Graphical abstract
Keywords: Microbial dysbiosis, Colorectal cancer, Short-chain fatty acids, High-fat diet (HFD), Non-invasive diagnosis, Faecal microbial transplant (FMT)
Highlights
-
•
Diet-mediated gut microbial dysbiosis in early & late onset of CRC was reviewed.
-
•
The potentiality of biomarkers for the diagnosis and treatment of CRC was discussed.
-
•
The role of prebiotics & probiotics in modulating the gut microbiota was discussed.
-
•
The fecal microbial transplantation for the treatment of CRC was discussed.
-
•
Future perspective targeting the dietary influence on gut microbiota was discussed.
Abstract
Background
Over the last decade, studies have shown an increased incidence of colorectal cancer (CRC), particularly early onset colorectal cancer (EOCRC). Researchers have demonstrated that dietary behavior, especially among young adults, influences alterations in the gut microbial community, leading to an increased accumulation of pathogenic gut microbiota and a decrease in beneficial ones. Unfortunately, CRC is likely to be diagnosed at a late stage, increasing CRC-related mortality. However, this alteration in the gut microbiota (gut dysbiosis) can be harnessed as a biomarker for non-invasive diagnosis, prognosis, prevention, and treatment of CRC in an effort to prevent late diagnosis and poor prognosis associated with CRC.
Aim of review
This review discusses identification of potential biomarkers by targeting diet-mediated gut dysbiosis for the stage-specific diagnosis, prognosis, treatment, and prevention of CRC. Our findings provide a comprehensive insight into the potential of protumorigenic bacteria (e.g. pathogenic Escherichia coli, enterotoxigenic Bacteroides fragilis and Fusobacterium nucleatum) and their metabolites (e.g., colibactin and B. fragilis toxin) from gut dysbiosis as biomarkers for the diagnosis of CRC.
Key scientific concepts of review
Collectively, a detailed understanding of the available data from current studies suggests that, further research on quantification of metabolites and stage-specific pathogenic microbial abundance is required for the diagnosis and treatment of CRC based on microbial dysbiosis. Specifically, future studies on faecal samples, from patient with CRC, should be conducted for F. nucleatum among different opportunistic bacteria, given its repeated occurrence in faecal samples and CRC biopsies in numerous studies. Finally, we discuss the potential of faecal microbial transplantation (FMT) as an intervention to restore damaged gut microbiota during CRC treatment and management.
Introduction
Colorectal cancer (CRC) is one of the most common types of cancer and is considered as a critical public health matter, especially in high-income countries and young adults (<50-years- old) worldwide [1]. Generally, CRC ranks third in terms of incidence per year, but it accounts for the second highest cancer-related mortality worldwide in both sexes [2], with higher incidence and mortality rates in men than in women [3]. According to the American Cancer Society’s projections, by the end of 2022, new CRC cases in the United States are expected to rise to 104,270 and 45,230 for colon and rectal cancers, respectively [3]. If the current trend continues, the global burden of this cancer will increase by 60% to more than 2.2 million cases and 1.1 million deaths yearly by 2030 [4]. Additionally, another study showed that CRC is likely to persist beyond 2035 [5]. Such a scenario looks alarming for policymakers and health forecasters; therefore, our maximum efforts are required for a better future.
The reasons for the increase CRC incidence are complex. However, CRC occurs in a multi factor and multistep process, that involves age, environment, lifestyle, physical inactivity, dietary patterns, gut microbiome, sex, and genetic factors, along with other factors related to the socioeconomic status of individuals [6], [7]. Economic status is reflected in the high incidence of CRC in developed countries, especially in the Western world [6] and other countries with a high human development index (HDI) compared to low-income countries [8], [9]. However, the ongoing increase in early-onset CRC (EOCRC) has gained attention [10]. Although the underlying factors driving this rising trend in EOCRC have not been fully elucidated, several scientists have hypothesized that dietary-mediated gut microbial dysbiosis to be a key contributing factor [11].
Youths and young adults have a different diet and lifestyle compared to those of their parents; most noticeably young adults tend to prefer Western diet over a traditional diet [12]. This preference could be due to work-related factors, the ease and comfort of a Western diet that matches their lifestyle, commercial advertisements, and others. Diet is an important modifiable factor that shapes the gut microbiome [13]. However, evidence from recent studies shows that changing from a traditional to a Western diet increases the abundance of CRC-associated bacteria [11], although the mechanisms connecting such bacteria directly to carcinogenesis have not yet been fully elucidated. Additionally, this alteration in the abundance and diversity of gut microbiota due to dietary changes has been linked to colorectal carcinogenesis [14], [15], [16], [17]. Therefore, dietary changes from a traditional to Western diet could be a contributing factor for the rising trend of CRC cases among the young adults.
Despite an increasing trend in CRC incidence, CRC related mortality rates are lower than the number of dragonized patients in Western and high HDI countries [18]. This can be attributed to considerable awareness among the population and advanced health care, providing affordable and widespread screening tests such as the faecal immunochemical test (FIT), faecal occult blood test (FOBT), and colonoscopy. These accessible tests allow early detection and excision of premalignant lesions, reducing mortality rates in these countries, despite higher rate of new cases [3], [19]. Colonoscopy is the gold standard for CRC screening owing to its high sensitivity and specificity [15]. However, the costs of laboratory equipment and labor coupled with low patient adherence to screening have created the need to develop more affordable and comfortable screening techniques to increase adherence, especially in high-risk and low-income countries. CRC is associated with DNA mutations, volatile organic compounds, microRNAs, and signature metabolites that are related to gut dysbiosis which can be harnessed as potential molecular biomarkers for CRC early detection, prevention, and treatment [14]. This review discusses how diet-mediated gut microbial dysbiosis and its signature metabolites can be potential biomarkers for the early detection, diagnosis, prognosis, and prevention of CRC at different stages. Additionally, we discuss the potential of modulating gut microbiota by faecal microbial transplantation (FMT) as a means of treating and managing CRC.
Diet-mediated changes in the gut microbial community
The human gut is estimated to harbor more than 1014 microorganisms, including bacteria, fungi, viruses, and protozoa [20]. These microorganisms provide the host with physiological and health benefits such as protection and pathogen resistance, metabolism, immunomodulation, tissue structural repair, and integrity maintenance [16], [20]. Some foods are rich in indigestible carbohydrate polymers such as xylans, fructans, plant cell walls, and pectins. Gut microbiota, such as Firmicutes, Bacteroidetes, and Actinobacteria, encode genes for enzymes that depolymerize complex carbohydrates; producing beneficial substrates such as anti-inflammatory and anticancer metabolites, for example short chain fatty acids (SCFAs) [21], [22]. This means that the human gut requires high abundance of these microbiota, especially when the diet is enriched with indigestible carbohydrates. Consequently, dietary changes in consumption of fiber rich as well as indigestible carbohydrate foods may affect this abundance.
Notably, the gut microbial composition is often affected by illness, developmental stage, and exposure to different environmental factors and this phenomenon varies among species [23]. The first microbial colonization of the human gut occurs immediately after birth. This colonization can be attributed mainly to the mode of delivery, method of breastfeeding, use of antimicrobials and mother’s hygiene during breastfeeding hours [24]. This microbiota subsequently undergoes drastic changes after birth and during the initial months of life, and it increases as the baby grows and becomes exposed to the surrounding environment as well as solid foods [25]. When the child is exposed to solid foods, a secondary shift in gut microbial composition occurs, which is critical because it defines the gut microbial stability of an individual later in life [26].
The food we consume feeds not only us but also the large and diverse microbial community within the gut. In theory, the gut microbiota could be considered parasitic for feeding on our food; however, the relationship is symbiotic and the gut microbiota maintains the health and integrity of the gut (hence boosting host immunity and facilitating host response to medication), while the host provides nutrition and shelter. However, studies have highlighted that a disrupted symbiotic relationship between the host and gut microbiota is the root cause of several health issues, including CRC [27]. Symbiotic gut microbiota thrives on the residues of undigested dietary materials and, in turn, yields various metabolites that are crucial in gut immunomodulation and synthesis of essential nutrients such as vitamins, including biotin and folic acid, which reinforce the mucosal defense barrier [28], [29].
An adult with a stable gut microbiota may experience a series of short or long-term shifts in gut microbiota and diet is one of the key factors that influence these changes in the microbial composition of gut microbiota. This means that any changes in diet can induce a shift in microbial composition. Studies have shown that stable gut microbial taxa are resilient to most short-term external factors; however, short-term dietary interventions have been indicated to cause rapid changes in microbial composition, especially at the species and family levels [30]. Although the enterotypes of the core gut microbiota profile remained consistent, they tend to shift when an individual undergoes an extreme dietary intervention.
For example, a dietary intervention with fiber in the form of fructans and galactooligosaccharides resulted in an increased faecal abundance of Bifidobacterium and Lactobacillus species, without affecting gut microbial diversity [31]. These findings indicated that brief dietary changes such as those experienced during short trips and events may induce an increase in the abundance of certain species in the gut microbiota, although the diversity remains unaffected. This suggests that the gut microbiota remains stable and performs its symbiotic roles despite sudden changes in the diet.
Although an individual attains a stable adult-like microbial profile during the first 2–3 years of life, it continues to change during childhood [32]. Studies have shown that the quality and quantity of nutrients continue to affect the microbiota despite their stability in adulthood [33]. Studies also suggest that the habitual diet is associated with gut microbial changes, especially in enterotypes, compared with short-term diet interventions [30]. This indicates that effective changes in the gut microbiota through diet require long-term or habitual dietary behavior. In the study of two child microbiomes from Europe and Burkina Faso, Wu et al. (2011) found that long-term dietary changes were associated with enterotype partitioning [34], which further illustrates that habitual and long-term dietary behaviors are the only interventions to reshape gut microbiota.
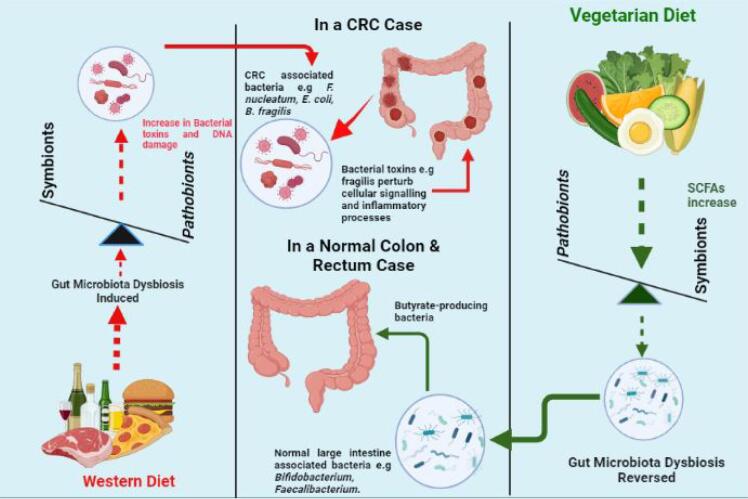
In another study, Fragiadakis et al. (2020), demonstrated that participants on a low carbohydrate diet had increased faecal abundance of Firmicutes, Bacteroidetes, and Proteobacteria at the phylum level [35]. In addition, the study showed that participants on a low-fat diet had a decrease in Actinobacteria and Firmicutes. This shows that a long-term shift to a low-carbohydrate diet can lead to an increase in the abundance of bacterial species associated with CRC carcinogenesis and progression. Given that a large proportion of individuals in the Western world would prefer a low-carb diet, it may be partly an explanation as to why Western countries are suffering from the burden of higher CRC risk; this increase in the risk could be due to their dietary intake, which is low in fiber and carbohydrates and high in red and processed meat [27]. This also explains why some countries such as Burkina Faso have low rate of CRC incidence; their habitual diet is based on low or no animal fats, and hence they have a low abundance of opportunistic bacterial phyla, such as Firmicutes. Studies have also indicated that transition from a traditional to a Western diet (Fig. 2) increases the risk of CRC [36]. For example, the number cases of CRC among of CRC first generation of Japanese migrants to Hawaii increased to levels similar to those of local Hawaiians, although traditionally the Japanese have low CRC cases [37], [38], [39]. Therefore, this evidence shows that diet is capable of causing gut microbiota dysbiosis and in response to long-term or habitual shift from traditional diet, gut microbiota may change completely. It also indicates that these habitual and long-term dietary interventions to the Western diet such as those among young adults, can lead to a high abundance of opportunistic gut microbes, many of which might be related to colorectal carcinogenesis.
Fig. 2.
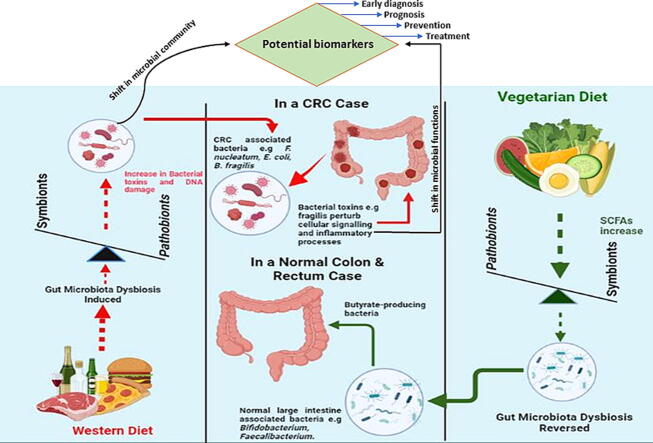
The role of diet-mediated dysbiosis in colorectal cancer (CRC). Effect of Western diet with low fiber, high sugar, salt and versus those of diets with high fiber and low animal fat on CRC onset.
Dietary effects on gut microbiota
Host diet composition, especially proteins, fat, fiber and probiotics, plays a crucial role in shaping the gut microbiota and fueling its involvement in the digestive process and synthesis of byproducts that are crucial to the host [17]. It influences the diversity and abundance of the gut microbiome [40], especially when a host consumes a balanced diet as well as on pre-probiotics, as shown in Table 3. In their study to assess the relative contribution of host genetics and diet to the shaping of the gut microbiota, Zhang et al. (2010) demonstrated that mice fed on a high-fat diet had key gut microbial community changes, such as the absence of the gut barrier-protecting population of Bifidobacteria spp. [41]. Additionally, in a humanized mouse model with transplanted adult human faecal microbiota fed on a low-fat and a polysaccharides rich in plant diets followed by a switch to a high-sugar and high-fat diet, the gut microbiota shifted to an overgrowth of Firmicutes and a significant reduction in the Bacteroides spp. population [42]. This demonstrates that dietary changes and interventions can influence the abundance and diversity of gut microbiota. Given the increasing incidence of early-onset CRC in the last decades [43], researchers have proposed a significant connection between CRC in younger adults and lifestyle characterized by dietary changes from traditional foods to a more Western-like diet. Here, we discuss how different dietary components affect the gut microbiota to enable young people to make informed food choices that will not expose them to early onset of CRC.
Table 3.
The effect of diet on common gut microbiota.
| Diet | Affected bacteria | Effect | Reference |
|---|---|---|---|
| Vegetarian | E. coli | Decreased | [148] |
| High fat | Bifidobacteria spp | Decreased / absent | [41] |
| High-fat and high-sugar | Firmicutes spp | Increased | [42] |
| Bacteroides spp | Decreased | ||
| Refined sugars | C. difficile and C. perfringens | Increased | [149] |
| Reduced-carbohydrate | Bacteroidetes spp | Increased | [150] |
| Calorie-restricted | Clostridium coccoides, Lactobacillus spp. And Bifidobacteria spp. | Restricted growth | [151] |
| Animal milk fat | δ-Proteobacteria | Increased | [152] |
Protein
Balanced diet containing a variety of nutrients is recommended to optimize health. However, proteins have recently received more attention than other nutrients. Proteins are the primary sources of amino acids and nitrogen in humans. Additionally, it provides substrates for symbiotic microbes in the gut that produce the beneficial SCFAs such as butyrate as well as pathogenic and opportunistic ones that produce harmful putrefactive metabolites such as ammonia, phenols, indoles, hydrogen sulphide and amines, through proteolytic fermentation processes in the gut [44]. The quality and quantity of protein consumed is key to the type of effects on both the host and its microbiota [45]. For example, Jantchou et al. (2010) demonstrated that a high-protein intake, especially from animal source, is associated with an increased risk of inflammatory bowel disease (IBD) [46]. This indicates that although protein deficiencies may cause diseases such as kwashiorkor especially in infants, high protein intake can also cause bowel diseases in addition to other excretory diseases and disorders, highlighting the importance of protein quantity on the outcomes of the host.
As mentioned above, the amount, type and source of dietary protein may also affect the abundance and diversity of the gut microbiota. For example, a high-protein diet causes a remarkable reduction in the production of acetate and SCFAs in the gut [47]. Acetate and SCFAs such as butyrate inhibit the growth of pathogenic and opportunistic bacteria in the gut [48]. In their absence, the gut environment becomes conducive to an increase in the abundance of pathogenic bacterial genera and a decrease in the abundance of beneficial bacteria. An example can be drawn from a study by Mu et al. (2016) in which rats fed a high-protein diet showed an increase in the abundance of pathogenic bacterial genera such as Escherichia/Shigella, Enterococcus, and Streptococcus, and a decrease in the abundance of beneficial bacteria such as Ruminococcus, Akkermansia and Faecalibacterium [47].
Additionally, rat models fed a high-protein diet by another research group showed an increased abundance of the pathogenic genus Escherichia and a decreased abundance of beneficial genera such as Akkermansia, Ruminococcus, Prevotella and Roseburia [49]. An increase in the abundance of pathogenic Escherichia species is disadvantageous because they are clinically pathogenic and are associated with one of the most powerful antibiotic resistance mechanisms of anaerobic pathogens [50]. Additionally, an increase in the abundance of Escherichia species is also undesirable because they are mostly opportunistic, which increases the risk of gastrointestinal diseases [47]. The decrease in the abundance of beneficial genera of carbohydrate degraders, including Akkermansia, Ruminococcus, Prevotella and Roseburia, in response to high-protein diet, highlights that such diets should be limited in favor of diets with a more appropriate macronutrient composition to avoid gut dysbiosis and a decrease in beneficial bacterial genera.
Food processing methods are essential for preservation and production. However, processing technologies used worldwide affect the structure of protein the processed foods. It should be noted that gut microbiota utilize undigested dietary proteins to derive cellular components and energy in some cases [51]. However, some food-processing technologies induce modifications to proteins, such as oxidation, aggregation, and denaturation, eventually influencing their digestibility and absorption. Therefore, processed foods with highly digestible proteins leave little or no indigestible substrates for proteolytic fermenting bacteria which decreases the relative abundance of various bacterial genera [51].
Fat
Dietary components affect the abundance and diversity of gut microbiota differently. Studies show that a high-fat diet (HFD) alters gut microbial composition as well as associated metabolites [52]. This alteration involves an increase in the pathogenic microbes in the gut, depletion of probiotics, and elevation of corresponding metabolites [52]. For example, a study by Wang et al. (2020) showed that a high-fat diet increased the biodiversity of the gut microbiota. However, mice fed a high-fat diet showed a reduced abundance of beneficial bacterial genera such as Lactobacillus, Bifidobacterium, Blautia and Akkermansia [53]. The above evidence suggests that in addition to inducing obesity, a high-fat diet discourages SCFAs and lactate-fermenting bacteria, and creates a favorable environment for pathogenic microbiota. In turn, this can disrupt the integrity of gut barrier protection mechanisms, thereby inducing bowel infections. Similarly, a recent study by Yang et al. (2022) showed that an HFD was associated with colorectal tumorigenesis by altering microbial abundance and associated metabolites, including an elevated production of lysophosphatidic acid which promotes CRC cell proliferation [52]. Additionally, studies have indicated that an HFD promotes CRC by stimulating and facilitating bile acid metabolism and its conversion into tumor-promoting deoxycholic acid by the gut microbiota [54].
Additionally, a study by Fava et al. (2013) showed that participants on a low-fat diet-fed had an increased abundance of Bifidobacterium, a beneficial bacterial genus [55]. This also aligns with the general trend regarding the effect of HFD on gut microbiota. Moreover, animal studies have indicated that HFD-fed animals have a significant reduction in the abundance of lactic acid bacteria such Bacillus bifidus [56]. These findings suggest that a diet rich in fat discourages the growth of beneficial bacteria, mainly because of a lack of their favorable nutritional substrates. This partly explains why individuals consuming a typical Western diet, which is mainly composed of protein and fat, are more likely to have a higher abundance of Bacteroides compared to that in individuals consuming other diets [34].
One of the most crucial activities of the gut microbiota is the production of SCFAs, which increase the abundance of the family Ruminococcaceae as well as Lactobacillus and Bifidobacterium at the genus level [57]. However, since the effects of an HFD on gut microbiota have not been extensively studied, future studies are required to establish a clear relationship between HFD and gut microbiota in humans and animals.
Fiber and non-digestible carbohydrates
The current dramatic decline in the consumption of fiber-rich foods in many parts of the world is believed to be the cause for the sharp increase in CRC cases. For example, it is estimated that a typical modern daily US diet contains approximately 15 g of fiber, which is substantially less than the recommended daily fiber allowance [58]. Individuals in non-industrialized societies such as those in the rural areas of South Africa and Uganda are estimated to consume 50 g of dietary fiber per day. This partly explains why such individuals are largely free from chronic bowel diseases such as CRC [59]. Additionally, shifting from a typical American diet to a typical African-style diet that is rich in fiber and contains more than 55 g per day can reverse the risks of CRC [60]. The importance of a high-fiber diet was first hypothesized by Burkitt, in 1970 s, who highlighted that cases of bowel diseases such as CRC were lower in Africa and rural tropical areas than in urbanized industrial countries [61]. He attributed his observation to a diet with limited dietary fiber among urban populations. Low dietary fiber intake not only affects an individual’s health directly but also indirectly through changes in the gut microbiota, which are crucial for digestion [58].
Dietary fibers are polysaccharides that do not undergo rapid endogenous enzyme digestion in the small intestine, but persist through the gut into the colon, where they are metabolized by resident microbes and microbial metabolites [62]. Some of the by-products of digestion in the colon are SCFAs, which affect human health when absorbed by epithelial cells in the colon [63]. Therefore, understanding the role of dietary fiber in structuring the gut microbial community can provide insights into the management of chronic diseases associated with dysbiosis.
Recently, Oliver et al. (2021) demonstrated that a short-term (2 weeks) increase in dietary fiber intake altered the composition of the gut microbial community, with a notable increase in fiber-degrading bacteria such as Lactobacillus and Bifidobacterium [58]. Furthermore, they showed that the dietary intervention altered the gut microbial community, but highlighted Bifidobacterium as the strongest predictor of the gut microbiome post-fiber [58]. However, the co-occurrence of Bifidobacterium with other genera was assessed because Bifidobacterium cross-feeds with other gut microorganisms [64]. Additionally, they re-established the important fact from previous studies that the abundance of Bifidobacterium increases following high fiber intake [31]. The gut microbiota ferments fiber in the colon into butyrate, a metabolite that has been increasingly associated with modulating the tumor microenvironment [65]. The increased abundance of beneficial bacterial genera as a result of fiber intervention suggests that an increased uptake of a diet rich in fibers modulates the gut microbiota by increasing the abundance of genera that maintain the integrity of the gut and promote good health.
Pre- and probiotics
Pre- and probiotics play crucial roles in modulating the gut microbial community of the host. They can restore the composition of gut microbiota, introduce beneficial functions to the gut microbiota, increase the diversity and abundance of beneficial gut microbiota, increase gut microbial metabolites, influence gut microbial colonization, and nourish the gut microbiota, among other benefits [66]. According to the International Scientific Association for Probiotics and Prebiotics (ISAPP), probiotics are live microorganisms that confer health benefits to hosts when ingested in sufficient quantities. Probiotics can be naturally occurring microbes in or on our body; however, they are usually consumed with the food we eat, especially fermented food such as dairy products. Although several microbes can be probiotics, the majority of probiotics are from the Lactobacillaceae family or species of Bacillus, Bifidobacterium, or Saccharomyces [67]. For microbes to function as probiotics, they must be alive when administered, with studies indicating health benefits to the target host [68]. Studies have shown that dietary supplementation of a patient with a disrupted gut microbial balance ensures recovery and reconstruction of the gut microbiota [69]. This is further supported by studies that show that treatments with a side effect on gut microbiota imbalance include probiotics in the treatment regimen improve restoration of gut microbial balance [70]. Dietary probiotics include kefir, yogurt, sauerkraut, sour pickles, tempeh, kimchi, and soft cheese, containing microbes of the genera Lactobacillus, Bifidobacterium and Streptococcus [71]. These lactic acid-producing bacterial genera ensure that lactate is produced in the gut. Lactate regulates the immune microenvironment in solid tumors such as CRC. Conversely, prebiotics, are dietary substrates in the form of carbohydrates and fiber, which are selectively utilized to feed ‘friendly’ microbiota conferring health benefits to the host [72]. Although there are few studies on prebiotics, the available literature shows that prebiotics improve the abundance of gut microbiota by feeding probiotics, thereby indirectly increasing the levels of beneficial microbes [73].
For dietary substances to be classified as a prebiotic, they should: (i) be resistant to gastric acidity, not be metabolized by the host enzymes, and not be absorbed in the gastrointestinal tract; (ii) selectively stimulate the activity and/or growth of gut microbiota, which improves the host’s health, and finally (iii) be fermented by the gut microbiota [74]. Although some studies have described dietary prebiotics as oligosaccharides (OSCs), other studies have indicated that prebiotics include dietary compounds other than carbohydrates [75]. Some of these are fructans, including inulin and fructooligosaccharides. These selectively stimulate the activity and growth of lactic acid bacteria and other gut microbes [76]. Galacto-saccharides are another type of prebiotics that significantly stimulate the growth and activity of Lactobacillus and Bifidobacterium, and to a lesser extent Firmicutes, Bacteroidetes, and Enterobacteria [77]. Prebiotics exist naturally in different forms in foods, such as cow’s milk, wheat, beans, Jerusalem artichoke, honey, onion, garlic, barley, soybean, asparagus, sugar beet, and peas [78]. By providing the ‘friendly’ gut microbiota with a source of energy, prebiotics can modulate the composition and function of these microbes for health benefits. Most prebiotic fermented products are acidic in nature [75]; therefore, they can act as pH-modulatory mechanisms for some gut microbiota, thereby, preventing the loss of SCFAs-producing bacteria, while inhibiting the growth of the pathogenic ones [79].
Typical gut microbiota dysbiosis associated with colorectal carcinogenesis
The human gut microbiota is susceptible to frequent variations, especially at lower taxonomic levels; however, the four phyla Proteobacteria, Firmicutes, Bacteroidetes and Actinobacteria are always dominant. Dietary changes cause dysbiosis because different food support specific microbial communities; therefore, a balanced diet can help avoid problems. When gut dysbiosis occurs, the diversity and abundance of beneficial and neutral microbes are lost, leading to an increase in pathogenic microbial communities [Table. 3], which cause chronic diseases [80]. This disrupts the symbiotic relationship between the host and gut microbiota, resulting in an undesirable inflammatory responses, cell damage, autoimmunity, and, consequently, chronic gastrointestinal tract diseases such as CRC. Maintaining a symbiotic relationship requires the host’s immune system, as well as the gut microbiota, to be stable and healthy, to eliminate pathogens or prevent them from colonizing through the maintenance of healthy self-tissue cells.
Given that our gut microbiota is susceptible to repeated damage by the environment and antibiotic use, it should be repaired swiftly to restore mucosal homeostasis. Otherwise, dysbiosis can disrupt the symbiotic relationship, thereby risking colonization by opportunistic bacteria as well as dysregulation of the immune system; if this occurs, healthy tissue cells can get mistakenly attacked and destroyed, leading to autoimmune and bowel-related diseases such as CRC [81], [82].
Studies have provided increasing evidence of gut microbial dysbiosis in CRC [83], [84]. As the gut microbiota and mucosa interact within colorectal tissues, epithelial cells form a protective barrier, which is continuously maintained by replenishing the epithelial cells with multipotent stem cells [85]. The gut microbiota is involved in the maintenance of the epithelial barrier by ensuring mucosal homeostasis and protection against pathogenic threats [11]. This is achieved by commensal bacteria and immune cells through a delicate balance between proinflammatory and oncogenes and anti-inflammatory genes and antioncogenes [86]. However, dysbiosis creates an imbalance between the beneficial and opportunistic microbiota. This causes frequent attacks on the protective epithelial barrier by pathogens, including toxin-producing bacteria, thereby weakening its integrity [87]. As integrity weakens, opportunistic bacteria bind to the epithelium, inducing the release of inflammatory cytokines, suppression of mucosal immunity, and DNA damage thereby driving CRC tumorigenesis [88]. Recent studies have demonstrated colorectal carcinogenesis by gut microbiota dysbiosis in carcinogenesis models, including germ-free rats [89], which confirms the close relationship between dysbiosis and CRC. Further evidence of this relationship can be drawn from studies by Yung et al. (2017) and Fan et al. (2016) in which opportunistic bacteria abundant during dysbiosis induced tumorigenesis in experimental subjects [90], [91].
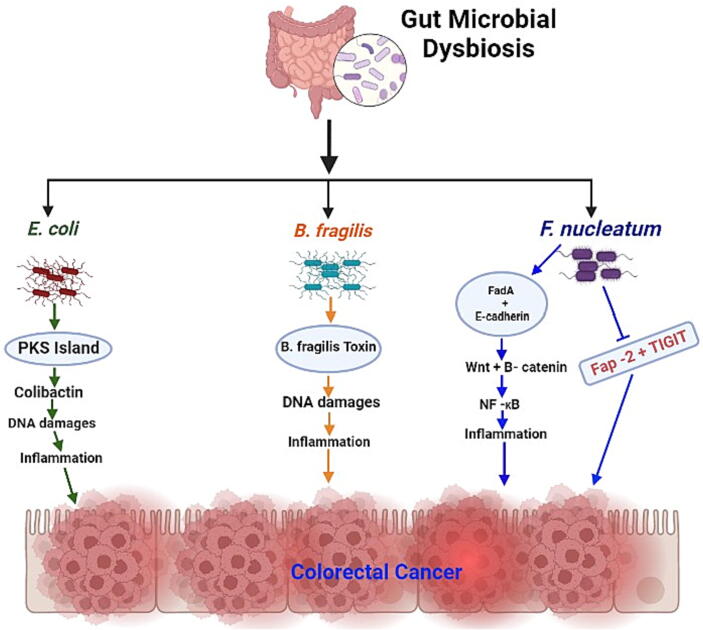
Faecal and metagenomic studies by different scientists [92], [93] indicated that some gut bacterial species are predominant in the faecal samples as well as tumor microenvironments of patients with CRC, in high abundance, compared to those of healthy individuals and normal tissues adjacent to tumor microenvironments. This provides insights into the possible correlation between these bacterial species and colorectal carcinogenesis. In previous studies, scientists have repeatedly associated these species with colorectal carcinogenesis [16], [94], although the exact mechanism of CRC initiation by these species has not yet been fully studied. Nevertheless, bacterial species such B. fragilis, F. nucleatum, E. coli, Helicobacter pylori, Streptococcus gallolyticus, and Enterococcus faecalis have been widely implicated in several studies as key players in colorectal carcinogenesis [95] through different mechanisms, some of which are depicted in Fig. 3.
Fig. 3.
Role of gut microbiota and their metabolites in colorectal carcinogenesis.
Unlike other repeatedly-mentioned bacterial species, recent studies have strongly implicated F. nucleatum in the carcinogenesis of CRC [96]. This finding implicates the high potential of this bacterium as a biomarker for CRC diagnosis, prognosis and prevention. This opportunistic gram-negative bacterium is indigenous to the human oral cavity and plays a key role there in periodontal disease [95]. This means that its presence in faecal samples could be harnessed as a non-invasive CRC screening technique. Studies have shown that this bacterium induces and promotes CRC visa various mechanisms. For example, the FadA adhesin of this bacterium binds to E-cadherin, thereby driving the activation of β-catenin and the Wnt signaling pathway [97]. This in turn induces oncogenic responses in CRC cells [98]. Additionally, this bacterium increases the phosphorylation of p65, which is a crucial component in the activation of NF-KB signaling [89]. This activation leads to increased expression of the carcinogenic microRNA such as miR21. Consequently, inflammation in the colon tissues is induced, resulting in colorectal tumorigenesis [99]. Moreover, the F. nucleatum Fap-2 has been shown to defend colorectal tumors by inhibiting NK cell activity in the tumor microenvironment [100]. Gur et al. (2015) showed that Fap-2, when bound to the T cell immunoreceptor with Ig and ITIM domains (TIGIT) on NK and T cells, interferes with the host immune system attack on tumors, thus protecting them from elimination by the immune system [101]. This allows F. nucleatum-induced colorectal tumors to progress, resulting in CRC. Therefore, this bacterium is indeed implicated in colorectal carcinogenesis, based on published literature.
B. fragilis, is a symbiotic bacterium that colonizes the human gut and contributes significantly to food digestion by the host. However, this bacterium exists in two strains, nonpathogenic and pathogenic. The pathogenic strain, enterotoxigenic B. fragilis (ETBF), which has been shown to be highly abundant in faecal samples of patients with CRC [102], produces B. fragilis toxin (BFT) that triggers DNA double-strand breaks, which disrupts the gut epithelial cells with repeated inflammation, thus promoting progression of CRC. Additionally, this toxin binds to colorectal epithelial cells, thereby stimulating the cleavage of E-cadherin, an important tumor suppressor [103]. As a result, pro-carcinogenic responses such as Wnt signaling pathway, and mucosal inflammation are triggered, which promotes colorectal tumor formation [104]. Additionally, this toxin upregulates the expression of spermine oxidase (SMO), a polyamine catabolic enzyme, in colorectal epithelial cells, which increases SMO-dependent reactive oxygen species (ROS) that promote DNA damage [102]. This increases ROS-dependent inflammation, DNA damage, and consequently carcinogenesis [104]. These and other mechanisms not described here implicate this bacterium as a major contributor to CRC carcinogenesis.
Another highly relevant bacterium in CRC carcinogenesis is E. coli. This facultative gram-negative anaerobic bacterium symbiotically colonizes the human gut. The bacterium has four major phylogenetic groups i.e., A, B1, B2, and D [105]; however, strains from groups D and B2 are known to be pathogenic. B2 group strains associated with CRC carcinogenesis via different mechanisms are an excellent example. Group B2 strains possess a unique genotoxicity gene island called pks which synthesizes a toxin colibactin, and the genotoxic properties of this toxin cause DNA double-strand breaks, cell cycle arrest, chromosomal aberration, and chromosomal instabilities (CSI) in tissue cells [106]. The resulting genome instability paves the way for pregulated expression of oncogenes such as c-Myc, which leads to the formation of adenomas and the development of CRC [107]. Recent evidence shows that colibactin is synthesized as a prodrug, that is activated by the peptidase enzyme; clbP, which is present on the pks island [108]. A study that aimed to identify inhibitors of this enzyme showed that targeting the enzyme prevented the deleterious effects of colibactin and pro-tumoral effects induced by the toxin [109]. This demonstrated the involvement of the toxin in tumorigenesis and E. coli phylogenetic group B2 involvement in CRC carcinogenesis. Therefore, these discussed mechanisms (and many others) partly explain our notion that the high abundance these bacteria in the faecal samples of patients with CRC is related to carcinogenesis.
There is some evidence that such opportunistic gut microbiota initiates colorectal carcinogenesis using the driver-passenger model [110]. According to this model, local bacteria called the “driver bacteria” are responsible for initiating the process by producing genotoxins that trigger DNA damage. For example, B. fragilis produces BFT, which stimulates inflammation in epithelial barrier cells, resulting from DNA damage. Additionally, tumor suppressor proteins such as E-cadherin are cleaved by these “driver bacteria”, which provides an opportunity for tumors to develop [111]. As these develop, rupture and bleed, the surrounding microenvironment changes, into a favorable environment that promotes the growth of a second set of opportunistic bacteria called as “passenger bacteria” such as the F. nucleatum, that mediate CRC tumorigenesis [110]. This model demonstrates that these bacteria play a central role in the initiation, promotion and progression of CRC.
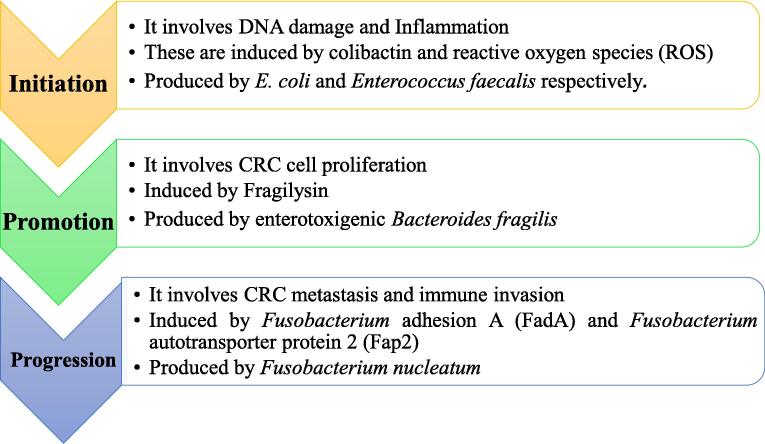
It should be noted that colorectal carcinogenesis can be divided into three stages i.e. initiation, promotion, and progression. There are specific microbes which have been repeatedly identified in carcinogenesis, as shown in Fig. 1, by causing initial inflammation of colorectal tissues and modulating signaling pathways, such as the Wnt/ β-catenin signaling pathway in active cells within these tissues [112].
Fig. 1.
Gut microbiota-mediated initiation, promotion and progression of colorectal cancer.
As mentioned above, the gut microbiota in a healthy person is always in a stable state where abundance and diversity, a balance between pro- and anti-inflammatory cytokines, and an intact mucosal barrier are all ensured. However, dysbiosis affects all these balanced parameters. These changes encourage an increased abundance of CRC-associated bacteria, which ultimately initiates carcinogenesis.
During the initiation of CRC, the gut microbiota influences DNA damage, inflammation, immune dysfunction including cleavage of antitumor suppressors, and modification of dietary metabolic components that lead to the production of oncometabolites and genotoxins [113]. Therefore, opportunistic bacteria such E. coli group B2 and enterotoxigenic B. fragilis producing colibactin and BFT respectively, are likely to be the prime initiators of colorectal carcinogenesis. During promotion, developing colorectal tumors undergo chronic inflammation [114], which enhances the rate of DNA damage and mutations, angiogenesis in the tumor mucosa and proliferation of adenomas. During progression, the formed colorectal tumors grow and metastasize to other organs [115]. Gut microbiota at this stage produce oncometabolites that downregulate antitumor T cell immunity and/or induce hepatocyte growth factor that promotes the growth and progression of tumors [116], [117]. Therefore, gut microbiota acts as mediator of CRC-progression. For example, studies have indicated that F. nucleatum inhibits the activity of natural killer cells targeting colorectal tumor cells by binding the protein Fap2 human T cell immunoglobulin and ITIM domain molecules, thereby protecting tumors from the immune system, which provides a chance for tumor cells to progress [118]. This bacterial species also creates a tumor-favorable microenvironment in the colorectal tissue by inducing the expression of tumor necrosis factor alpha, and the activation of NF-KB, favoring tumor proliferation and progression [118]. This mediation role of F. nucleatum could partly explain a high abundance of this bacterium in the advanced and metastatic stages of CRC reported in some studies [119].
Microbial community and its metabolites as biomarkers for early diagnosis of CRC at different stages
Having observed the potential involvement of specific gut microbiota in the initiation, promotion and progression of CRC, we can harness this observation for clinical purposes in CRC treatment and management. Under clinical descriptions, CRC is categorized into different pathological stages that enable physicians and oncologists to make informed medical care decisions for patients with CRC [120]. These pathological stages provides an assessment of the local, regional, and distant extent of the cancer. CRC oncologists generally use the American Joint Committee on Cancer (AJCC) TNM system for staging the condition of the patient [3], based on the following information i.e.,:
-
i.
Size and depth of the tumor (T) in the colon or rectal tissue
-
ii.
Spread of cancer to the nearby lymph nodes (N),
-
iii.
Spread of cancer (metastasis) to distant organs or body parts (M)
CRC stage is determined as one of five stages: 0 and I through IV, with the details of each stage in the CRC TNM system (Eighth edition), as shown in Table 1.
Table 1.
The TNM system of colorectal cancer stage classification.
| Group Staging | Stage Description |
|---|---|
| Primary Tumor (T) | |
| T Category | T Criterion |
| TX | The primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Cancer in situ, intramucosal carcinoma. The tumor has not grown beyond the inner layer of the colorectum |
| T1 | The tumor has invaded the submucosa layer of the colorectum |
| T2 | The tumor has invaded the muscularis propria |
| T3 | The tumor has invaded the peri-colorectal tissue through the muscularis propria |
| T4 | The tumor has invaded the visceral peritoneum |
| T4a | The tumor has invaded through the visceral peritoneum, that is, the tumor grows through all layers of the colon |
| T4b | The tumor has grown directly invaded adjacent organs or structures |
| Lymph node (N) | |
| N Category | N Criterion |
| NX | The regional lymph nodes cannot be evaluated |
| N0 | The tumor has not spread to regional lymph nodes |
| N1 | Presence of one, two or three positive regional lymph nodes (containing tumor cells) |
| N1a | One regional lymph node contains tumor cells |
| N1b | Two or three regional lymph nodes contain tumor cells |
| N1c | Lymph node-like structures near the colon or rectum contain tumor cells |
| N2a | The tumor cells have spread to 4 to 6 nearby/ regional lymph nodes |
| N2b | More than 7 regional lymph nodes contain tumor cells |
| Metastasis (M) | |
| M Category | M Criterion |
| M0 | Colorectal cancer has not spread to other body parts/ organs beyond the colon or rectum such as the liver or lungs |
| M1a | The disease has spread to one other body part/organ beyond the colon or the rectum |
| M1b | The disease has spread to more than one body part/ organ beyond the colon or rectum |
| M1c | The disease has spread to the peritoneal surface in the peritoneal cavity |
Before effecting to colorectal tissues, opportunistic microbiota produces metabolites and networks of functional features for their survival. These metabolites and network features can be harnessed as biomarkers for the detection and prognosis of CRC at different stages [121]. For example, analyzing patient faecal samples and genotoxins load can show if the patient has actively developing and proliferating tumors, and therefore, has a higher chance of survival upon immediate treatment [Table 2]. Moreover, pathogenic E. coli that produces a genotoxin called colibactin (CoPEC) was more abundantly observed in the mucosa of patients with CRC with stage III/IV tumors than those in stage I [122]. This shows the potential of genotoxin detection in patient samples as a diagnostic technique for CRC stages III and IV. Additionally, secondary gut microbial metabolites such as phenolic compounds, antibiotics, SCFAs, and bile acids such as deoxycholic acid (DCA), sulfurated hydrogen, and alkaloids [123], have a great potential for diagnosis and screening. Some of these metabolites such as SCFAs eg., butyrate enter tumor cell nuclei and act as histone deacetylase inhibitors, suppressing tumor cell proliferation [2]. Therefore, loads of SCFAs in faecal samples could signify healthy colorectal tissues. Additionally, secondary metabolites such as deoxycholic acid (DCA) have been identified as CRC promoters in several studies [124], [125]. Therefore, the presence of DCA load in patient faecal samples could indicate advanced stages of CRC.
Table 2.
Five-year relative survival rates for colorectal cancer. The numbers are based on the number of people diagnosed between 2011 and 2017 with colorectal cancers in the United States.
| SEER Stage |
The 5-year relative survival rate |
|
|---|---|---|
| Rectal Cancer | Colon Cancer | |
| Localized | 90% | 91% |
| Regional | 73% | 72% |
| Distant | 17% | 14% |
| Combined SEER stages | 67% | 64% |
Similarly, a patient’s faecal sample with F. nucleatum load could signify an advanced stage of CRC. This is based on evidence from several studies demonstrating that the relative abundances of opportunistic gut microbiota differ with the CRC stage. For example, Fang et al. (2021) demonstrated a decrease in the abundance of the phylum Synergistetes in their research participants with polyps compared to that in healthy participants. However, they showed that the abundance of this phylum increased in participants with advanced colorectal tumors [2], a trend that was also reported in previous studies [121]. The shift in the abundance of this or any other specific phylum can be harnessed as a biomarker to detect the extent of colorectal tumors. A previous study showed that faecal samples enriched with biofilm-forming genera (such as Peptostreptococcus) could signify advanced stages of CRC [126]. Biofilms have been reported to have pro-carcinogenic activities [127] and protect tumor environments against host immunity, which is one of the hallmarks of advanced cancer. Several other studies have indicated that the presence of human oral microbiome in high abundance (such as Fusobacterium) in the faecal samples confirms advanced CRC stages [128], which can be possibly another potential biomarker to indicate advanced stages of CRC. Additionally, lactic acid lowers gut pH, thereby inhibiting the growth of numerous opportunistic bacteria. Studies have noted that patients with advanced stages of CRC, such as adenoma and carcinoma, lack lactic acid-producing bacteria [129], [130]. This means that in addition to the presence of CRC bacteria, lack of lactic acid-producing bacterial genera, such as Bifidobacterium and Lactobacillus in these patient faecal samples can be a confirmatory test for a positive microbiota-based CRC diagnosis.
Therefore, microbial communities and their metabolites have a strong potential as non-invasive diagnostic techniques for CRC with stage-specific screening results. This can be harnessed to solve the issues of late diagnosis in young adults [131] and poor prognosis [132] which are associated with CRC. Therefore, a patient whose faecal samples contain high abundance of any of the bacterial species F. nucleatum, S. gallolyticus, E. coli, Peptostreptococcus anaerobius, E. faecalis and enterotoxigenic B. fragilis (ETBF) can be declared positive for CRC [133], without the need for restrictive pre-diagnosis preparations. The results are delivered faster, easier, and are more cost effective. Abundance studies can be time consuming; however, test results are required in a timely manner. Further research is needed to evaluate the potential of any of these most abundant species in combination with serology and/or molecular techniques for efficient, effective, sensitive, and timely diagnosis of CRC. Further research is also needed to fully understand stage-specific microbial communities and their signature metabolites for the effective and accurate use of gut microbiota as a non-invasive stage-specific diagnosis of CRC.
Faecal microbiota transplant (FMT) in CRC management
In addition to diet, gut microbiota can be modulated by other factors such as faecal microbiota transplantation (FMT) [134]. In FMT, faecal material, including bacteria and natural antibacterial agents, is transferred from a healthy individual to a patient’s intestine as treatment [135], which is possibly to be the most direct means manipulating the gut microbiota [136]. The prepared FMT doses can be administered to the target patients orally through freeze-dried or frozen capsules, or directly by infusing a faecal suspension via colonoscopy [134]. FMT is currently being tested for the treatment of several diseases. A case in point is the application of this technique in the treatment of patients with Clostridium difficile infection (CDI), who were resistant to conventional therapies [135]. Following this application, CDI was successfully treated with a cure rate of greater than 90%. As the most direct way to manipulate the gut microbiota [16], [137], such manipulations have been performed in various disease models and clinical trials, thus attracting public health attention [138]. Due to the high cure rate (greater than90%) and the rarity of associated side effects, this technique has been considered by many (including approval by the US Food and Drug Administration) as a potentially life-saving treatment option for various infections, such as recurrent CDIs [135], [139]. Studies have also indicated the use of FMT to restore the gut microbial diversity and composition [138], [140], [141]. Several studies have indicated a close relationship between gut dysbiosis and CRC initiation and progression [142], [143], and FMT can be used as an intervention, to restore the gut microbiota, especially beneficial genera with homeostasis and tumor suppressor activity [144].
In CRC management, modulation of the gut microbiota through FMT can restore chemotherapy-mediated gut dysbiosis. Chang et al. (2020) showed that FOLFOX, on 5-fluorouracil-based chemotherapy, altered the abundance of major gut microbiota phyla such as Bacteroidetes and Firmicutes [135]. However, administration of FMT restored the abundance of these phyla, indicating that FMT may have the clinical potential to restore chemotherapy-induced gut dysbiosis [145]. Their study also revealed that FMT ameliorated the severity of chemotherapy-induced mucositis, a painful side effect of chemotherapy [134]. This suggests that FMT can be used in CRC management to reverse the chemotherapy-induced mucosal barrier damage during cancer treatment [135].
Unfortunately, the use of FMT as a therapy for CRC and other bowel infections has been associated with several concerns regarding its safety [138]. Wang et al. (2016) noted that FMT might have adverse effects that should be monitored throughout the process [138]. They may include the dissemination of unknown pathogens and disease-causing genes from the donor’s stool to the recipient, however, all these uncertainties are still understudied, with lack of large-scale and long-term prospective trials. Additionally, the entire process of selecting and screening donors, blood and stool testing, and collection, preparation, and storage of FMT faeces could be laborious, inconvenient and time consuming [146]. This uncertainty regarding the safety of FMT might limit its use, especially in patients with compromised immunity and those receiving treatment with antineoplastic agents [147]. Therefore, further studies on the use of FMT treatment strategy should be conducted to understand the long- and short-term effects and prevention of post screening occult infections.
Future prospects and recommendations
Colonoscopy is the gold standard for CRC screening worldwide. However, expenses involving laboratory equipment and skilled personnel have made CRC screening costly in low-income countries, leading to late detection and reduced chances of survival. In developed countries, patient adherence is low owing to the discomfort of the colonoscopy screening process; yet, the early-onset CRC incidence per year is the highest in these countries. This creates a need for the development of less invasive screening techniques to encourage screening and early detection of CRC. Our aim was to show how gut dysbiosis can be harnessed as a potential biomarker for the diagnosis, prognosis, and treatment of CRC in the near future.
Studies of microbial profiles among patients with CRC and healthy individuals have demonstrated an increased abundance of F. nucleatum, B. fragilis and E. coli among patients with CRC compared to that in healthy individuals using metagenomics. However, diagnoses based on these tools are poorly timed. These highly abundant microbes among patients should be further studied using serology and molecular diagnostic techniques to ensure timely diagnosis. FMT has been reported to restore gut microbiota after dysbiosis; however, the skepticism regarding its safety in the form of pathogens and disease-causing gene transmission has limited its clinical application. Large-scale studies should be conducted in the near future to address the safety issues associated with this technique. Given that dietary behavior in young adults is a key risk factor for dysbiosis-induced early-onset CRC, safe intervention mechanisms such as probiotics and FMT to restore the frequently damaged gut microbiota need to be developed in the near future.
Gut microbiota ferment complex non-digestible dietary components such as fiber, producing large amounts of metabolites including SCFAs and essential vitamins, which provide immunological and homeostatic benefits to the host. Short-chain fatty acids with antitumor benefits, such as butyrate, should be investigated for potential application as an addition to the treatment package of patients with CRC. Additionally, microbial metabolites and toxins, such as deoxycholic acid, B. fragilis toxin (BFT), and colibactin should be further studied to establish quantification procedures in future, the presence of specific quantities of these molecules in faecal samples could be used to represent a specific stage of CRC.
Conclusion
A healthy gut microbiome confers crucial benefits to the host including immunomodulation, metabolism and maintenance of the gut epithelial structural integrity. However, different interventions that alter the gut microbiota encourage opportunistic species to invade the gut, causing serious health issues such as inflammation, and gut epithelial damage, which consequently leads to chronic diseases such as CRC. Diet is a factor that alters the gut microbiota (gut dysbiosis), leading to overgrowth of opportunistic microbes as well as their metabolites that are associated with colon and rectal cancers. However, these opportunistic microbes and their signature metabolites can be harnessed as non-invasive CRC diagnostic techniques, as well as biomarkers for the prognosis, prevention, and treatment of CRC. To fully exploit the potential of dysbiosis microbes, further studies are required to quantify stage specific metabolites, in addition to molecular and serological studies that will ensure timely non-invasive diagnosis results. Restoring the dysbiosis-affected gut microbiota has been observed to have anti-CRC benefits; however, to fully exploit the potential of FMT in restoring damaged gut microbiota, we suggest that large-scale prospective studies should be conducted to address the safety issues associated with faecal microbial transplantation.
Compliance with Ethics Requirements.
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Mutebi John Kenneth: Methodology, Investigation, Writing – original draft. Hsin-Chi Tsai: Conceptualization, Writing – review & editing, Supervision. Chuan-Yin Fang: Methodology, Writing – review & editing, Supervision. Bashir Hussain: Conceptualization, Investigation, Writing – original draft. Yi-Chou Chiu: Investigation, Writing – original draft. Bing-Mu Hsu: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the Ministry of Science and Technology (MOST), Taiwan (110-2116-M-194-010), the Ditmanson Medical Foundation Chia-Yi Christian Hospital, and the Center for Innovative Research on Aging Society (CIRAS) from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE), Taiwan.
Biographies

Mutebi John Kenneth Department of Earth and Environmental Sciences, National Chung Cheng University, Taiwan. Mutebi John Kenneth is the Ph.D. student in the Department of Earth and Environmental Sciences, National Chung Cheng University, Taiwan. He received a bachelor in Biotechnology (Sep, 2015–Dec, 2019) and master of science in Molecular Biology (Jun, 2020–Aug, 2021) from BRAC University, Dhaka, Bangladesh. His research interests lie in biotechnology and bioinformatics.

Hsin-Chi Tsai Attending Physician, Department of Psychiatry, Tzu-Chi General Hospital, Taiwan. Assistant professor, School of Medicine, Tzu-Chi University, Hualien, Taiwan. Hsin-Chi Tsai is the senior attending physician in the Department of Psychiatry, Tzu-Chi General Hospital, Taiwan. He received a medical doctor degree from Kaohsiung Medical University in 2001 and a Ph.D. degree in medical sciences from Tzu-Chi University in 2014. He has long experience of working with anxiety disorders, neurocognitive disorders, dementia, and forensic psychiatry.

Chuan-Yin Fang Surgical Oncologist, Division of Colon and Rectal Surgery, Department of Surgery, Ditmanson Medical Foundation, Chia-Yi Christian Hospital, Taiwan. Chuan-Yin Fang is the senior attending physician in the Division of Colon and Rectal Surgery, Department of Surgery, Ditmanson Medical Foundation, Chia-Yi Christian Hospital, Taiwan. He was graduated from College of Medicine, National Yang-Ming University in 1994 and received postgraduate resident training in Kaohsiung Veterans General Hospital and Taipei Veterans General Hospital from 1996 to 2001. Then, he had been an attending physician at Chiayi Branch, Taichung Veterans General Hospital (2001∼2003) and Ditmanson Medical Foundation Chia-Yi Christian Hospital till now. Chuan-Yin Fang also had a fellowship program at Japan National Cancer Center in 2008. Dr. Fang is a surgical oncologist focused on colorectal cancer treatment and his recent research focuses on relationship of microbiota and colorectal cancer.

Bashir Hussain Department of Biomedical Sciences, National Chung Cheng University, Taiwan. Bashir Hussain is the Ph.D. candidate in the Department of Biomedical Sciences, National Chung Cheng University, Taiwan. He received a master degree from PMAS-Arid Agriculture University, Pakistan. His research interests lie in microbial ecology, clinical microbiology, and bioinformatics.

Yi-Chou Chiu Genearl Surgery, Division of General Surgery, Department of Surgery, Cheng Hsin General Hospital, Taiwan. Yi-Chou Chiu is the senior attending physician in the Division of General Surgery, Department of Surgery, Chen Hsin Genearl Hospital, Taiwan. He was graduated from College of Medicine, China Medical College in 1995 and received resident training in Taipei Veterans General Hospital from 1995 to 2000. Then, he had been an attending physician at Cheng Hsin General hospital till now. Yi-Chou Chiu also had a fellowship program at Ohio university hospital in 2008. Dr. Chiu is a surgical oncologist focused on hepatogastric cancer treatment and his recent research focuses on relationship of microbiota and hepatic cancer.

Bing-Mu Hsu Distinguished Professor, Department of Earth and Environmental Sciences, National Chung Cheng University, Taiwan. Bing-Mu Hsu received a Ph.D. degree in Environmental Engineering from the National Chiao Tung University in 2000. He then joined the faculty of the Yuanpei University of Science and Technology as an assistant professor in 2000. In 2004, he joined the faculty of the National Chung Cheng University in the Department of Earth and Environmental Sciences and was promoted to professor in 2010. He has published more than 100 original papers (75% corresponding author, 33% first author) since 1996. His current research interests include environmental and geological medicine, environmental biotechnology, and environmental risk assessment.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Akimoto N., et al. Rising incidence of early-onset colorectal cancer—A call to action. Nat Rev Clin Oncol. 2021;18(4):230–243. doi: 10.1038/s41571-020-00445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang C.-Y., et al. Colorectal Cancer Stage-Specific Fecal Bacterial Community Fingerprinting of the Taiwanese Population and Underpinning of Potential Taxonomic Biomarkers. Microorganisms. 2021;9(8):1548. doi: 10.3390/microorganisms9081548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL., et al., Colorectal cancer statistics, 2020. CA: A Cancer J Clin; 2020. 70(3): p. 145-164. [DOI] [PubMed]
- 4.Arnold M., et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 5.Xi Y., Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10) doi: 10.1016/j.tranon.2021.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawicki T., et al. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers. 2021;13(9):2025. doi: 10.3390/cancers13092025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibáñez-Sanz G., et al. Positive impact of a faecal-based screening programme on colorectal cancer mortality risk. PLoS One. 2021;16(6):e0253369. doi: 10.1371/journal.pone.0253369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douaiher J., et al. Colorectal cancer—global burden, trends, and geographical variations. J Surg Oncol. 2017;115(5):619–630. doi: 10.1002/jso.24578. [DOI] [PubMed] [Google Scholar]
- 9.Deo S., et al. Colorectal Cancers in Low-and Middle-Income Countries—Demographic Pattern and Clinical Profile of 970 Patients Treated at a Tertiary Care Cancer Center in India. JCO Global Oncol. 2021;7:1110–1115. doi: 10.1200/GO.21.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C.-W.-K., Lui R.N. Early-onset colorectal cancer: Current insights and future directions. World J Gastrointest Oncol. 2022;14(1):230. doi: 10.4251/wjgo.v14.i1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad Kendong S.M., et al. Gut dysbiosis and intestinal barrier dysfunction: potential explanation for early-onset colorectal cancer. Front Cell Infect Microbiol. 2021;11:1244. doi: 10.3389/fcimb.2021.744606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pira C., et al. Comparative Study Regarding the Adherence to the Mediterranean Diet and the Eating Habits of Two Groups—The Romanian Children and Adolescents Living in Nord-West of Romania and Their Romanian Counterparts Living in Italy. Foods. 2021;10(9):2045. doi: 10.3390/foods10092045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leeming E.R., et al. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients. 2019;11(12):2862. doi: 10.3390/nu11122862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zygulska A.L., Pierzchalski P. Novel Diagnostic Biomarkers in Colorectal Cancer. Int J Mol Sci. 2022;23(2):852. doi: 10.3390/ijms23020852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy B.T., et al. Comparative effectiveness of five fecal immunochemical tests using colonoscopy as the gold standard: study protocol. Contemp Clin Trials. 2021;106 doi: 10.1016/j.cct.2021.106430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebersek M. Gut microbiome and its role in colorectal cancer. BMC Cancer. 2021;21(1):1–13. doi: 10.1186/s12885-021-09054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appunni S., et al. Emerging Evidence on the Effects of Dietary Factors on the Gut Microbiome in Colorectal Cancer. Front Nutr. 2021;8:752. doi: 10.3389/fnut.2021.718389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong MC., et al., Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin Gastroenterol Hepatol; 2021. 19(5): p. 955-966. e61. [DOI] [PubMed]
- 19.Onyoh E.F., et al. The rise of colorectal cancer in Asia: epidemiology, screening, and management. Curr Gastroenterol Rep. 2019;21(8):1–10. doi: 10.1007/s11894-019-0703-8. [DOI] [PubMed] [Google Scholar]
- 20.Wong S.H., Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16(11):690–704. doi: 10.1038/s41575-019-0209-8. [DOI] [PubMed] [Google Scholar]
- 21.Esgalhado M., et al. Short-chain fatty acids: A link between prebiotics and microbiota in chronic kidney disease. Future Microbiol. 2017;12(15):1413–1425. doi: 10.2217/fmb-2017-0059. [DOI] [PubMed] [Google Scholar]
- 22.Abdullah M., et al. Gut microbiota profiles in early-and late-onset colorectal cancer: a potential diagnostic biomarker in the future. Digestion. 2021;102(6):823–832. doi: 10.1159/000516689. [DOI] [PubMed] [Google Scholar]
- 23.Lozupone C.A., et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho N.T., et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun. 2018;9(1):1–13. doi: 10.1038/s41467-018-06473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day A.W., Kumamoto C.A. Gut Microbiome Dysbiosis in Alcoholism: Consequences for Health and Recovery. Front Cell Infect Microbiol. 2022;12:198. doi: 10.3389/fcimb.2022.840164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matamoros S., et al. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013;21(4):167–173. doi: 10.1016/j.tim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Vipperla K., O'Keefe S.J. Diet, microbiota, and dysbiosis: a ‘recipe’for colorectal cancer. Food Funct. 2016;7(4):1731–1740. doi: 10.1039/c5fo01276g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wardwell L.H., Huttenhower C., Garrett W.S. Current concepts of the intestinal microbiota and the pathogenesis of infection. Curr Infect Dis Rep. 2011;13(1):28–34. doi: 10.1007/s11908-010-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Keefe S.J., et al. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr. 2009;139(11):2044–2048. doi: 10.3945/jn.109.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David L.A., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.So D., et al. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107(6):965–983. doi: 10.1093/ajcn/nqy041. [DOI] [PubMed] [Google Scholar]
- 32.Xiao X., Wu Z.-C., Chou K.-C. A multi-label classifier for predicting the subcellular localization of gram-negative bacterial proteins with both single and multiple sites. PLoS One. 2011;6(6):e20592. doi: 10.1371/journal.pone.0020592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voreades N., Kozil A., Weir T.L. Diet and the development of the human intestinal microbiome. Front Microbiol. 2014;5:494. doi: 10.3389/fmicb.2014.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu G.D., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fragiadakis G.K., et al. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am J Clin Nutr. 2020;111(6):1127–1136. doi: 10.1093/ajcn/nqaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Marchand L., Kolonel L. Cancer in Japanese migrants to Hawaii: interaction between genes and environment. Rev Epidemiol Sante Publique. 1992;40(6):425–430. [PubMed] [Google Scholar]
- 37.Marchand L.L. Combined Influence of Genetic and Dietary Factors on Colorectal Cancer Incidence in Japanese Americans. JNCI Monographs. 1999;1999(26):101–105. doi: 10.1093/oxfordjournals.jncimonographs.a024220. [DOI] [PubMed] [Google Scholar]
- 38.Park S.-Y., Le Marchand L. In: Cancer Epidemiology Among Asian Americans. Wu A.H., Stram D.O., editors. Springer International Publishing; Cham: 2016. Colorectal Cancer Among Asian Americans; pp. 137–160. [Google Scholar]
- 39.Cologne J., et al. Stepwise approach to SNP-set analysis illustrated with the Metabochip and colorectal cancer in Japanese Americans of the Multiethnic Cohort. BMC Genomics. 2018;19(1):524. doi: 10.1186/s12864-018-4910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis E.C., et al. Microbiome composition in pediatric populations from birth to adolescence: impact of diet and prebiotic and probiotic interventions. Dig Dis Sci. 2020;65(3):706–722. doi: 10.1007/s10620-020-06092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C., et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4(2):232–241. doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- 42.Turnbaugh PJ., et al., The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Translat Med, 2009. 1(6): p. 6ra14-6ra14. [DOI] [PMC free article] [PubMed]
- 43.Swartjes H., et al. Incidence, treatment and relative survival of early-onset colorectal cancer in the Netherlands since 1989. Eur J Cancer. 2022;166:134–144. doi: 10.1016/j.ejca.2022.01.029. [DOI] [PubMed] [Google Scholar]
- 44.Diether N.E., Willing B.P. Microbial fermentation of dietary protein: an important factor in diet–microbe–host interaction. Microorganisms. 2019;7(1):19. doi: 10.3390/microorganisms7010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hentges D.J., et al. Effect of a high-beef diet on the fecal bacterial flora of humans. Cancer Res. 1977;37(2):568–571. [PubMed] [Google Scholar]
- 46.Jantchou P., et al. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Off J Am College Gastroenterol| ACG. 2010;105(10):2195–2201. doi: 10.1038/ajg.2010.192. [DOI] [PubMed] [Google Scholar]
- 47.Mu C., et al. The colonic microbiome and epithelial transcriptome are altered in rats fed a high-protein diet compared with a normal-protein diet. J Nutr. 2016;146(3):474–483. doi: 10.3945/jn.115.223990. [DOI] [PubMed] [Google Scholar]
- 48.Stecher B., Hardt W.-D. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011;14(1):82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Mu C., et al. Temporal microbiota changes of high-protein diet intake in a rat model. Anaerobe. 2017;47:218–225. doi: 10.1016/j.anaerobe.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Wu D., et al. Antimicrobial resistance analysis of clinical Escherichia coli isolates in neonatal ward. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.670470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frank D.N., et al. Investigating the biological and clinical significance of human dysbioses. Trends Microbiol. 2011;19(9):427–434. doi: 10.1016/j.tim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J., et al., High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology; 2022. 162(1): p. 135-149. e2. [DOI] [PubMed]
- 53.Wang B., et al. A high-fat diet increases gut microbiota biodiversity and energy expenditure due to nutrient difference. Nutrients. 2020;12(10):3197. doi: 10.3390/nu12103197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ocvirk S., et al. Fiber, fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21(11):1–7. doi: 10.1007/s11894-019-0725-2. [DOI] [PubMed] [Google Scholar]
- 55.Fava F., et al. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’population. Int J Obes (Lond) 2013;37(2):216–223. doi: 10.1038/ijo.2012.33. [DOI] [PubMed] [Google Scholar]
- 56.Liu X., Chen Q., Yan Y. Effect of high-fat diet on intestinal flora in mice. Food Sci. 2011;32:306–311. [Google Scholar]
- 57.Machate D.J., et al. Fatty acid diets: regulation of gut microbiota composition and obesity and its related metabolic dysbiosis. Int J Mol Sci. 2020;21(11):4093. doi: 10.3390/ijms21114093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliver A., et al. High-fiber, whole-food dietary intervention alters the human gut microbiome but not fecal short-chain fatty acids. Msystems. 2021;6(2):e00115–e121. doi: 10.1128/mSystems.00115-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makki K., et al. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 60.Purves D.W., Turnbull L.A. Different but equal: the implausible assumption at the heart of neutral theory. J Anim Ecol. 2010;79(6):1215–1225. doi: 10.1111/j.1365-2656.2010.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burkitt D.P. Some Leads to the Etiology of Cancer of the Large Bowel: Possible Relationships between Bowel Cancer and Dietary Habits. SAGE Publications. 1971;64:964–965. doi: 10.1177/003591577106400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr J. 2014;13(1):1–10. doi: 10.1186/1475-2891-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scott K.P., et al. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69(1):52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 64.Flint H.J., et al. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3(4):289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Encarnação J., et al. Revisit dietary fiber on colorectal cancer: butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015;34(3):465–478. doi: 10.1007/s10555-015-9578-9. [DOI] [PubMed] [Google Scholar]
- 66.Lin C.-S., et al. Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. Biomedical journal. 2014;37(5):259–268. doi: 10.4103/2319-4170.138314. [DOI] [PubMed] [Google Scholar]
- 67.Swanson K.S., et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17(11):687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hill C., et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 69.Kåhrström C.T., Pariente N., Weiss U. Intestinal microbiota in health and disease. Nature. 2016;535(7610):47. doi: 10.1038/535047a. [DOI] [PubMed] [Google Scholar]
- 70.Derikx L.A., Dieleman L.A., Hoentjen F. Probiotics and prebiotics in ulcerative colitis. Best Pract Res Clin Gastroenterol. 2016;30(1):55–71. doi: 10.1016/j.bpg.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Hemarajata P., Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6(1):39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibson G.R., et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. 2017;14::491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y.-N., et al. Effects of probiotics and prebiotics on intestinal microbiota in mice with acute colitis based on 16S rRNA gene sequencing. Chin Med J (Engl) 2019;132(15):1833–1842. doi: 10.1097/CM9.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gibson G.R., et al. Dietary prebiotics: current status and new definition. Food Sci Technol Bull Funct Foods. 2010;7(1):1–19. [Google Scholar]
- 75.Davani-Davari D., et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scott K.P., et al. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87(1):30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- 77.Louis P., Flint H.J., Michel C. How to manipulate the microbiota: prebiotics. Microbiota Human Body. 2016:119–142. doi: 10.1007/978-3-319-31248-4_9. [DOI] [PubMed] [Google Scholar]
- 78.Davani-Davari D., et al., Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods; 2019. 8(3). [DOI] [PMC free article] [PubMed]
- 79.Walker A.W., et al. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. 2005;71(7):3692–3700. doi: 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackson M.A., et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun. 2018;9(1):1–8. doi: 10.1038/s41467-018-05184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu H., et al. The dynamic interplay between the gut microbiota and autoimmune diseases. J Immunol Res. 2019;2019:7546047. doi: 10.1155/2019/7546047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zou S., Fang L., Lee M.-H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterology report. 2018;6(1):1–12. doi: 10.1093/gastro/gox031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castellarin M., et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fan X., et al. Gut microbiota dysbiosis drives the development of colorectal cancer. Digestion. 2021;102(4):508–515. doi: 10.1159/000508328. [DOI] [PubMed] [Google Scholar]
- 85.Gehart H., Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16(1):19–34. doi: 10.1038/s41575-018-0081-y. [DOI] [PubMed] [Google Scholar]
- 86.Plaza-Diaz J., et al. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol. 2014;20(42):15632–15649. doi: 10.3748/wjg.v20.i42.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu L.-C.-H. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci. 2018;25(1):79. doi: 10.1186/s12929-018-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brennan C.A., Garrett W.S. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu Rev Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu L.C., Wei S.C., Ni Y.H. Impact of microbiota in colorectal carcinogenesis: lessons from experimental models. Intest Res. 2018;16(3):346–357. doi: 10.5217/ir.2018.16.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang Y., et al., Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor− κB, and up-regulating expression of microRNA-21. Gastroenterology; 2017. 152(4): p. 851-866. e24. [DOI] [PMC free article] [PubMed]
- 91.Fan H., et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 2016;10(2):421–433. doi: 10.1038/mi.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fang H., et al. Screening for vancomycin-resistant enterococci: an efficient and economical laboratory-developed test. Eur J Clin Microbiol Infect Dis. 2012;31(3):261–265. doi: 10.1007/s10096-011-1304-0. [DOI] [PubMed] [Google Scholar]
- 93.Berbert L., et al. Metagenomics analysis reveals universal signatures of the intestinal microbiota in colorectal cancer, regardless of regional differences. Braz J Med Biol Res. 2022;55:e11832. doi: 10.1590/1414-431X2022e11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan B., et al. Fecal Bacteria as Non-Invasive Biomarkers for Colorectal Adenocarcinoma. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.664321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dai Z., et al. The role of microbiota in the development of colorectal cancer. Int J Cancer. 2019;145(8):2032–2041. doi: 10.1002/ijc.32017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu J., Li Q., Fu X. Fusobacterium nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and suppressing host immunity. Transl Oncol. 2019;12(6):846–851. doi: 10.1016/j.tranon.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rubinstein M.R., et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu J., et al. Invasive Fusobacterium nucleatum may play a role in the carcinogenesis of proximal colon cancer through the serrated neoplasia pathway. Int J Cancer. 2016;139(6):1318–1326. doi: 10.1002/ijc.30168. [DOI] [PubMed] [Google Scholar]
- 99.Ma C.T., et al. Fusobacterium nucleatum promotes the progression of colorectal cancer by interacting with E-cadherin. Oncol Lett. 2018;16(2):2606–2612. doi: 10.3892/ol.2018.8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bashir A., et al. Fusobacterium nucleatum. Eur J Cancer Prev. 2015;24(5):373–385. doi: 10.1097/CEJ.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 101.Gur C., et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shariati A., et al. Association between colorectal cancer and Fusobacterium nucleatum and Bacteroides fragilis bacteria in Iranian patients: a preliminary study. Infectious Agents and Cancer. 2021;16(1):1–10. doi: 10.1186/s13027-021-00381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu S., et al. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and γ-secretase-dependent E-cadherin cleavage. J Cell Sci. 2007;120(11):1944–1952. doi: 10.1242/jcs.03455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sears C.L., Geis A.L., Housseau F. Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J Clin Invest. 2014;124(10):4166–4172. doi: 10.1172/JCI72334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olesen B. Characterization of four Escherichia coli clonal groups. APMIS. 2017;125(Suppl 139):1–28. doi: 10.1111/apm.12737. [DOI] [PubMed] [Google Scholar]
- 106.Cuevas-Ramos G., et al., Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci; 2010. 107(25): p. 11537-11542. [DOI] [PMC free article] [PubMed]
- 107.Arthur J.C., et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338(6103):120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Velilla J.A., Kenney G.E., Gaudet R. Structure and function of prodrug-activating peptidases. Biochimie. 2022 doi: 10.1016/j.biochi.2022.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cougnoux A., et al. Small-molecule inhibitors prevent the genotoxic and protumoural effects induced by colibactin-producing bacteria. Gut. 2016;65(2):278–285. doi: 10.1136/gutjnl-2014-307241. [DOI] [PubMed] [Google Scholar]
- 110.Tjalsma H., et al. A bacterial driver–passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012;10(8):575–582. doi: 10.1038/nrmicro2819. [DOI] [PubMed] [Google Scholar]
- 111.Viljoen K.S., et al. Quantitative profiling of colorectal cancer-associated bacteria reveals associations between fusobacterium spp., enterotoxigenic Bacteroides fragilis (ETBF) and clinicopathological features of colorectal cancer. PLoS One. 2015;10(3):e0119462. doi: 10.1371/journal.pone.0119462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maisonneuve C., et al. The impact of the gut microbiome on colorectal cancer. Ann Rev Cancer Biol. 2018;2:229–249. [Google Scholar]
- 113.Montalban-Arques A., Scharl M. Intestinal microbiota and colorectal carcinoma: Implications for pathogenesis, diagnosis, and therapy. EBioMedicine. 2019;48:648–655. doi: 10.1016/j.ebiom.2019.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grivennikov S.I. Vol. 35. Springer; 2013. Inflammation and colorectal cancer: colitis-associated neoplasia; pp. 229–244. (Seminars in immunopathology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lopez L.R., Bleich R.M., Arthur J.C. Microbiota effects on carcinogenesis: initiation, promotion, and progression. Annu Rev Med. 2021;72:243–261. doi: 10.1146/annurev-med-080719-091604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim J., Lee H.K. Potential role of the gut microbiome in colorectal cancer progression. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.807648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matsumoto K., et al. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017;108(3):296–307. doi: 10.1111/cas.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guevarra L.A., et al. Immunogenicity of a Fap2 peptide mimotope of Fusobacterium nucleatum and its potential use in the diagnosis of colorectal cancer. Infectious Agents Cancer. 2018;13(1):1–6. doi: 10.1186/s13027-018-0184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu C., et al. Fusobacterium nucleatum promotes colorectal cancer metastasis through miR-1322/CCL20 axis and M2 polarization. Gut Microbes. 2021;13(1):1980347. doi: 10.1080/19490976.2021.1980347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ponnatapura J., Lalwani N. Imaging of Colorectal Cancer: Screening, Staging, and Surveillance. Semin Roentgenol Elsevier. 2021;56(2):128–139. doi: 10.1053/j.ro.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 121.Liu W., et al. Study of the relationship between microbiome and colorectal cancer susceptibility using 16SrRNA sequencing. Biomed Res Int. 2020;2020:7828392. doi: 10.1155/2020/7828392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bonnet M., et al. Colonization of the human gut by E. coli and colorectal cancer risk. Clin Cancer Res. 2014;20(4):859–867. doi: 10.1158/1078-0432.CCR-13-1343. [DOI] [PubMed] [Google Scholar]
- 123.Han S., et al. Progress in research on colorectal cancer-related microorganisms and metabolites. Cancer Manag Res. 2020;12:8703. doi: 10.2147/CMAR.S268943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Song X., et al. Microbial metabolite deoxycholic acid promotes vasculogenic mimicry formation in intestinal carcinogenesis. Cancer Sci. 2022;113(2):459. doi: 10.1111/cas.15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cao H., et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int J Cancer. 2017;140(11):2545–2556. doi: 10.1002/ijc.30643. [DOI] [PubMed] [Google Scholar]
- 126.Osman M.A., et al. Parvimonas micra, Peptostreptococcus stomatis, Fusobacterium nucleatum and Akkermansia muciniphila as a four-bacteria biomarker panel of colorectal cancer. Sci Rep. 2021;11(1):1–12. doi: 10.1038/s41598-021-82465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dejea C.M., Sears C.L. Do biofilms confer a pro-carcinogenic state? Gut Microbes. 2016;7(1):54–57. doi: 10.1080/19490976.2015.1121363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakatsu G., et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6(1):1–9. doi: 10.1038/ncomms9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stadlmayr A., et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Z Gastroenterol. 2015;53(05):P41. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- 130.Feng Q., et al. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat Commun. 2015;6(1):6528. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- 131.Campos F.G. Colorectal cancer in young adults: A difficult challenge. World J Gastroenterol. 2017;23(28):5041–5044. doi: 10.3748/wjg.v23.i28.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ganesh K., et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16(6):361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Warren R.L., et al. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome. 2013;1(1):1–12. doi: 10.1186/2049-2618-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang F., et al. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell. 2018;9(5):462–473. doi: 10.1007/s13238-018-0541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chang C.-W., et al. Fecal microbiota transplantation prevents intestinal injury, upregulation of toll-like receptors, and 5-fluorouracil/oxaliplatin-induced toxicity in colorectal cancer. Int J Mol Sci. 2020;21(2):386. doi: 10.3390/ijms21020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cammarota G., et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68(12):2111–2121. doi: 10.1136/gutjnl-2019-319548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gupta S., Allen-Vercoe E., Petrof E.O. Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol. 2016;9(2):229–239. doi: 10.1177/1756283X15607414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang S., et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11(8):e0161174. doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang J.-W., et al. Fecal microbiota transplantation: Review and update. J Formos Med Assoc. 2019;118:S23–S31. doi: 10.1016/j.jfma.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 140.Fuentes S, Vos WMd. How to manipulate the microbiota: fecal microbiota transplantation. Microbiota of the Human Body; 2016: p. 143-153. [DOI] [PubMed]
- 141.Kim J.-H., Kim K., Kim W. Gut microbiota restoration through fecal microbiota transplantation: a new atopic dermatitis therapy. Exp Mol Med. 2021;53(5):907–916. doi: 10.1038/s12276-021-00627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim K., et al. Differences regarding the molecular features and gut microbiota between right and left colon cancer. Annals Coloproctol. 2018;34(6):280. doi: 10.3393/ac.2018.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tarashi S., et al. Gut bacteria and their metabolites: which one is the defendant for colorectal cancer? Microorganisms. 2019;7(11):561. doi: 10.3390/microorganisms7110561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Janney A., Powrie F., Mann E.H. Host–microbiota maladaptation in colorectal cancer. Nature. 2020;585(7826):509–517. doi: 10.1038/s41586-020-2729-3. [DOI] [PubMed] [Google Scholar]
- 145.Xu M.-Q., et al. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J Gastroenterol: WJG. 2015;21(1):102. doi: 10.3748/wjg.v21.i1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McQuade J.L., et al. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20(2):e77–e91. doi: 10.1016/S1470-2045(18)30952-5. [DOI] [PubMed] [Google Scholar]
- 147.Moss E.L., et al. Long-term taxonomic and functional divergence from donor bacterial strains following fecal microbiota transplantation in immunocompromised patients. PLoS One. 2017;12(8):e0182585. doi: 10.1371/journal.pone.0182585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zimmer J., et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr. 2012;66(1):53–60. doi: 10.1038/ejcn.2011.141. [DOI] [PubMed] [Google Scholar]
- 149.Berg A.M., Kelly C.P., Farraye F.A. Clostridium difficile infection in the inflammatory bowel disease patient. Inflamm Bowel Dis. 2013;19:194–204. doi: 10.1002/ibd.22964. [DOI] [PubMed] [Google Scholar]
- 150.Walker A.W., et al. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11(1):1–12. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Santacruz A., et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity. 2009;17(10):1906–1915. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- 152.Devkota S., et al. 43 Dietary Fat-Induced Taurocholic Acid Production Promotes Pathobiont and Colitis in IL-10-/-Mice. Gastroenterology. 2012;142(5):p. S-12. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]