Abstract
Acyclovir (ACV) has been used for more than 15 years in the management of herpes simplex virus (HSV) and varicella-zoster virus (VZV) disease. The present survey was undertaken to assess the level of ACV resistance in the population. More than 2,000 HSV isolates from both immunocompetent and immunocompromised patients in northwest England were collected over a 2-year period and tested for sensitivity to ACV. These studies suggested a prevalence of resistance of approximately 0.1 to 0.6% in immunocompetent individuals, with no apparent difference in prevalence between treated and untreated groups. In line with previous studies, the prevalence of resistance in treated immunocompromised individuals was approximately 6%.
Acyclovir (ACV) has been in clinical use for more than 15 years and is accepted as the treatment of choice for the management of herpes simplex virus (HSV)-related and varicella-zoster virus-related diseases (1). However, resistant HSV variants were readily isolated in culture following multiple passage in the presence of increasing concentrations of ACV (for a review, see reference 16) and this raised concerns that the clinical use of ACV would be associated with the emergence of drug-resistant virus.
Resistance occurs as a result of mutations in the virus-specific genes responsible for the action of ACV, namely, thymidine kinase (TK) and DNA polymerase (for a review, see reference 2). The most frequently observed resistant variants are unable to express TK. Variants of this type are relatively avirulent in animal models of disease and are generally unable to reactivate from the latent state (7, 11). Resistance may be acquired less frequently by selection of TK- or DNA polymerase-encoding variants which no longer recognize ACV or ACV triphosphate, respectively, as substrates but which otherwise retain normal functions. These variants generally show slightly reduced virulence in animal models (4, 8, 15, 17).
Extensive sensitivity monitoring was carried out alongside the early trials evaluating the clinical efficacy of ACV. Those surveys revealed that among isolates from treated immunocompromised individuals there is a low (4 to 10%) incidence of resistance (3, 21). However, there appeared to be no impact of treatment or prophylaxis on the prevalence of resistant virus in the immunocompetent population (3). What is unclear from these early surveys is whether in the years since the introduction of the drug there has been any rise in the prevalence of drug-resistant virus in the general population. The aim of this study was to carry out a sensitivity survey of all HSV isolates collected during a 2-year period (1991 to 1993) from northwest England. The objectives were threefold. First, the main objective was to provide a reliable estimate of the prevalence of resistance in various patient groups in that period in northwest England, especially in the general immunocompetent population. This could then be compared retrospectively with historical data (3) and, more importantly, can be used in the future as a baseline against which to assess changes in prevalence. The second objective was to confirm earlier studies which demonstrated the emergence of resistance in the severely immunocompromised population and to look for any differences between different patient groups. Finally, the survey was to provide information on the relative frequency of appearance of different types of ACV-resistant variants in diverse patient groups.
In these studies, 2,012 clinical HSV isolates were collected and assayed for susceptibility to ACV. Resistant variants were identified and phenotypically characterized. The survey involved collaboration with consultants in infectious diseases at Monsall Hospital, physicians from genitourinary medicine (GUM) clinics, consultant physicians from the Respiratory Medicine Unit at Wythenshawe Hospital, and general practitioners and clinical virologists at local public health laboratories.
MATERIALS AND METHODS
Survey.
From June 1991 to November 1993, all HSV isolates from the northwest region of England were collected at the Manchester Public Health Laboratory (PHL), Booth Hall Regional Virus Laboratory, and Preston Public Health Laboratory and were screened for resistance to ACV.
Basic clinical information was obtained for selected patients by means of a two-part questionnaire. Part A was completed by the patient’s doctor, who noted details of the site and severity of the patient’s HSV infection and whether it was initial or recurrent. Information was also requested about any underlying medical conditions, particularly immunodeficiency, and about previous treatment with ACV or any other antiviral or immunomodulatory therapy. Individual patients of interest were followed up further. Additional details of their treatment and information about their sexual and other contacts were sought, and this information was further supplemented by hospital and clinic visits to gain access to the patients’ records. This additional information, collected by a hospital-based nurse associated with the project, was entered into part B of the questionnaire. However, despite these efforts it was often not possible to differentiate between primary and recurrent disease or to exclude the possibility that some patients may have received antiviral therapy during previous disease episodes.
ACV sensitivity assay.
HSV isolates were tested for their sensitivity to ACV by a plaque reduction assay with Vero cells (African Green Monkey kidney cells; ICN Biomedicals Ltd.). Primary screening for resistance was performed at Manchester PHL. Each well of one 24-well tissue culture plate per isolate was seeded with 2 × 105 cells in 1 ml of growth medium. On the following day the medium was removed, each row of six wells was washed with phosphate-buffered saline (PBS), and each well was inoculated with 50 μl of one of four dilutions of virus. After adsorption for 1 h at 37°C the inocula were removed and the wells were overlaid with medium containing 1% carboxymethyl cellulose. ACV was added at half log dilutions over a range of from 0.04 to 4 μM. The standard HSV strains SC16 (wild type) and R5C1 (TK-deficient SC16 mutant) were also tested with every batch of isolates. After 72 h at 37°C the overlay was removed, and the cell sheets were washed with PBS and fixed and stained with 0.125% crystal violet in 20% ethanol. Plaques were counted with the aid of a dissecting microscope, and an approximate estimate of the 50% inhibitory concentration (IC50) was made.
Secondary assessment of resistance was carried out at Manchester PHL for all isolates for which approximate IC50s were greater than 1.3 μM, as follows. Confluent Vero cell monolayers in six-well plates were prepared as described above and were infected with 100 to 200 PFU of test virus per well. Duplicate wells were overlaid with medium containing 1% carboxymethyl cellulose and a range of ACV concentrations on the basis of the data obtained in the primary screening test. Control wells containing no drug were also included. Plaque counts, expressed as a percentage of the counts in the control wells, were plotted against the log10 ACV concentration, and the concentration of drug which reduced the plaque count to 50% of the control value (IC50) was determined.
Confirmatory plaque reduction assays were then carried out at Wellcome Research Laboratories for those isolates judged to be resistant or for which the IC50 fell just below the cutoff value of 3 μM. The method used was the same as that used in Manchester, and again, the results were used to derive IC50s. Isolates for which the IC50s were above the cutoff value of 3 μM (5) were classified as resistant.
HSV typing.
At Manchester HSV typing was performed by direct immunofluorescence with an Imagen kit (Dako AS, Glostrup, Denmark). At Wellcome isolates were typed by enzyme-linked immunosorbent assay (20), also with a commercial kit (Dako AS). In both cases the tests were carried out according to the manufacturer’s instructions.
Phenotypic characterization of isolates.
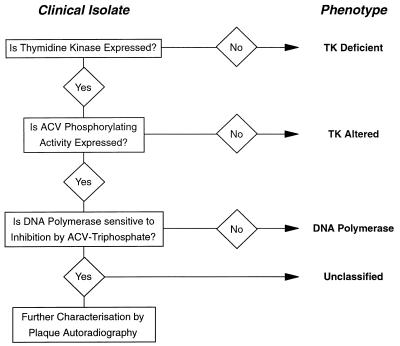
The procedure used to characterize the drug resistance phenotypes of the variants is illustrated in Fig. 1. The procedure is briefly described below.
FIG. 1.
Characterization of resistant isolates of HSV. Resistant viruses were classified phenotypically by the illustrated protocol.
(i) TK assay.
The TK activities of the isolates were determined by measuring the phosphorylation of [14C]thymidine as described previously (13) by using infected extracts from TK-deficient BHK cells. Those expressing <10% of the activity expressed by the HSV type 1 (HSV-1) wild-type virus, SC16, were classified as TK deficient (TKD). This group would be expected to contain true TK-negative viruses, low-level TK producers, and possibly mixtures of these variants with low levels of wild-type viruses. For those isolates with significant levels of TK activity, the levels of ACV phosphorylating activity were measured in the same manner but substituting [3H]ACV for labelled thymidine. If an infected cell extract was TK positive but unable to phosphorylate ACV it was classified as TK altered (TKA). Variants expressing ACV phosphorylating activity were further investigated.
(ii) DNA polymerase assay.
Viral DNA polymerase activity was assayed at 37°C by using calf thymus DNA as a template (15). Inhibition of polymerase by ACV triphosphate was expressed as an IC50 and was compared to the values obtained with standard laboratory HSV strains. A variant expressing a polymerase insensitive to inhibition by ACV triphosphate would be classified as DNA Pol. Other variants, those expressing phenotypically normal TK and DNA polymerase, were categorized as unclassified. They were further investigated by plaque autoradiography.
Plaque autoradiography.
The phenotypic mix within a HSV isolate was assessed by 125I-labelled 5-iodo-2′-deoxycytidine (IdC) plaque autoradiography (18). Confluent Vero cell monolayers were grown in 60-mm flexible petri dishes (LUX; Miles Scientific). The plates were infected with approximately 100 PFU of HSV and were incubated for 48 h at 37°C in a 5% CO2 atmosphere. The cultures were then labelled with [125I]IdC (NEN, du Pont Co.) for 2 h. The monolayers were washed with PBS and fixed and stained with 1% crystal violet in 10% formalin, 5% acetic acid, 60% methanol, and 24% H2O. The dishes were dried and the plaques were counted, and then the labelled cell sheets were cut out of their dishes and exposed to X-ray film for 7 days. The numbers of visible plaques were compared with the numbers of black plaques (wild type, normal TK) and grey plaques (TK altered) visualized on the film. The TKD population does not produce visible plaques on the X-ray film and is seen only by staining.
RESULTS
Study population.
Over a period of 2 years, all HSV isolates collected from the northwest region of England were assessed for their resistance to ACV. Although a small proportion did not grow on reisolation, data were obtained for 2,012 isolates from 1,870 patients (Table 1). Roughly two-thirds of the patients (n = 1,171) had genital herpes and attended GUM clinics. Comprehensive clinical records were available for 857 of these GUM patients. The majority of these patients (n = 708) had not received antiviral treatment, but the remainder (n = 149) were receiving ACV treatment, mainly episodic. A number of isolates (n = 146) were from 95 severely immunocompromised patients who were being treated at local hospitals and who were also on ACV treatment, and again, for this group good clinical data were available. The remaining isolates were from 918 individuals whose treatment histories were unclear. Diagnoses were only available for those patients (n = 314) who were attending GUM clinics.
TABLE 1.
Characteristics of study populationa
| No. of patients | No. of isolates | Immune status | Treatment status | Diagnosis |
|---|---|---|---|---|
| 708 | 760 | Immunocompetent | Untreated | Genital herpes |
| 149 | 162 | Immunocompetent | ACV treatedb | Genital herpes |
| 95 | 146 | Immunocompromised | ACV treated | Varied |
| 918 | 944 | Immunocompetent | Unknown | Unknownc |
A total of 2,012 isolates from 1,870 patients were tested.
Most patients were receiving episodic treatment.
This group contained 314 patients with genital herpes.
Range of drug susceptibilities.
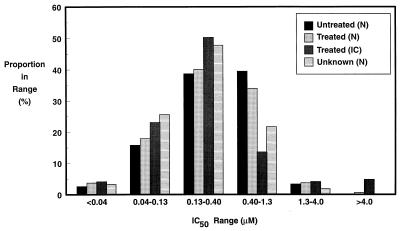
All isolates were tested in a primary screening to assess their approximate ACV susceptibilities. The distribution of isolates by drug sensitivity, treatment history, and immunologic status is presented in Fig. 2. For the majority of isolates in all groups IC50s were in the range of 0.04 to 1.3 μM. The only major difference seen between the groups was that there appeared to be a higher proportion of isolates from treated immunocompromised individuals for which IC50s were above 4 μM.
FIG. 2.
Sensitivity distribution of 2,012 HSV isolates tested by a primary assay for sensitivity to ACV. The ACV treatment history and immunologic status (N, normal; IC, immunocompromised) are also indicated.
Identification of resistant isolates.
Historically, a cutoff value of 3 μM has been used to discriminate between drug-sensitive and drug-resistant isolates (5). To ensure that all resistant isolates were captured, those for which IC50s were above 1.3 μM in the primary assessment were tested more rigorously and precise IC50s were determined. Subsequently, the IC50s were confirmed in independent assays at Wellcome Research Laboratories. The numbers of patients shedding resistant virus are presented in Table 2 by ACV treatment history and immunologic status.
TABLE 2.
ACV-resistant isolates identified in survey, by ACV treatment history and immunologic status
| Immune status | Treatment status | No. of patients | No. (%) of patients infected with resistant isolates |
|---|---|---|---|
| Immunocompetent | Untreated | 708 | 3 (0.42)a |
| Immunocompetent | ACV treatedb | 149 | 1 (0.67) |
| Immunocompromised | ACV treated | 95 | 6 (6.3) |
| Immunocompetent | Unknown | 918 | 1 (0.11) |
These numbers relate to the number of patients shedding ACV-resistant virus. A series of resistant isolates from a single patient were scored only once.
Most patients were receiving episodic treatment.
As expected, the highest proportion of resistant isolates was identified in the isolates collected from the group of immunocompromised, treated individuals. The total number of patients in that group was 95, and of that number 6 (6.3%) shed resistant virus.
Far fewer resistant isolates were identified in the other patient groups. Among all 857 immunocompetent patients with comprehensive clinical records (whether they were treated with ACV or not), resistant isolates were identified from only 4 (0.47%) patients. A history of ACV treatment did not appear to influence the frequency of resistant isolates (3 of 708 isolates from untreated patients were classified as resistant, whereas 1 of 149 isolates from patients treated with ACV were classified as resistant), although the numbers were too small for a meaningful comparison. Among the isolates from those patients whose treatment history was unknown (604 patients not attending GUM clinics and 314 patients attending GUM clinics) only one resistant isolate was identified, giving a prevalence of approximately 0.1%.
Phenotypic characterization of resistant isolates.
Each resistant isolate was investigated to determine its phenotype (Fig. 1). The results of this analysis are presented in Table 3. The majority of isolates (five of six) from treated, immunocompromised patients were TKD, although a single TKA variant was also identified. Two other TKD viruses were identified; one was isolated from an immunocompetent individual undergoing ACV therapy, and the other was isolated from a patient in the group of patients with unknown treatment histories. Referral to the clinical notes for the latter patient indicated that the isolate was obtained from an ocular infection. Little other information was available. Further characterization of the TKD variants is under way, and this will be the subject of a later publication.
TABLE 3.
Phenotypic characterization of ACV-resistant isolates
| Immune status | Treatment status | No. of isolates
|
|||
|---|---|---|---|---|---|
| TK variants
|
DNA Pol | Unclas- sified | |||
| Deficient | Altered | ||||
| Immunocompetent | Untreated | 0 | 1 | 0 | 2 |
| Immunocompetent | ACV treateda | 1 | 0 | 0 | 0 |
| Immunocompromised | ACV treated | 5 | 1 | 0 | 0 |
| Immunocompetent | Unknown | 1 | 0 | 0 | 0 |
Most patients were receiving episodic treatment.
The three isolates from untreated immunocompetent individuals comprised one TKA isolate and two isolates which could not be classified. One of the unclassified isolates was furher investigated by plaque autoradiography and was compared with the wild-type virus. With the wild-type virus, plaques were uniformly heavily labelled, suggesting efficient incorporation of radiolabelled IdC in infected cells. With the clinical isolate there were a small number of heavily labelled plaques, but the remainder were lightly grained, suggesting either that an altered TK was expressed or that a low level of the wild-type enzyme was induced. The most likely explanation is therefore that this particular isolate is a heterogeneous mixture of variants containing a small proportion of wild-type virus, with the remainder of the isolates expressing TK only weakly.
Summary data for all resistant isolates.
The clinical and treatment histories for all those individuals who shed resistant viruses are presented in Table 4. Table 4 also indicates the virus types. As expected, the genital isolates from the patients attending GUM clinics were all HSV-2 (patients 1 to 4). Similarly, all oral isolates and the single lung isolate from immunocompromised individuals were HSV-1 (patients 7 to 11). Perhaps surprisingly, the isolate from the eye of the otherwise healthy individual (patient 5) was type 2.
TABLE 4.
Summary data for patients shedding ACV-resistant virusa
| Immune status | Patient no. | Disease status | Isolation site | Virus type | Phenotype | IC50 (μM) | Treatment history | Dose |
|---|---|---|---|---|---|---|---|---|
| Immunocompetent | 1 | Genital | Genital | 2 | UC | 12 | None | |
| 2 | Genital | Genital | 2 | TKD | 18 | ACV cream | ||
| 3 | Genital | Genital | 2 | TKA | 5.5 | None | ||
| 4 | Genital | Genital | 2 | UC | 19 | None | ||
| 5 | Keratitis | Eye | 2 | TKD | 110 | No information | ||
| Immunocompromised | 6 | HIV positive | Genital | 1 | TKD | 22 | Prophylaxis (oral) | 400 mg BID |
| 7 | Lung transplant | Lung | 1 | TKD | 43 | Prophylaxis (oral) | 200 mg BID | |
| 8 | AML | Oral | 1 | TKD | 19 | Treatment (oral) | Not known | |
| 9 | BMT | Oral | 1 | TKA | 40 | Treatment (oral) | 200 mg QID | |
| 10 | BMT | Oral | 1 | TKD | 11 | Prophylaxis (oral) | 200 mg 5× daily | |
| 11 | Heart transplant | Oral | 1 | TKD | 3 | Treatment (oral)b | 200 mg TID |
Abbreviations: UC, uncharacterized phenotype; BID, twice daily; QID, four times daily; TID, three times daily; AML, acute myelocytic leukemia; BMT, bone marrow transplant.
This patient also received ganciclovir.
Clinical status of immunocompromised individuals.
The incidence of resistance among isolates from individuals with different underlying causes of immunosuppression is presented in Table 5. Of the 46 patients who were undergoing treatment for cancer, 3 (6.5%) shed resistant virus. A further 20 patients were on immunosuppressive therapy following heart or lung transplantation. Two of these (10%) shed resistant virus. Finally, there were 29 human immunodeficiency virus (HIV)-infected individuals, and of these only 1 (3.4%) shed a resistant variant. Of the HIV-infected individuals, 17 were diagnosed with AIDS, and it was one of these individuals who shed resistant virus. Thus, the approximate incidence of ACV-resistant virus in AIDS patients was 6%.
TABLE 5.
Prevalence of ACV resistance in immunocompromised groups of patients
| Clinical status | No. of patients | No. of patients shedding resist- ant isolates | % of patients shedding resist- ant isolates |
|---|---|---|---|
| Oncology or bone marrow transplant | 46 | 3 | 6.5 |
| Heart or lung transplant | 20 | 2 | 10 |
| HIV positive | 29 (17)a | 1 (1) | 3.4 (5.9) |
Data in parentheses are for patients diagnosed with AIDS. The majority of patients with AIDS (15 of 17) had CD4 counts of less than 200, although the single resistant isolate was obtained from an individual with a CD4 count in the range of 200 to 500.
Interestingly, unusual clinical features were reported for only two of the immunocompromised patients who shed resistant viruses. The lung transplant recipient developed pneumonitis which eventually resolved, and one leukemia patient developed severe mucositis which responded to intensive ACV therapy.
DISCUSSION
The main objective of this study was to determine the prevalence of resistance to ACV in the general population, focusing especially on HSV isolates from patients with genital herpes. To obtain a true measure of prevalence, isolates should be obtained from ACV-naive patients. In the present study, isolates were obtained from 708 untreated immunocompetent individuals with comprehensive clinical records who were suffering from genital herpes and who attended GUM clinics in the northwest region of England. Among this group of isolates, only three isolates were characterized as resistant, a prevalence of 0.4%. Because of the uncertainty of the patients’ treatment histories in this type of study, this prevalence value could be an overestimate.
All three ACV-resistant HSV isolates from untreated immunocompetent patients were type 2. One had an unambiguous resistance phenotype, and this was a TKA variant isolated from patient 3. The IC50 for this isolate was only 5 μM, indicating a marginal level of resistance (5). The other two isolates had unclassified phenotypes, due in one case, at least, to a heterogeneous mixture of phenotypes.
The second patient group of interest comprised ACV-treated, immunocompetent subjects. Among these individuals (n = 149), only one resistant isolate, a TKD variant, was identified.
A third group of patients were immunocompetent individuals with unknown treatment histories (918 in all, including 314 patients attending GUM clinics and 604 patients not attending GUM clinics). Only one resistant isolate, another TKD variant, was obtained from these patients. This virus was unusual in that it was obtained from a patient with herpes keratitis and was HSV-2. The prevalence of resistance of 0.1% (1 of 918) in this group was not different from the prevalence seen in other immunocompetent groups, although the numbers were too small to make a meaningful comparison.
In view of the very small number of resistant isolates from the various immunocompetent patient groups, the prevalence values are unreliable. Overall, there were five resistant isolates from 1,775 patients (a prevalence of 0.28%). Of these isolates, two were TKD and thus were likely to be relatively avirulent (10, 12, 14) and one had a TKA phenotype and would be expected to be similar in virulence to the wild-type virus (6), although, as noted above, this isolate showed only marginal resistance. The remaining variants were unclassified, probably contained mixtures of phenotypes, and thus had unknown virulence. We conclude that if reliable measures of resistance prevalence and incidence among isolates in the immunocompetent population are required, far larger surveys will be needed.
Since it appears that there is a low prevalence of resistance among isolates in the general population, it would be expected that, rarely, some individuals would fail to respond to ACV treatment. However, since in the immunocompetent population herpetic disease is generally self-limiting and is of relatively short duration, a failure to respond to therapy would be unlikely to be noticed. However, if ACV is used prophylactically for the suppression of genital herpes, resistance would be expected to lead to the failure of suppression. It therefore may not be surprising that the only well-documented case of a resistant virus causing a response failure in an immunocompetent individual was in an individual receiving ACV for the suppression of genital herpes (14).
The final patient group consisted of immunocompromised patients, all of whom were treated with ACV. This was a relatively small collection of patients (n = 95), but in line with previous experience, there was a significant prevalence of ACV resistance among the isolates from this group (9, 10, 12, 21). Our finding of a prevalence of 6% is in reasonably good agreement with the values derived from the data of Wade et al. (21) (7.9%) and Englund et al. (9) (10.9%). Again, as expected, the majority of patients (five of six) shed TKD virus. Virus will not be recovered from all ACV-treated individuals infected with ACV-sensitive strains. Earlier studies (9, 21) had shown that the true incidence of resistance (the proportion of treated patients who shed resistant virus) was approximately half that observed if the only patients considered were those who shed virus. Thus, the true incidence of resistance in this population is likely to be significantly less than the value of 6 to 10.9% which studies to date have shown.
Patients in the severely immunocompromised group could be divided into three subgroups (Table 5): oncology and bone marrow transplant patients, heart and/or lung transplant patients, and HIV-positive individuals. Because the number of resistant isolates obtained in each subgroup was very small (n = 1 to 3), a reliable assessment of the relative risk to each patient population could not be made, although it appeared to be similar.
Our cutoff value for the IC50 in the plaque reduction assay was 3 μM, a value which had been established several years ago on the basis of a smaller study with isolates from ACV-naive patients (5). The selection of this value had been somewhat arbitrary and not related to the level at which resistance becomes clinically significant in the face of drug treatment. On the basis of a recent study with a similar in vitro plaque reduction assay, Safrin and colleagues (19) suggested that a more realistic cutoff value was 10 μM, since the IC50s for isolates from individuals who failed to respond to treatment were generally above this level. Interestingly, if this higher cutoff value had been used in the present study, only 2 of the total of 16 isolates classified as resistant would have been reclassified as sensitive. These were TKA isolates from an untreated immunocompetent individual and an immunocompromised heart transplant recipient (patients 3 and 11, respectively; Table 4).
In summary, the proportion of patients infected with naturally occurring ACV-resistant virus is low, approximately 0.4%. This proportion appears to have been relatively stable and does not appear to have been influenced significantly by the widespread use of ACV. Over a period of 12 years, from 1980 to 1992, isolates from hospitals and regional health centers throughout the United Kingdom and Europe were tested for sensitivity to ACV. Of the isolates collected from 657 immunocompetent patients, two (0.3%) were classified as resistant (3). In contrast, we found that isolates from up to 6% of immunocompromised patients develop resistance during treatment, data again which are broadly in line with those from earlier studies. The virus responsible generally has a TKD phenotype and will therefore be relatively avirulent in immunocompetent individuals and unlikely to pose a threat to the general population. Although there are no indications from these studies that the situation in relation to resistance is changing, in view of the low prevalence values, far larger studies will be required to confirm or refute this.
ACKNOWLEDGMENTS
Financial support for this study was kindly provided by Wellcome Foundation Ltd.
We thank Lynne Mallard for cell culture and the staff of the Virus Isolation benches at Manchester and Preston Public Health Laboratories and Booth Hall Regional Virus Laboratory for all their help. Valuable technical support was provided by Kate Rowley and David Gower at Wellcome Research Laboratories.
The collaborative group consisted of the following individuals in the United Kingdom. GUM physicians were S. Chandiok and P. J. Woolley, South Manchester NHS Trust, Withington Hospital; E. Curless, Bolton NHS Trust, Bolton District Hospital; D. Coker, Royal Infirmary, Lancaster; F. H. Choudhury, M. N. Bhattacharyya, and K. R. Haye, Manchester Royal Infirmary; T. K. Chatterjee, St Thomas’ Hospital, Stockport; B. P. Goorney, Hope Hospital, Salford; P. J. Woolley, Trafford General Hospital, Urmston; M. H. Khan, Leighton Hospital, Crewe; R. M. Girgis, Royal Oldham Hospital, Oldham; J. Forrer, Royal Albert Edward Infirmary, Wigan; M. A. Saeed, Royal Preston Hospital, Preston; and H. B. Lacey, Rochdale. Consultant physicians from the Monsall Unit were as follows: B. K. Mandall, E. Wilkins, and E. M. Dunbar; D. J. Morris, clinical virologist; G. Corbitt, Clinical Virology, Manchester Royal Infirmary; J. H. Scarffe, The Christie Hospital, Manchester; K. Carroll, Wythenshawe Hospital, Manchester; and P. Morgan-Capner, PHL Preston.
REFERENCES
- 1.Baker D A, editor. Acyclovir therapy for herpesvirus infections. New York, N.Y: Dekker Publications; 1990. [Google Scholar]
- 2.Collins P, Darby G. Laboratory studies of herpes simplex virus strains resistant to acyclovir. Rev Med Virol. 1991;1:19–28. [Google Scholar]
- 3.Collins P, Ellis N M. Sensitivity monitoring of clinical isolates of herpes simplex virus to acyclovir. J Med Virol Suppl. 1993;1:58–66. doi: 10.1002/jmv.1890410512. [DOI] [PubMed] [Google Scholar]
- 4.Collins P, Larder B A, Oliver N M, Kemp S, Smith I W, Darby G. Characterisation of a DNA polymerase mutant of herpes simplex virus from a severely immunocompromised patient receiving acyclovir. J Gen Virol. 1989;70:375–382. doi: 10.1099/0022-1317-70-2-375. [DOI] [PubMed] [Google Scholar]
- 5.Collins, P., and N. M. Oliver. 1986. Sensitivity monitoring of herpes simplex virus isolates from patients receiving acyclovir. J. Antimicrob. Chemother. 18(Suppl. B):103–112. [DOI] [PubMed]
- 6.Darby G, Field H J, Salisbury S A. Altered substrate specificity of herpes simplex virus thymidine kinase confers acyclovir resistance. Nature. 1981;289:81–83. doi: 10.1038/289081a0. [DOI] [PubMed] [Google Scholar]
- 7.Efstathiou S, Kemp S, Darby G, Minson A C. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J Gen Virol. 1989;70:869–879. doi: 10.1099/0022-1317-70-4-869. [DOI] [PubMed] [Google Scholar]
- 8.Ellis M N, Keller P M, Fyfe J A, Martin J L, Rooney J F, Straus S E, Nusinoff-Lehrman S, Barry D W. Clinical isolate of herpes simplex virus type 2 that induces a thymidine kinase with altered substrate specificity. Antimicrob Agents Chemother. 1987;31:1117–1125. doi: 10.1128/aac.31.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englund J A, Zimmerman M E, Swierkosz E M, Goodman J L, Scholl D R, Balfour H H. Herpes simplex virus resistance to acyclovir. A study in a tertiary care center. Ann Intern Med. 1990;112:416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- 10.Erlich K S, Mills J, Chatis P, Mertz G J, Busch D F, Follansbee S E, Grant R M, Crumpacker C S. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1989;320:293–296. doi: 10.1056/NEJM198902023200506. [DOI] [PubMed] [Google Scholar]
- 11.Field H J, Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg. 1978;81:267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray J J, Wreghitt T G, Baglin T P. Susceptibility to acyclovir of herpes simplex virus: emergence of resistance in patients with lymphoid and myeloid neoplasia. J Infect. 1989;19:31–40. doi: 10.1016/s0163-4453(89)94798-1. [DOI] [PubMed] [Google Scholar]
- 13.Klemperer H G, Haynes G R, Shedden W I H, Watson D H. A virus specific thymidine kinase in BHK21 cells infected with herpes simplex virus. Virology. 1967;31:120–128. doi: 10.1016/0042-6822(67)90015-3. [DOI] [PubMed] [Google Scholar]
- 14.Kost R G, Hill E L, Tigges M, Straus S E. Brief report: recurrent acyclovir-resistant genital herpes in an immunocompetent patient. N Engl J Med. 1993;329:1777–1781. doi: 10.1056/NEJM199312093292405. [DOI] [PubMed] [Google Scholar]
- 15.Larder B A, Derse D, Cheng Y-C, Darby G. Properties of purified enzymes induced by pathogenic drug-resistant mutants of herpes simplex virus: evidence for virus variants expressing normal DNA polymerase and altered thymidine kinase. J Biol Chem. 1983;258:2027–2033. [PubMed] [Google Scholar]
- 16.Larder B A, Darby G. Virus drug-resistance: mechanisms and consequences. Antivir Res. 1984;4:1–42. doi: 10.1016/0166-3542(84)90023-8. [DOI] [PubMed] [Google Scholar]
- 17.Larder B A, Darby G. Selection and characterisation of acyclovir-resistant herpes simplex virus type 1 mutants inducing altered DNA polymerase activities. Virology. 1985;146:262–271. doi: 10.1016/0042-6822(85)90009-1. [DOI] [PubMed] [Google Scholar]
- 18.Martin J L, Ellis N M, Keller P M, Biron K K, Lehrman S N, Barry D W, Furman P A. Plaque autoradiography assay for the detection and quantitation of thymidine kinase deficient and thymidine kinase altered mutants of herpes simplex virus in clinical isolates. Antimicrob Agents Chemother. 1985;28:181–187. doi: 10.1128/aac.28.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safrin S, Tarek E, Lienhanh P, Robinson D, Rush J, Elbaggari A, Mills J. Correlation between response to acyclovir and foscarnet therapy and in vitro susceptibility result for isolates of herpes simplex virus from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1994;38:1246–1250. doi: 10.1128/aac.38.6.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vestergard B F, Jenson O. Diagnosis and typing of herpes simplex virus in clinical specimens by the enzyme-linked immunosorbent assay (ELISA) In: Nahmias A J, Dowdle W R, Schinazi R F, editors. The human herpes viruses. New York, N.Y: Elsevier, North-Holland Inc.; 1981. pp. 391–394. [Google Scholar]
- 21.Wade J C, Newton B, McLaren C, Flournoy N, Keeney R E, Meyers J D. Intravenous acyclovir to treat mucocutaneous herpes simplex virus infection after marrow transplantation: a double blind test. Ann Intern Med. 1983;96:265–269. doi: 10.7326/0003-4819-96-3-265. [DOI] [PubMed] [Google Scholar]