Abstract
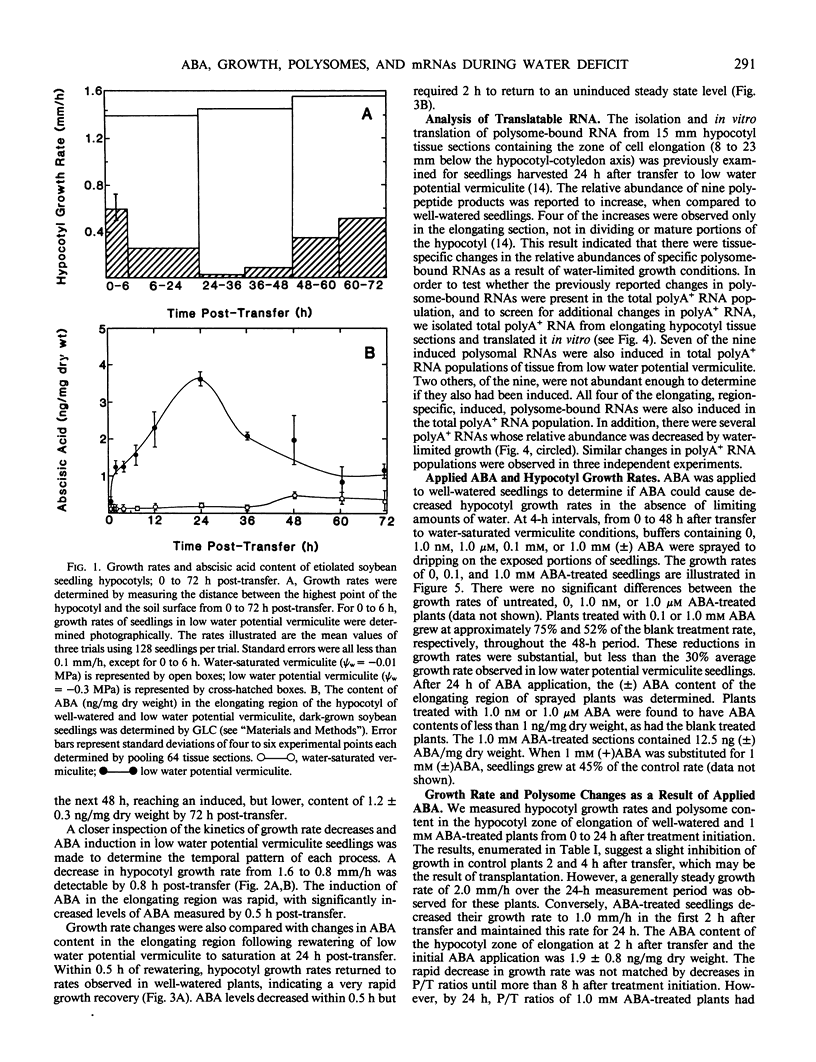
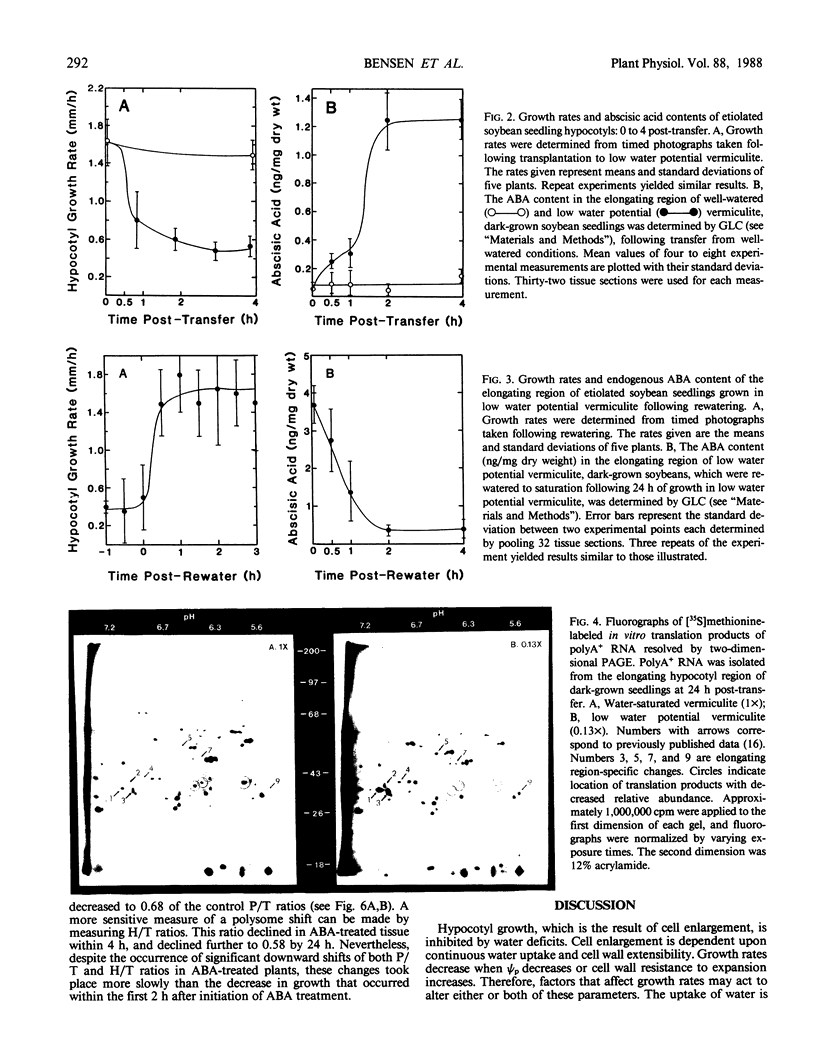
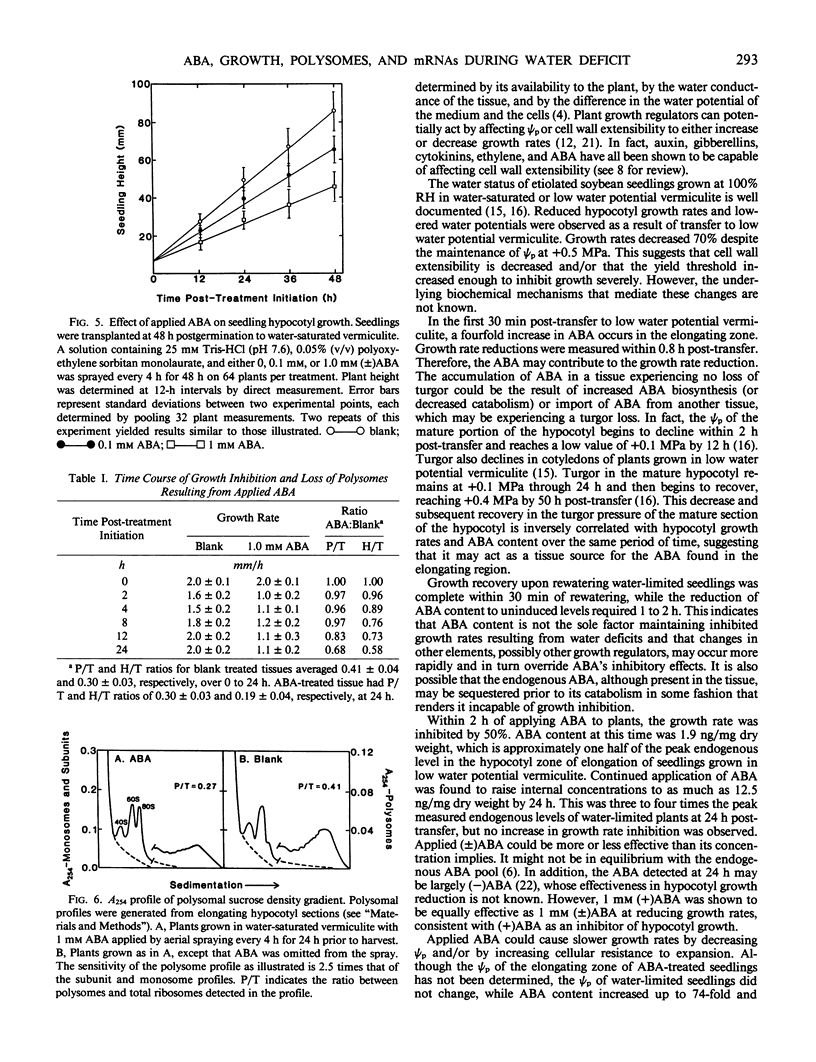
Soybean seedlings (Glycine max L.) were germinated and dark-grown in water-saturated vermiculite (water potential = −0.01 megapascal) for 48 hours, then transferred either to water-saturated vermiculite or to low water potential vermiculite (water potential = −0.30 megapascal). A decrease in growth rate was detectable within 0.8 hour post-transfer to low water potential vermiculite. A fourfold increase in the abscisic acid content of the elongating region was observed within 0.5 hour. At 24 hours post-transfer, hypocotyl elongation was severely arrested and abscisic acid reached its highest measured level: 3.7 nanograms per milligram dry weight (74-fold increase). A comparison of the polyA+ RNA populations isolated at 24 hours post-transfer from the elongating region of water-saturated and low water potential vermiculite-grown seedlings was made by two-dimensional (isoelectric focusing-sodium dodecyl sulfate) polyacrylamide gel analysis of in vitro translation products. It revealed both increases and decreases in the relative amounts of a number of translation products. Rewatering seedlings grown in low water potential vermiculite at 24 hours post-transfer led to a total recovery in growth rate within 0.5 hour, while abscisic acid in the elongating hypocotyl region required 1 to 2 hours to return to uninduced levels. Application of 1.0 millimolar (±) abscisic acid to well-watered seedlings resulted in a 48% reduction in hypocotyl growth rate during the first 2 hours after treatment. Plants treated with abscisic acid for 24 hours had a lower polysome content than control plants. However, hypocotyl growth inhibition in abscisic acid-treated seedlings preceded the decline in polysome content.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boyer J. S., Bowen B. L. Inhibition of oxygen evolution in chloroplasts isolated from leaves with low water potentials. Plant Physiol. 1970 May;45(5):612–615. doi: 10.1104/pp.45.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth C. S., Mullet J. E., Boyer J. S. Cell wall proteins at low water potentials. Plant Physiol. 1987 Sep;85(1):261–267. doi: 10.1104/pp.85.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray E. A., Zeevaart J. A. Compartmentation and equilibration of abscisic Acid in isolated xanthium cells. Plant Physiol. 1986 Jan;80(1):105–109. doi: 10.1104/pp.80.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri A. J., Boyer J. S. Water potentials induced by growth in soybean hypocotyls. Plant Physiol. 1982 Feb;69(2):492–496. doi: 10.1104/pp.69.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z. Abscisic Acid promotes both volume flow and ion release to the xylem in sunflower roots. Plant Physiol. 1980 Mar;65(3):537–540. doi: 10.1104/pp.65.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E., Boyer J. S. Polysomes, Messenger RNA, and Growth in Soybean Stems during Development and Water Deficit. Plant Physiol. 1988 Mar;86(3):725–733. doi: 10.1104/pp.86.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Schopfer P., Plachy C. Control of Seed Germination by Abscisic Acid : III. Effect on Embryo Growth Potential (Minimum Turgor Pressure) and Growth Coefficient (Cell Wall Extensibility) in Brassica napus L. Plant Physiol. 1985 Mar;77(3):676–686. doi: 10.1104/pp.77.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer E., Galson E. C., Chang Y. P., Walton D. C. Asymmetry, its importance to the action and metabolism of abscisic Acid. Science. 1971 Nov 19;174(4011):829–831. doi: 10.1126/science.174.4011.829. [DOI] [PubMed] [Google Scholar]



