supplementary data at IJE online). The analysis protocol for these GWAS have been described previously in detail.24 Briefly, UKB participants of non-European descent based on K-means clustering (K = 4) were excluded from analyses along with individuals with withdrawn consent, mismatch between genetic and reported sex, and putative sex chromosome aneuploidy. GWAS were conducted using the BOLT-LMM software that applied a linear mixed model to account for population structure and cryptic relatedness in UKB.25 Analyses were undertaken after applying rank inverse normal transformations to liver traits with adjustment for age and sex, resulting in final sample sizes of n = 441 209 (ALT) and n = 439 757 (AST).
Genotypes and genetic risk scores
Three genetic variants were selected based on previous studies that had elucidated their role in FLD.8,10,12 These single nucleotide polymorphisms (SNPs) were rs738409 (PNPLA3), rs58542926 (TM6SF2) and rs13130041 (HSD17B13), which were selected because they were previously used to construct a genetic risk score for FLD in an adult population.19 rs13130041 was identified as a proxy for the original SNP rs72613567 as this was not available in the ALSPAC genotype data (r2=0.95). Whereas the variants in PNPLA3 and TM6SF2 influence the entire FLD spectrum, the HSD17B13 variant only affects the later stages of the disorders [non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis] but not steatosis per se.12 We also selected 17 variants robustly associated with elevated ALT and with imaging or histologically defined FLD16 (Supplementary Table S2, available as Supplementary data at IJE online).
We conducted four analyses. First, we analysed the association between the three variants in PNPLA3, TM6SF2 and HSD17B13 individually against ALSPAC measures of ALT and AST, stratified by age. Second, to increase power we combined the three variants (each weighted by its effects on ALT and AST in UKB) into weighted genetic risk scores. We tested the association between increasing genetic risk score and ALT and AST in ALSPAC, again stratified by age. Third, we tested the association with ALT of a 17-variant weighted genetic risk score based on the 17 variants robustly associated with FLD mentioned above. The weights of the individual variants in this risk score were derived from beta ALT as reported in the GWAS. Our fourth set of analyses was based on whole-genome PRS for ALT and AST, based on the full set of genome-wide results from our UKB GWAS. PRS were constructed by applying linkage disequilibrium (LD) clumping to our UKB GWAS results to identify genetic variants based on an r2<0.1, window distance of 1000 kb and P <0.05. A reference panel of 10 000 random UKB European participants was used to estimate correlation of variants for LD clumping.26 PRS were then built using ALSPAC genotype data for ALT and AST as separate scores, by summing trait-increasing alleles weighted by their GWAS effect estimates. PLINK v2.0 was used for all LD clumping and PRS construction.27
Statistical analysis
Rank inverse normal transformations were applied to all liver biomarker data and measures of fibrosis in ALSPAC prior to analyses. The association between each of the three genetic variants was then evaluated with ALT and AST in turn in ALSPAC, using linear regression with adjustment for age and sex. The genotypes were coded 0, 1 and 2 for number of ALT-increasing alleles in the single variant analyses. Previous analyses have demonstrated that adjusting for principal components in ALSPAC does not make meaningful differences to results, given that study participants were recruited from the same geographical area (county of Avon in the UK).28 We therefore did not adjust for principal components in the present study. The three-variant and 17-variant scores and whole-genome PRS were entered as continuous variables in linear regression.
Evaluations of an interaction with time points in ALSPAC were conducted by stacking liver biomarker observations (i.e. so that each individual had multiple measures) and then regressing this against genetic variables with adjustment for age, sex and time point. Analyses were then repeated, along with adjustment for the interaction between genetic variables and time point as undertaken previously,29 by stacking observations (i.e. so that individuals had multiple ALT/AST measurements) and then regressing the liver enzymes measures on genetic variables with adjustment for age, sex and time point. An inherent feature of quantitative interactions (i.e. interactions that affect the magnitude but not the direction of an association) is that they depend on the scale on which they are analysed. To assess the effects of scale transformations, we repeated all interaction tests using untransformed or logarithmically transformed values of ALT or AST.
Associations of the ALT-PRS with BMI and plasma lipid levels were tested at the four time points, using linear regression adjusted for age and sex. Furthermore, given previous evidence of an interaction between genetic variants which influence risk of FLD and body BMI,30 we subsequently included an interaction term between liver biomarkers and BMI in models to formally assess this. Analyses were repeated to evaluate interactions with DXA-assessed fat mass substituted for BMI in the model. Last, we evaluated the association between individual genetic variants, three-variant and 17-variant scores and PRS, and the Fibroscan-measures of fibrosis and steatosis taken at the early adulthood time point in ALSPAC, as before using linear regression adjusting for age and sex. Finally, we used the whole-genome PRS to stratify the ALSPAC population into deciles, and for their respective biomarkers assessed linear trends across groups using linear regression.
Figures in this paper were generated using the R package ‘ggplot2’.31 All analyses were conducted using R (version 3.5.3).
Results
Baseline characteristics
The baseline characteristics of up to 4018 participants from the ALSPAC cohort, stratified by age group, are given in Supplementary Table S3 (available as Supplementary data at IJE online).
PNPLA3, TM6SF2 and HSD17B13 variants and liver enzymes during childhood through to early adulthood
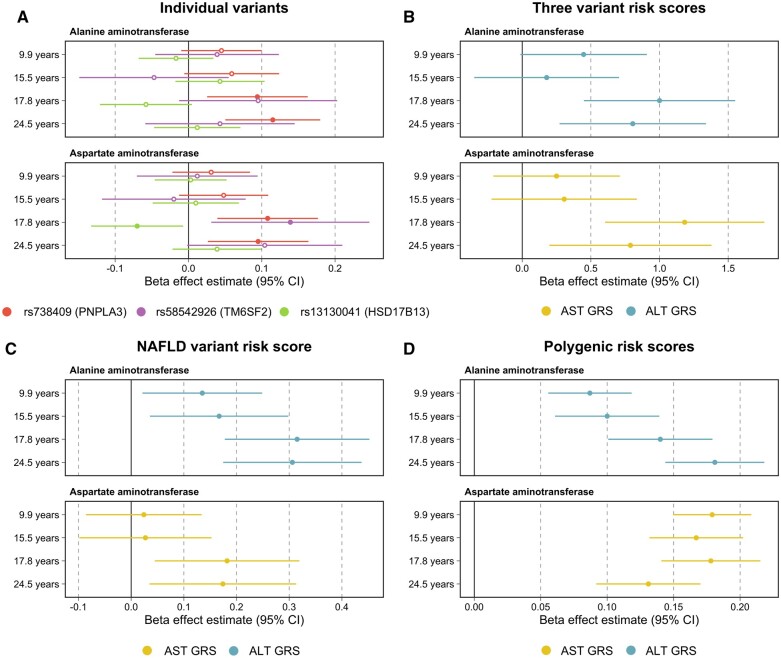
We first tested the associations of three variants known to associate robustly with FLD in adults. The strongest evidence of association was found between the PNPLA3 variant rs738409 and ALT measured during early adulthood (i.e. mean age = 24.5 years) (beta = 0.12 per SD change in ALT levels, 95% CI = 0.05 to 0.18, P = 5.8 × 10-4). Furthermore, we found that the magnitude of effect for this variant on both ALT and AST levels increased incrementally across time points in the ALSPAC cohort (Figure 1A). For example, the per-allele effect on inverse-normalized ALT was 0.045 at 9.9 years old, 0.059 at 15.5 years old, 0.094 at 17.8 years old and 0.115 by 24.5 years old (P for interaction between time and PNPLA3 genotype on ALT = 0.048). A similar, albeit less consistent, pattern of increasing effects on inverse-normalized AST with age was seen for the TM6F2-variant (P for interaction between time and TM6SF2 genotype on inverse normalized AST = 0.075). The interactions were similar when analysing ALT or AST either as raw, untransformed values or after logarithmical transformation (Supplementary Table S4, available as Supplementary data at IJE online). The full results underlying Figure 1A can be found in Supplementary Table S5 (available as Supplementary data at IJE online).
Figure 1.
Forest plots illustrating the effect estimates per one standard deviation change in liver enzymes, and corresponding 95% confidence intervals for A) single variant analyses encoded 0, 1 and 2 per ALT-increasing allele. Filled circles: P <0.05, unfilled circles: P ≥0.05. B) Genetic risk scores consisting of all three variants encoded additively per ALT-increasing allele. C) Genetic risk score consisting of 17 variants associated with ALT as well as histologically or imaging-verified fatty liver disease in adults. D) Polygenic risk scores weighted by effect estimates derived from UK Biobank genome-wide association studies for corresponding traits after rank inverse normal transformation. Analyses were conducted at four time points across childhood, adolescence and early adulthood using liver enzyme data from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort. ALT, alanine transaminase; GRS, genetic risk score; NAFLD, non-alcoholic fatty liver disease
Three-variant risk score and liver enzymes during childhood through to early adulthood
Evidence of an association between the three-variant genetic risk score and liver enzymes was strongest when analysing data from the late adolescent (i.e. mean age = 17.8 years) and early adulthood (i.e. mean age = 24.5 years) time points (Figure 1B). For example, the AST risk score was most strongly associated with AST levels during late adolescence (beta = 1.18 per SD change in AST levels, 95% CI = 0.60 to 1.76, P = 6.7 × 10-5). Evidence of an interaction with time for the three variant risk scores for inverse normalized ALT and AST was P = 0.062 and P = 0.021, respectively (Supplementary Table S4). The evidence for interaction was stronger when analysing ALT or AST on the untransformed scale or after logarithmical transformation (all P-values <0.02; Supplementary Table S4). The full results underlying Figure 1B can be found in Supplementary Table S6 (available as Supplementary data at IJE online).
The 17-variant risk score and plasma ALT during childhood through to early adulthood
Similarly, we found that the 17-variant risk score provided stronger evidence of association with plasma ALT at the late adolescent (beta = 0.32 per SD change in ALT levels, 95% CI = 0.18 to 0.45, P = 6.1 × 10-6) and early adulthood (beta = 0.31, 95% CI = 0.17 to 0.44, P = 6.0 × 10-6) time points. Comparatively, the association of this score with AST levels was weaker than with ALT, which was to be expected given that these 17 variants were identified based on elevated ALT levels (Figure 1C). Full results underlying this figure can be found in Supplementary Table S7 (available as Supplementary data at IJE online).
Genome-wide PRS and liver enzymes during childhood and early adulthood
There was strong evidence of an association for both the ALT and AST whole-genome PRS and their corresponding traits measured at each of the four time points in the ALSPAC cohort (Supplementary Table S8, available as Supplementary data at IJE online). The ALT-PRS and AST-PRS explained 0.2% to 1.5% of and 0.2% to 2.1% of the total variation in plasma ALT and AST, respectively, across the four time points (Supplementary Table S9, available as Supplementary data at IJE online). The association between the genome-wide ALT-PRS and ALT plasma levels increased with age and was strongest among the young adults (Figure 1C, P for interaction between time and PRS on inverse normalized ALT = 1.5 × 10-4). The evidence for interaction was similar when analysing ALT on the untransformed scale (P for interaction = 2.6 × 10-4) but was stronger when analysing ALT on the logarithmical scale (P for interaction = 2.1 × 10-7, Supplementary Table S4). In contrast, the association between the AST-PRS and AST was similar during childhood and adolescence, and slightly weaker during young adulthood (P for interaction between time and AST-PRS on inverse normalized AST = 0.136).
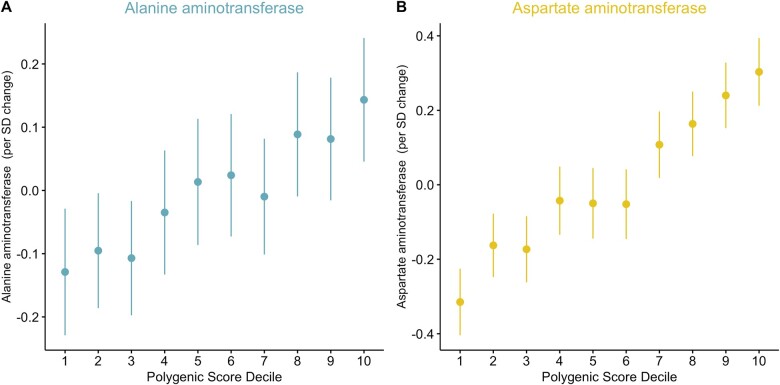
Using these genome-wide PRS to stratify the ALSPAC population resulted in a clear incremental trend observed across risk deciles for each ,as illustrated using data from the childhood (i.e. mean age = 9.9 years) in Figure 2. Plots generated using findings from the other three time points can be found in Supplementary Figures S1–S3 (available as Supplementary data at IJE online). Tests for linearity in effects on ALT and AST across deciles are shown in Supplementary Table S10 (available as Supplementary data at IJE online). The percentage of individuals with liver enzyme levels greater than the 95th percentile across each PRS decile can be found in Supplementary Table S11 (available as Supplementary data at IJE online).
Figure 2.
Error plots presenting the mean measurement and 95% confidence intervals for A) alanine aminotransferase and B) aspartate aminotransferase measured in a cohort of young individuals (mean age 9.9 years, range = 8.8 to 11.7 years) from the Avon Longitudinal Study of Parents and Children (ALSPAC) study, across deciles determined using whole-genome polygenic risk scores (PRS) for their corresponding traits. Weights for polygenic risk scores were derived using findings from genome-wide association studies undertaken in populations of adults (mean age 56.9 years, range = 39 to 73 years) enrolled in the UK Biobank study. Similar plots generated using findings from the other three timepoints can be found in Supplementary Figures S1–S3 (available as Supplementary data at IJE online).
Associations between genetic scores with BMI and plasma lipid levels during childhood and early adulthood
We found that the association between the genome-wide PRS for ALT and BMI increased in magnitude across time points in ALSPAC from mean age 9.9 years (beta = 0.28, 95% CI = 0.06 to 0.49, P = 0.01) to mean age 24.5 years (beta = 0.75, 95% CI = 0.44 to 1.06, P = 1.7 × 10-6). The ALT PRS was likewise associated with triglycerides most strongly at the early adulthood time point (beta = 0.57, 95% CI = 0.23 to 0.91, P = 9.6 × 10-4). In terms of individual genetic variants, the TM6SF2 variant (rs58542926) was associated consistently with LDL cholesterol across time points in ALSPAC (Supplementary Table S12, available as Supplementary data at IJE online).
Interaction between genetics and adiposity on liver enzymes during childhood through to early adulthood
Previous studies in adults have found that the effects of individual risk variants and polygenic risk scores for liver disease are robustly amplified by underlying obesity or insulin resistance (i.e. interaction). We attempted to identify similar interaction in the ALSPAC cohort. Fitting interaction terms between genetic variants (and scores) with measured BMI and DXA-assessed fat mass at each time point in the ALSPAC cohort provided little evidence that BMI may modulate the association of these genetic variables with the early life measures of liver biomarkers (Supplementary Table S13, available as Supplementary data at IJE online).
Genetic effects on liver fat and fibrosis at 24.5 years of age
Fibroscan-quantified hepatic steatosis and fibrosis were available in approximately n = 2400 from the young adulthood time point in ALSPAC (mean age: 24.5, range: 22.4 to 26.5). Evaluating the relationship between individual genetic variants and polygenic scores with measures of steatosis or fibrosis provided the strongest evidence of association when analysing the ALT PRS with the measure of steatosis based on the controlled attenuation parameter (CAP) (beta = 0.08 per SD change in CAP measure, 95% CI = 0.04 to 0.12, P = 2.5 × 10-5). However, no other findings from this analysis were robust to multiple testing comparisons (Supplementary Table S14, available as Supplementary data at IJE online).
Discussion
The main finding of this study is that genetic variants known to confer a higher risk of FLD in adults also associate with elevated liver enzymes during early childhood, adolescence and early adulthood. The liver enzyme-increasing effects of these variants were evident already among the 10-year-old children in our cohort and they seemed to become stronger with increasing age.
Unravelling the genetic underpinning of FLD has progressed rapidly during the past decade, mainly owing to the burgeoning of large-scale GWAS conducted in cohorts of adults. In the latest and largest of these, 172 different genetic loci were found to associate with elevated plasma ALT levels.17 Comparatively little is known about the genetic basis of FLD in children. The most robustly validated risk variants identified in adults have been found to confer risk of FLD in children too. For example, Goyal and colleagues recently reported that the PNPLA3 and TM6SF2 variants were associated with hepatic steatosis among 822 children with histologically verified non-alcoholic FLD.32 Earlier studies have reported similar results.33,34 The results reported in our study align with and extend the findings from these previous studies. Taken together, these data support the notion that genetic risk factors that promote the development of FLD exert their effects from childhood and throughout adolescence and early life.
An unexpected finding in our study was that the liver enzyme-increasing effects of the genetic variants increased with higher age. This was true for the individual variants (in PNPLA3 and TM6SF2), for a three-variant score comprising the risk alleles in PNPLA3, TM6SF2 and HSD17B13, a 17-variant score based on alleles with robust effects on FLD in adults, and most clearly for a genome-wide ALT-PRS. The mechanisms underlying these time-gene interactions are not obvious. We speculate that they might reflect the effects of certain permissive factors that gradually emerge during childhood and adolescence, effectively ‘unmasking’ the genetic risk factors.
In support of this notion is the fact that previous studies have consistently found that the deleterious effect of genetic risk factors for FLD in adults are amplified (i.e. ‘un-masked’) by the co-occurrence of environmental risk factors such as obesity, heavy alcohol intake or insulin resistance.19,30 These synergistic effects are among the most robust examples of gene-environment interaction documented in humans.35 Some moderately-sized studies have found that adiposity augments the steatogenic effect of the PNPLA3 variant in children.36,37 It is, however, unclear if these interactions are generalizable to all genetic risk variants during early life. This is an important question. If obesity ‘unmasks’ the genetic risk of FLD during early life, the rapidly rising prevalence of obesity in children could herald a future of widespread paediatric and adolescent FLD, especially among ethnic groups genetically predisposed to develop the disorder (e.g. Hispanics).10,38
There are limitations to our study that should be considered. First, with N = 4018 in the full cohort, power was limited for stratified sub-analyses. For example, although we were able to detect gene-time interactions affecting liver enzyme levels, we did not detect any evidence of BMI-gene or DXA-assessed fat mass-gene interactions. This may reflect lack of power, given that such interactions are well described in adults, and have been hinted at in previous studies of children.36,37 Additionally, we note that the estimates for our genetic scores derived from the UK Biobank are likely to suffer from ‘winner’s curse’ and that this source of bias may variably affect findings across time points in ALSPAC. Full comparisons of estimates derived in the UK Biobank with those derived in ALSPAC can be found in Supplementary Tables S15–S22 (available as Supplementary data at IJE online). Another limitation is that plasma levels of liver enzymes are neither specific nor sensitive markers of FLD. Whereas the variants in PNPLA3, TM6SF2 and HSD17B13 and those included in the 17-variant score are known to be robustly associated with ALT and FLD, we cannot rule out that some of the variants used in the whole-genome PRS (derived from associations with ALT or AST) may affect liver enzyme levels via other pathways than FLD. The exact fraction of ‘true FLD-variants’ in the PRS is unknown. That said, recent GWASs in adults have reported high correlations between genetic effects on plasma ALT and their effects on FLD.16 Finally, ALSPAC primarily (>95%) comprises children and adolescents of White European ethnicity recruited from the vicinity of Bristol in the UK. This potentially limits the generalizability of our findings to other ethnic groups and geographies.
Conclusion
In conclusion, the genetic risk factors that promote FLD in adults begin to exert their deleterious effects already during childhood, and these effects appear to grow stronger with increasing age. These data highlight the potential of FLD to become a major health problem among children, adolescents and young adults, potentially due to an ‘unmasking’ of heritable risk by permissive factors such as obesity.
Ethics approval
Written informed consent was obtained for all study participants. Ethical approval for this study was obtained from the ALSPAC Ethics and Law Committee and the local research ethics committees. All research was conducted in accordance with the Declarations of Helsinki and of Istanbul.
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council and Wellcome (grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. Genetic data were generated by Sample Logistics and Genotyping Facilities at the Wellcome Trust Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe. This research was conducted at the NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. This publication is the work of the authors and T.G.R. will serve as guarantor for the contents of this paper.
Contributor Information
Stefan Stender, Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
George Davey Smith, MRC Integrative Epidemiology Unit (IEU), Population Health Sciences, University of Bristol, Bristol, UK.
Tom G Richardson, MRC Integrative Epidemiology Unit (IEU), Population Health Sciences, University of Bristol, Bristol, UK.
Data availability
All individual-level data analysed in this study can be accessed via an approved application to ALSPAC [http://www.bristol.ac.uk/alspac/researchers/access/]and the UK Biobank study [https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access].
Supplementary data
Supplementary data are available at IJE online.
Author contributions
S.S.: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding. G.D.S.: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; obtained funding; administrative, technical or material support. T.G.R.: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding. All authors approved the final version of the draft to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Funding
This work was supported by the Integrative Epidemiology Unit which receives funding from the UK Medical Research Council and the University of Bristol (grant number MC_UU_00011/1); Independent Research Fund Denmark (grant number 9060-00012B) to SS; and Novo Nordisk Fonden (grant number NNF22OC0075038) to SS.
Conflict of interest
T.G.R. is an employee of GlaxoSmithKline outside this research. All other authors declare no conflict of interest.
References
- 1. Povsic M, Wong OY, Perry R, Bottomley J.. A structured literature review of the epidemiology and disease burden of Non-Alcoholic Steatohepatitis (NASH). Adv Ther 2019;36:1574–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M.. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 3. Browning JD, Szczepaniak LS, Dobbins R. et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–95. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Henry L.. Fatty liver through the ages: nonalcoholic steatohepatitis. Endocr Pract 2022;28:204–13. [DOI] [PubMed] [Google Scholar]
- 5. Li J, Ha A, Rui F. et al. Meta-analysis: global prevalence, trend and forecasting of non-alcoholic fatty liver disease in children and adolescents, 2000-2021. Aliment Pharmacol Ther 2022;56:396–406. [DOI] [PubMed] [Google Scholar]
- 6. Anderson EL, Howe LD, Fraser A. et al. Weight trajectories through infancy and childhood and risk of non-alcoholic fatty liver disease in adolescence: the ALSPAC study. J Hepatol 2014;61:626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haas ME, Pirruccello JP, Friedman SN. et al. Machine learning enables new insights into genetic contributions to liver fat accumulation. Cell Genom 2021;1:100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kozlitina J, Smagris E, Stender S. et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2014;46:352–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Basty N, Whitcher B. et al. Genetic architecture of 11 organ traits derived from abdominal MRI using deep learning. Elife 2021;10:e65554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romeo S, Kozlitina J, Xing C. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Speliotes EK, Yerges-Armstrong LM, Wu J. et al. ; GOLD Consortium. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet 2011;7:e1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abul-Husn NS, Cheng X, Li AH. et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med 2018;378:1096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buch S, Stickel F, Trepo E. et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet 2015;47:1443–48. [DOI] [PubMed] [Google Scholar]
- 14. Emdin CA, Haas M, Ajmera V. et al. Association of genetic variation with cirrhosis: a multi-trait genome-wide association and gene-environment interaction study. Gastroenterology 2021;160:1620–33.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Innes H, Buch S, Hutchinson S. et al. Genome-wide association study for alcohol-related cirrhosis identifies risk loci in MARC1 and HNRNPUL1. Gastroenterology 2020;159:1276–89.e7. [DOI] [PubMed] [Google Scholar]
- 16. Vujkovic M, Ramdas S, Lorenz KM. et al. ; VA Million Veteran Program. A multiancestry genome-wide association study of unexplained chronic ALT elevation as a proxy for nonalcoholic fatty liver disease with histological and radiological validation. Nat Genet 2022;54:761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen VL, Du X, Chen Y. et al. Genome-wide association study of serum liver enzymes implicates diverse metabolic and liver pathology. Nat Commun 2021;12:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chambers JC, Zhang W, Sehmi J. et al. ; Meta-analyses of Glucose and Insulin-Related Traits Consortium (MAGIC). Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet 2011;43:1131–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gellert-Kristensen H, Richardson TG, Davey Smith G, Nordestgaard BG, Tybjaerg-Hansen A, Stender S.. Combined effect of PNPLA3, TM6SF2, and HSD17B13 variants on risk of cirrhosis and hepatocellular carcinoma in the general population. Hepatology 2020;72:845–56. [DOI] [PubMed] [Google Scholar]
- 20. Helgeland O, Vaudel M, Sole-Navais P. et al. Characterization of the genetic architecture of infant and early childhood body mass index. Nat Metab 2022;4:344–58. [DOI] [PubMed] [Google Scholar]
- 21. Boyd A, Golding J, Macleod J. et al. Cohort Profile: ‘The children of the 90s' the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013;42:111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fraser A, Macdonald-Wallis C, Tilling K. et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013;42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ness AR, Leary SD, Mattocks C. et al. Objectively measured physical activity and fat mass in a large cohort of children. PLoS Med 2007;4:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richardson TG, Sanderson E, Palmer TM. et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med 2020;17:e1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loh PR, Tucker G, Bulik-Sullivan BK. et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet 2015;47:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kibinge NK, Relton CL, Gaunt TR, Richardson TG.. Characterizing the causal pathway for genetic variants associated with neurological phenotypes using human brain-derived proteome data. Am J Hum Genet 2020;106:885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ.. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richardson TG, O'Nunain K, Relton CL, Davey Smith G.. Harnessing whole genome polygenic risk scores to stratify individuals based on cardiometabolic risk factors and biomarkers at age 10 in the lifecourse-brief report. Arterioscler Thromb Vasc Biol 2022;42:362–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richardson TG, Sanderson E, Elsworth B, Tilling K, Davey Smith G.. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: Mendelian randomisation study. BMJ 2020;369:m1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stender S, Kozlitina J, Nordestgaard BG, Tybjærg-Hansen A, Hobbs HH, Cohen JC.. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat Genet 2017;49:842–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ginestet C. ggplot2: elegant graphics for data analysis. J R Stat Soc Stat 2011;174:245–46. [Google Scholar]
- 32. Goyal NP, Rosenthal SB, Nasamran C. et al. ; NASH Clinical Research Network. Nonalcoholic fatty liver disease risk and histologic severity are associated with genetic polymorphisms in children. Hepatology 2023;77:197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riccio S, Melone R, Vitulano C. et al. Advances in pediatric non-alcoholic fatty liver disease: from genetics to lipidomics. World J Clin Pediatr 2022;11:221–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nobili V, Alisi A, Valenti L, Miele L, Feldstein AE, Alkhouri N.. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat Rev Gastroenterol Hepatol 2019;16:517–30. [DOI] [PubMed] [Google Scholar]
- 35. Lyon MS, Millard LAC, Davey Smith G, Gaunt TG, Tilling K. Hypothesis-free detection of gene-interaction effects on biomarker concentration in UK Biobank using variance prioritisation. medRxiv; doi: 10.1101/2022.01.05.21268406, 5 January 2022. Preprint: not peer reviewed. [DOI]
- 36. Giudice EM, Grandone A, Cirillo G. et al. The association of PNPLA3 variants with liver enzymes in childhood obesity is driven by the interaction with abdominal fat. PLoS One 2011;6:e27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Romeo S, Sentinelli F, Cambuli VM. et al. The 148M allele of the PNPLA3 gene is associated with indices of liver damage early in life. J Hepatol 2010;53:335–38. [DOI] [PubMed] [Google Scholar]
- 38. Kubiliun MJ, Cohen JC, Hobbs HH, Kozlitina J.. Contribution of a genetic risk score to ethnic differences in fatty liver disease. Liver Int 2022;42:2227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All individual-level data analysed in this study can be accessed via an approved application to ALSPAC [http://www.bristol.ac.uk/alspac/researchers/access/]and the UK Biobank study [https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access].