Abstract
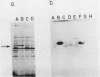
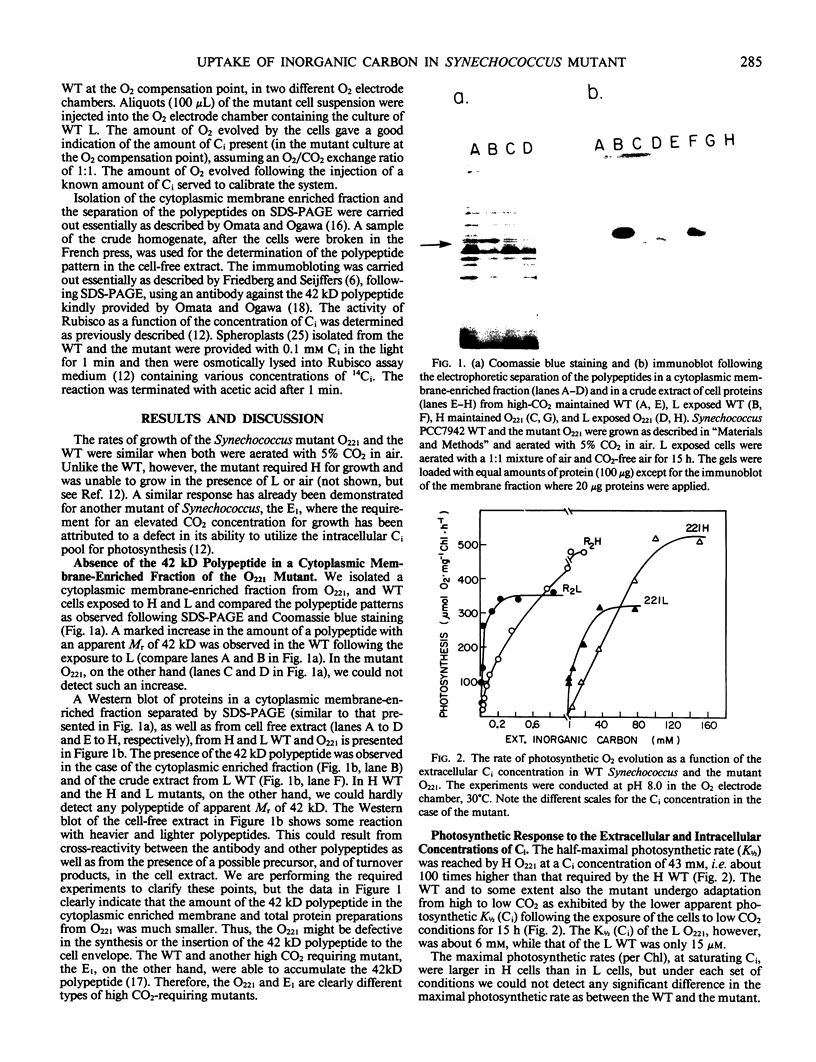
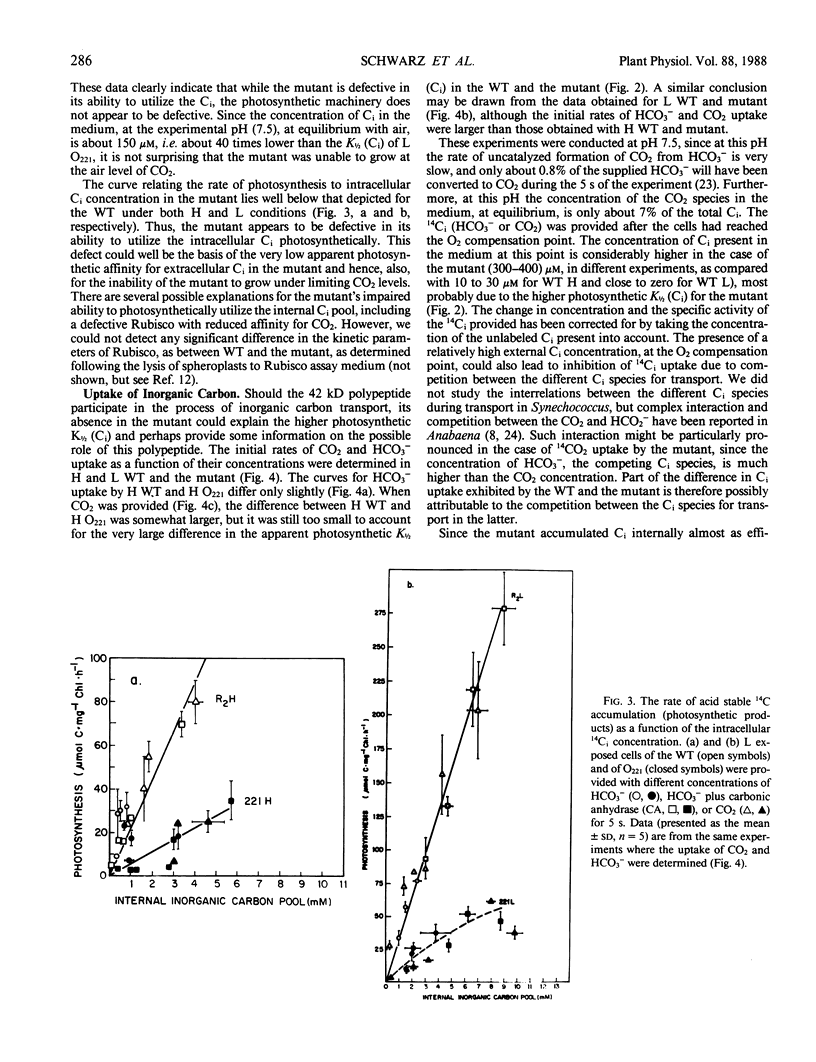
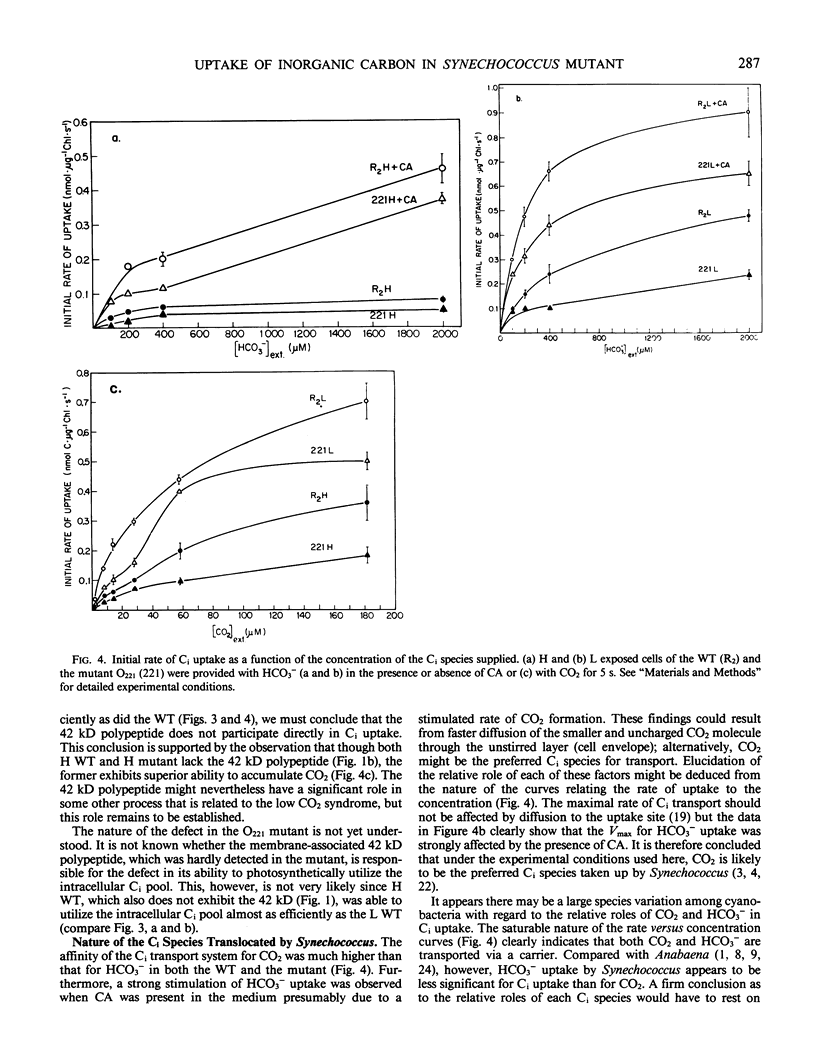
Cyanobacterial cells accumulate substantial amounts of a membrane-associated 42 kilodalton polypeptide during adaptation to low CO2 conditions. The role of this polypeptide in the process of adaptation and in particular in the large increase in the ability to accumulate inorganic carbon (Ci), which accompanies this process, is not yet understood. We have isolated a mutant Synechococcus PCC7942 that does not accumulate the 42 kilodalton polypeptide. The mutant requires a high-CO2 concentration for growth and exhibits a very low apparent photosynthetic affinity for extracellular Ci. The latter might be attributable to the observed defective ability of the mutant to utilize the intracellular Ci pool for photosynthesis. The 42 kilodalton polypeptide does not appear to participate directly in the active transport of Ci, since the difference between the observed capabilities for CO2 and HCO3− uptake of the mutant and the wild type is not sufficient to account for their different growth and photosynthetic performance. Furthermore, high CO2-grown wild-type cells, where we could not detect the 42 kilodalton polypeptide, transported CO2 faster than the mutant. An analysis of the curves relating the rate of accumulation of Ci to the concentration of CO2 or HCO3− supplied, in the presence or absence of carbonic anhydrase, indicated that under the experimental conditions used here, CO2 was the preferred Ci species taken up by Synechococcus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Bassett M., Comins H. N. A Model for HCO(3) Accumulation and Photosynthesis in the Cyanobacterium Synechococcus sp: Theoretical Predictions and Experimental Observations. Plant Physiol. 1985 Feb;77(2):465–471. doi: 10.1104/pp.77.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R. Kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase from Anabaena variabilis. Arch Biochem Biophys. 1980 Apr 15;201(1):247–254. doi: 10.1016/0003-9861(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Espie G. S., Canvin D. T. Evidence for Na-Independent HCO(3) Uptake by the Cyanobacterium Synechococcus leopoliensis. Plant Physiol. 1987 May;84(1):125–130. doi: 10.1104/pp.84.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg D., Seijffers J. Controlled gene expression utilising lambda phage regulatory signals in a cyanobacterium host. Mol Gen Genet. 1986 Jun;203(3):505–510. doi: 10.1007/BF00422077. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Zenvirth D., Marcus Y., Omata T., Ogawa T. Energization and activation of inorganic carbon uptake by light in cyanobacteria. Plant Physiol. 1987 Jun;84(2):210–213. doi: 10.1104/pp.84.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Schwarz R., Friedberg D., Kaplan A. High CO(2) Requiring Mutant of Anacystis nidulans R(2). Plant Physiol. 1986 Oct;82(2):610–612. doi: 10.1104/pp.82.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Zenvirth D., Harel E., Kaplan A. Induction of HCO(3) Transporting Capability and High Photosynthetic Affinity to Inorganic Carbon by Low Concentration of CO(2) in Anabaena variabilis. Plant Physiol. 1982 May;69(5):1008–1012. doi: 10.1104/pp.69.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kaneda T., Omata T. A Mutant of Synechococcus PCC7942 Incapable of Adapting to Low CO(2) Concentration. Plant Physiol. 1987 Jul;84(3):711–715. doi: 10.1104/pp.84.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kaplan A. The Stoichiometry between CO(2) and H Fluxes Involved in the Transport of Inorganic Carbon in Cyanobacteria. Plant Physiol. 1987 Apr;83(4):888–891. doi: 10.1104/pp.83.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T., Ogawa T. Biosynthesis of a 42-kD Polypeptide in the Cytoplasmic Membrane of the Cyanobacterium Anacystis nidulans Strain R2 during Adaptation to Low CO(2) Concentration. Plant Physiol. 1986 Feb;80(2):525–530. doi: 10.1104/pp.80.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T., Ogawa T., Marcus Y., Friedberg D., Kaplan A. Adaptation to Low CO(2) Level in a Mutant of Anacystis nidulans R(2) which Requires High CO(2) for Growth. Plant Physiol. 1987 Apr;83(4):892–894. doi: 10.1104/pp.83.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Spiller H., Wynns G. C., Silverman D. N. Carbonic Anhydrase and the Uptake of Inorganic Carbon by Synechococcus sp. (UTEX-2380). Plant Physiol. 1987 Sep;85(1):72–77. doi: 10.1104/pp.85.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Kaplan A., Reinhold L. Nature of the rate-limiting step in the supply of inorganic carbon for photosynthesis in isolated asparagus mesophyll cells. Plant Physiol. 1983 Jul;72(3):886–890. doi: 10.1104/pp.72.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Zenvirth D., Kaplan A., Reinhold L. Nature of the Inorganic Carbon Species Actively Taken Up by the Cyanobacterium Anabaena variabilis. Plant Physiol. 1984 Nov;76(3):599–602. doi: 10.1104/pp.76.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]