Abstract
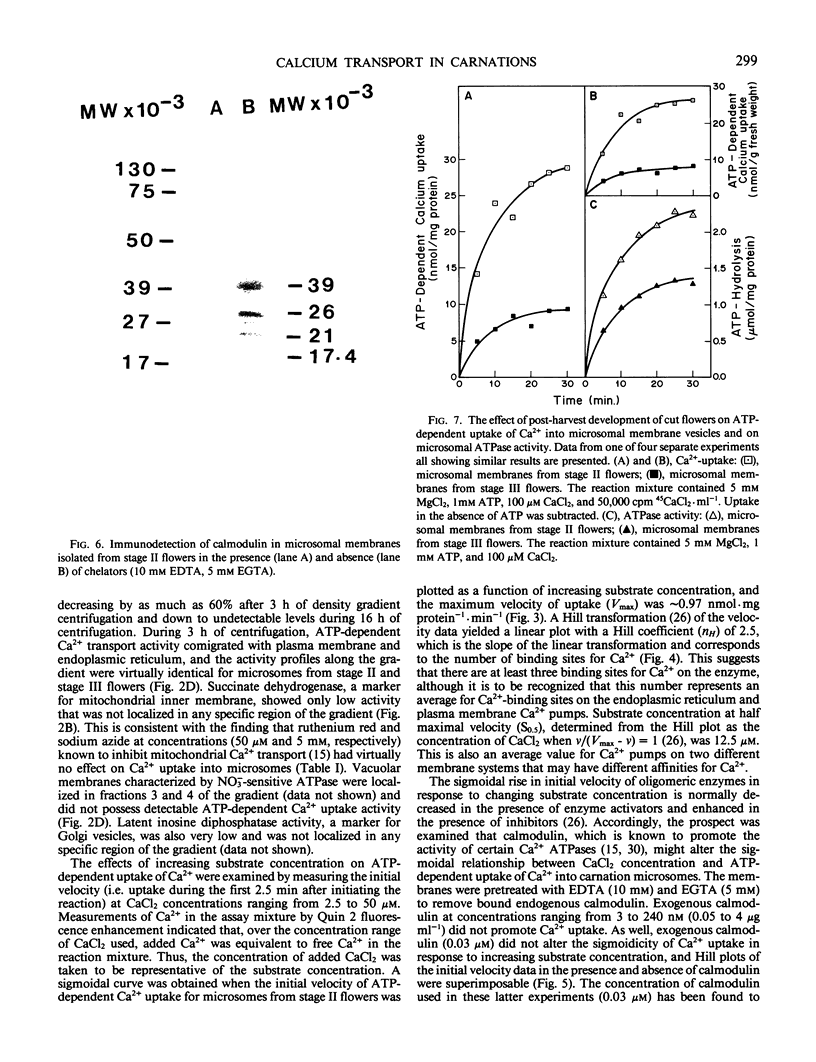
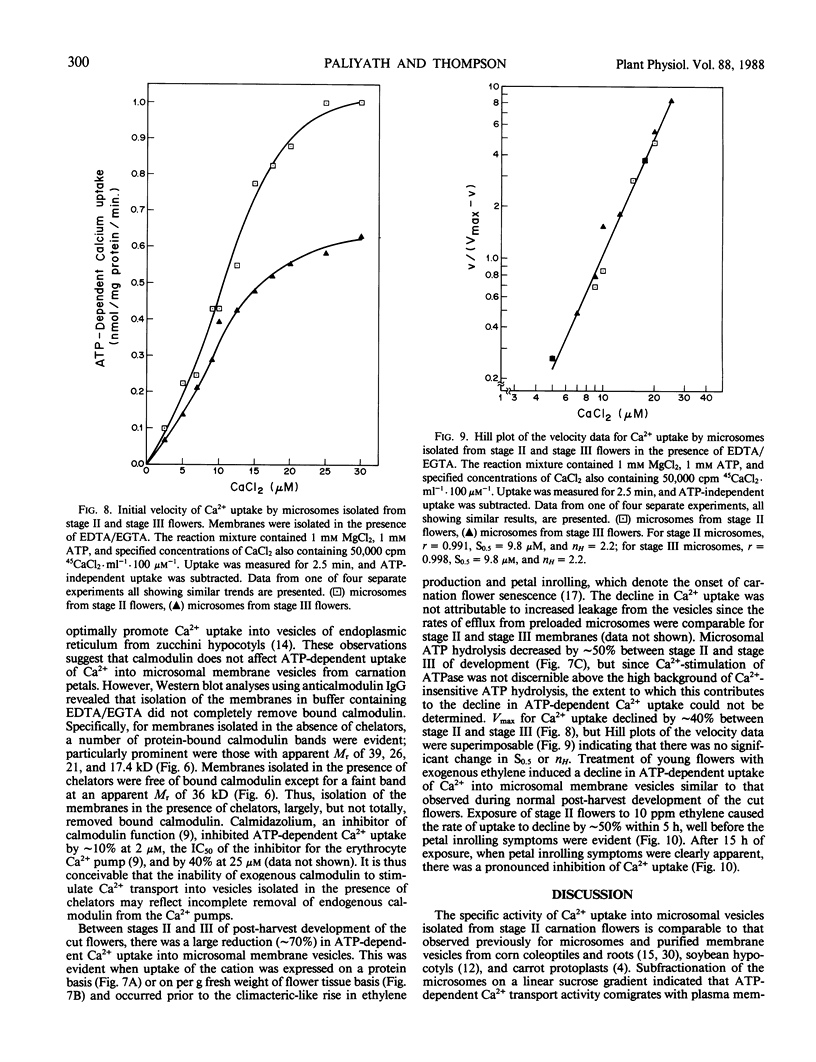
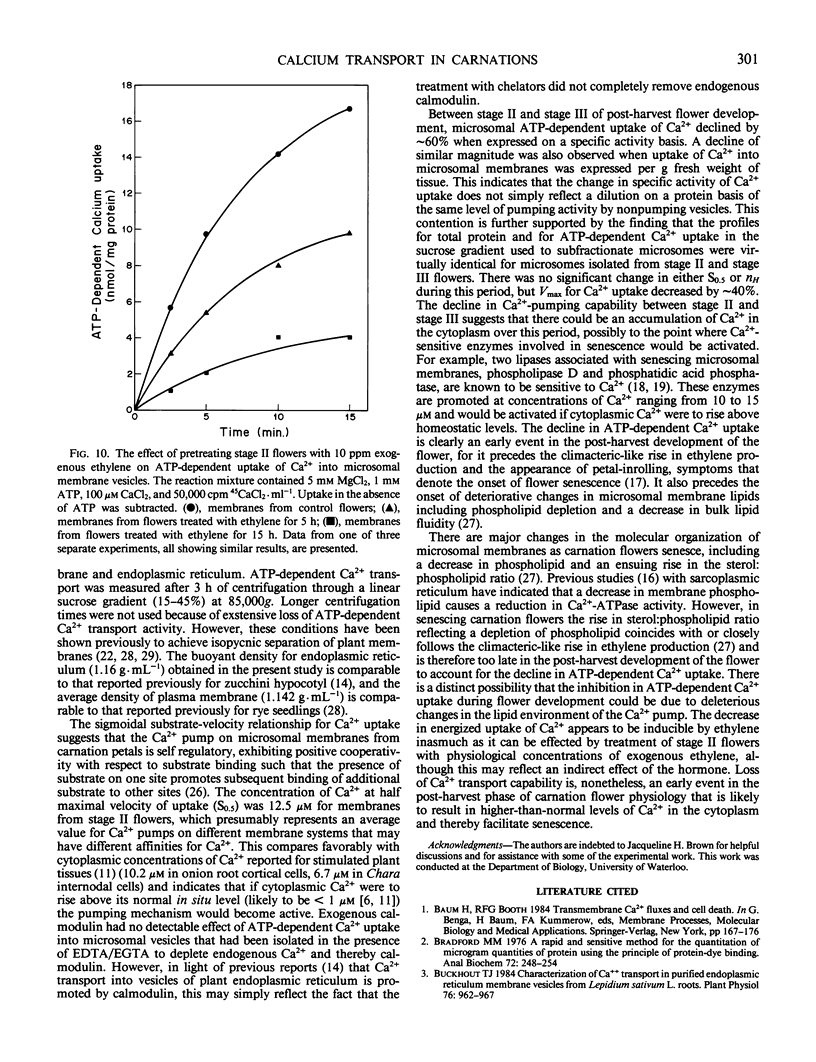
Microsomal membrane vesicles isolated from the petals of young carnation (Dianthus caryophyllus L. cv White Sim) flowers accumulate Ca2+ in the presence of ATP. The specific activity of ATP-dependent uptake is ∼20 nanomoles per milligram of protein per 30 minutes. The membranes also hydrolyze ATP, but Ca2+ stimulation of ATP hydrolysis was not discernible above the high background of Ca2+-insensitive ATPase activity. The initial velocity of uptake showed a sigmoidal rise with increasing Ca2+ concentration, suggesting that Ca2+ serves both as substrate and activator for the enzyme complex mediating its uptake. The concentration of Ca2+ at half maximal velocity of uptake (S0.5) was 12.5 micromolar and the Hill coefficient (nH) was 2.5. The addition of calmodulin to membrane preparations that had been isolated in the presence of chelators did not promote ATP-dependent accumulation of Ca2+, although this may reflect the fact that the treatment with chelators did not fully remove endogenous calmodulin. Transport of Ca2+ into membrane vesicles was unaffected by 50 micromolar ruthenium red and 5 micromolar sodium azide, indicating that uptake is primarily into vesicles of non-mitochondrial origin. By subfractionating the microsomes on a linear sucrose gradient, it was established that the ATP-dependent Ca2+ transport activity comigrates with endoplasmic reticulum and plasma membrane. During post-harvest development of cut flowers, ATP-dependent uptake of Ca2+ into microsomal vesicles declined by ∼70%. This occurred before the appearance of petal-inrolling and the climacteric-like rise in ethylene production, parameters that denote the onset of senescence. There were no significant changes during this period in S0.5 or nH, but Vmax for ATP-dependent Ca2+ uptake decreased by ∼40%. A similar decline in ATP-dependent uptake of Ca2+ into microsomal vesicles was induced by treating young flowers with physiological levels of exogenous ethylene.
Full text
PDF
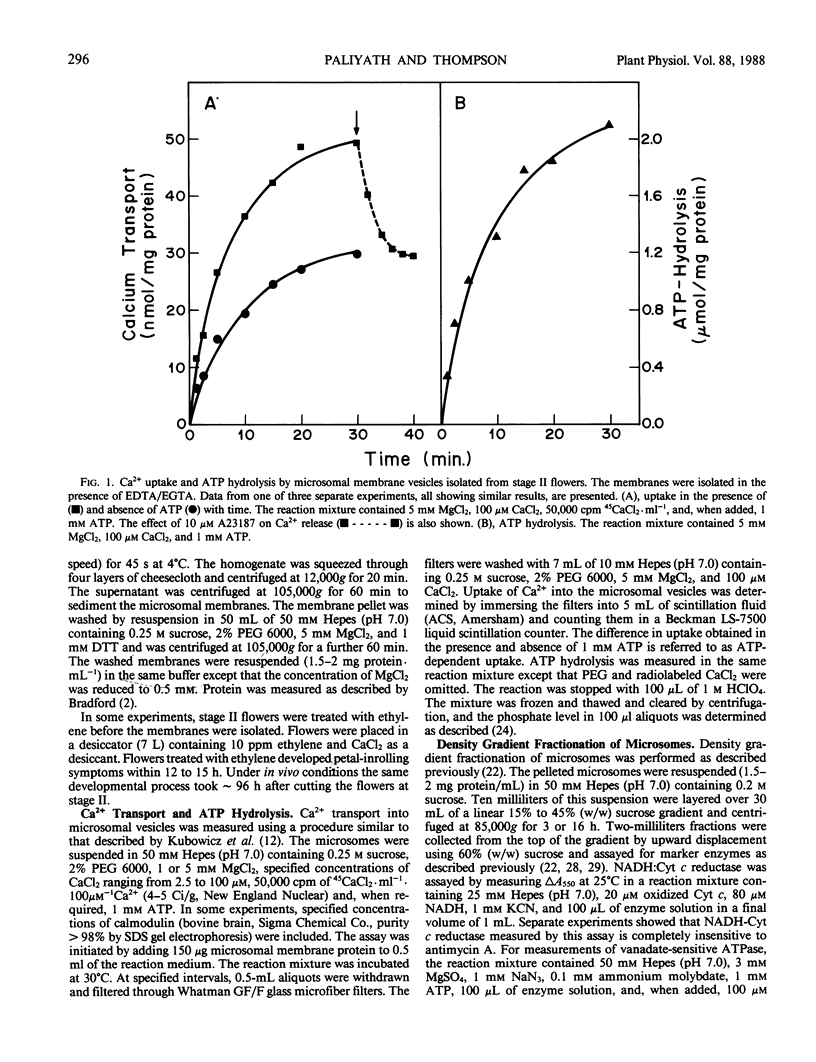
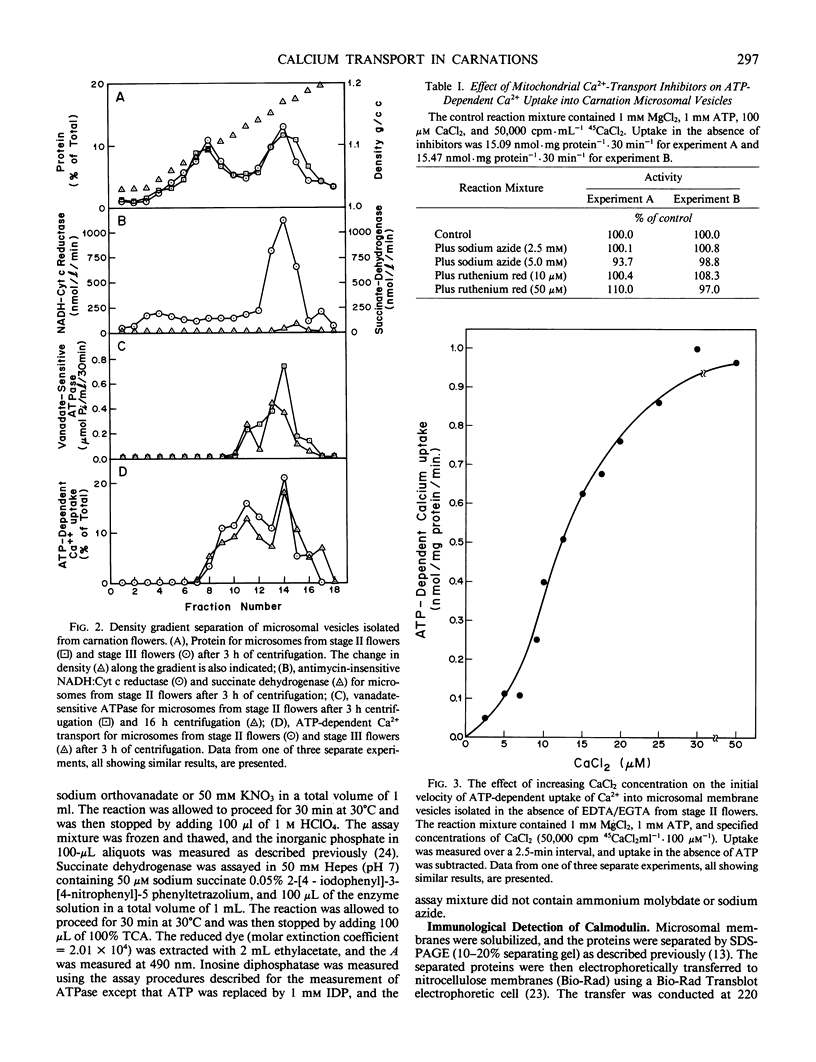
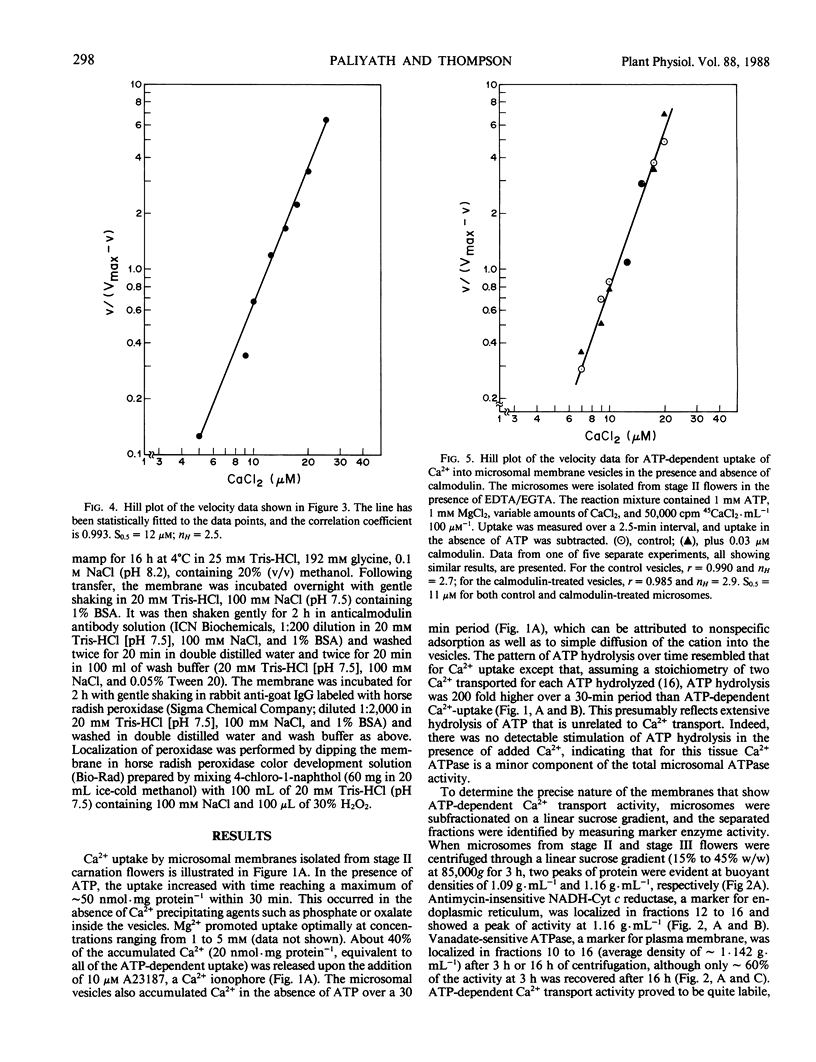

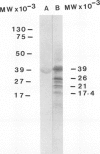
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buckhout T. J. Characterization of Ca Transport in Purified Endoplasmic Reticulum Membrane Vesicles from Lepidium sativum L. Roots. Plant Physiol. 1984 Dec;76(4):962–967. doi: 10.1104/pp.76.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. R., Sze H. Calcium transport in tonoplast and endoplasmic reticulum vesicles isolated from cultured carrot cells. Plant Physiol. 1986 Feb;80(2):549–555. doi: 10.1104/pp.80.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Lehninger A. L. Ca 2+ transport activity in mitochondria from some plant tissues. Arch Biochem Biophys. 1973 Jul;157(1):183–196. doi: 10.1016/0003-9861(73)90404-9. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Wüthrich A., Bader H. R 24571: a new powerful inhibitor of red blood cell Ca++-transport ATPase and of calmodulin-regulated functions. Biochem Biophys Res Commun. 1981 Jul 30;101(2):418–425. doi: 10.1016/0006-291x(81)91276-6. [DOI] [PubMed] [Google Scholar]
- Hale C. C., Roux S. J. Photoreversible calcium fluxes induced by phytochrome in oat coleoptile cells. Plant Physiol. 1980 Apr;65(4):658–662. doi: 10.1104/pp.65.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubowicz B. D., Vanderhoef L. N., Hanson J. B. ATP-Dependent Calcium Transport in Plasmalemma Preparations from Soybean Hypocotyls : EFFECT OF HORMONE TREATMENTS. Plant Physiol. 1982 Jan;69(1):187–191. doi: 10.1104/pp.69.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lew R. R., Briskin D. P., Wyse R. E. Ca uptake by endoplasmic reticulum from zucchini hypocotyls : the use of chlorotetracycline as a probe for ca uptake. Plant Physiol. 1986 Sep;82(1):47–53. doi: 10.1104/pp.82.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliyath G., Thompson J. E. Calcium- and calmodulin-regulated breakdown of phospholipid by microsomal membranes from bean cotyledons. Plant Physiol. 1987 Jan;83(1):63–68. doi: 10.1104/pp.83.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., De Michelis M. I. The Ca-Transport ATPase of Plant Plasma Membrane Catalyzes a nH/Ca Exchange. Plant Physiol. 1987 Apr;83(4):994–1000. doi: 10.1104/pp.83.4.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Calcium transport into the vacuole of oat roots. Characterization of H+/Ca2+ exchange activity. J Biol Chem. 1986 Sep 15;261(26):12172–12178. [PubMed] [Google Scholar]
- Thompson J. E., Mayak S., Shinitzky M., Halevy A. H. Acceleration of membrane senescence in cut carnation flowers by treatment with ethylene. Plant Physiol. 1982 Apr;69(4):859–863. doi: 10.1104/pp.69.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Isolation and Identification of Plasma Membrane from Light-Grown Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1983 Nov;73(3):586–597. doi: 10.1104/pp.73.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kawata T., Uemura M., Niki T. Isolation and Characterization of Tonoplast from Chilling-Sensitive Etiolated Seedlings of Vigna radiata L. Plant Physiol. 1986 Jan;80(1):161–166. doi: 10.1104/pp.80.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]