Graphical abstract
Keywords: Neoagarotetraose, Longevity, Neuronal injury, Gut microbiota, Alzheimer’s disease
Highlights
-
•
Neoagarotetraose extended the lifespan by up to 33.3 % of natural aging mice.
-
•
Neoagarotetraose improved the cerebral neuronal injuries of natural aging mice.
-
•
Neoagarotetraose increased abundance of short-chain fatty acids-producing bacteria.
-
•
Neoagarotetraose regulated the glucolipid and bile acid metabolism-related metabolic pathways.
-
•
Neoagarotetraose ameliorated cognitive impairment of Alzheimer’s disease mice.
Abstract
Introduction
Dietary oligosaccharides can impact the gut microbiota and confer tremendous health benefits.
Objectives
The aim of this study was to determine the impact of a novel functional oligosaccharide, neoagarotetraose (NAT), on aging in mice.
Methods
8-month-old C57BL/6J mice as the natural aging mice model were orally administered with NAT for 12 months. The preventive effect of NAT in Alzheimer’s disease (AD) mice was further evaluated. Aging related indicators, neuropathology, gut microbiota and short-chain fatty acids (SCFAs) in cecal contents were analyzed.
Results
NAT treatment extended the lifespan of these mice by up to 33.3 %. Furthermore, these mice showed the improved aging characteristics and decreased injuries in cerebral neurons. Dietary NAT significantly delayed DNA damage in the brain, and inhibited reduction of tight junction protein in the colon. A significant increase at gut bacterial genus level (such as Lactobacillus, Butyricimonas, and Akkermansia) accompanied by increasing concentrations of SCFAs in cecal contents was observed after NAT treatment. Functional profiling of gut microbiota composition indicated that NAT treatment regulated the glucolipid and bile acid-related metabolic pathways. Interestingly, NAT treatment ameliorated cognitive impairment, attenuated amyloid-β (Aβ) and Tau pathology, and regulated the gut microbiota composition and SCFAs receptor-related pathway of Alzheimer’s disease (AD) mice.
Conclusion
NAT mitigated age-associated cerebral injury in mice through gut-brain axis. The findings provide novel evidence for the effect of NAT on anti-aging, and highlight the potential application of NAT as an effective intervention against age-related diseases.
Introduction
Aging is characterized by the time-dependent functional decline in different organs, which results in several chronic and age-related diseases including cardiovascular disorders, type II diabetes, cancers, hypertension, and Alzheimer's disease (AD) [1], [2]. These diseases account for up to 70 % of deaths globally, and are undisputedly the leading causes of mortality [3], [4]. Diet is an important factor for maintaining health. Unhealthy dietary habits are associated with many chronic diseases, which are major contributors to aging [5]. Proper diet and nutrition management have been shown to reduce the rate of age-associated diseases and prolong lifespan [6]. Thus, a healthy diet is a safe and effective option to alleviate aging and age-related diseases.
The gut microbiota is emerging as a key regulator of metabolic, immune, and neuroendocrine pathways in the host [7]. Deregulation of the gut microbiota is related to numerous diseases like obesity, diabetes, atherosclerosis, cancer, and aging [8]. Intriguingly, the gut microbiota can influence the enteric and autonomic nervous system, and the immunity of the central nervous system. The microbiota-gut-brain axis has an essential regulatory role in pathogenesis of several chronic diseases. The main participants of this axis are the gut microbiota, its bacterial products, the intestinal barrier, and the nervous system [9].
Prebiotics can induce specific changes of gastrointestinal microbiota, thereby conferring several benefits to host health [10]. Functional oligosaccharides, as majorly known prebiotics, improve the intestinal flora and related metabolites to impact host health by maintaining metabolic balance [11]. Neoagarooligosaccharides (NAOS) are a new type of β-1, 4 bond-linked functional oligosaccharides derived from agarose [12]. NAOS are reported to be safe [13], and possess several biological benefits, including prebiotic [14], anti-oxidant [15], [16], moisturizing and whitening [17], anti-inflammatory [18], [19], and anti-tumor activities [20]. NAOS, such as neoagarotetraose (NAT) and neoagarohexaose (NAH), have been reported to improve obesity-related metabolic defects by improving glucose tolerance and adiponectin induction [21]. In particular, NAT was more useful in scavenging hydroxyl radicals in comparison with neoagarobiose (NAB), NAH, and neoagarooctaose (NAO) [22]. Recently, we demonstrated that NAT can mediate lifespan extension by regulating the insulin/IGF1 (IIS)-AMPK signaling pathway in Caenorhabditis elegans [23]. Therefore, NAT may regulate host physiology, and serve as a functional food material for health management.
Numerous prebiotic supplementation trials have demonstrated their beneficial effects on microbiota composition and metabolic activity in humans [24], [25]. In the previous study, NAT treatment profoundly altered the microbiome composition and remarkably enhanced the concentration of total SCFAs in feces [12]. However, the effect of NAT on aging, particularly of the neurological system, has not been investigated in mammals. Further, the specific signaling pathways of the gut microbiota involved in this process remain unknown. Therefore, we investigated the impact of NAT on aging and age-related neuro-degeneration. The effect of long-term NAT intervention on age-associated cerebral injury and intestinal flora changes was examined in C57BL/6J mice. We further evaluated the influence of NAT treatment on gut physiology, and illustrated potential metabolic pathways of the gut microbiota that were impacted during aging in mice. We further evaluated the preventive effect of NAT in AD mice. Our results thus provide the evidence for the influence of NAT on anti-aging, particularly in the AD model; the findings gained in this study provide important insights for using functional oligosaccharides to alleviate aging and age-associated diseases in the future.
Materials and methods
Ethics statement
All experiments involving mice were performed in accordance with the ethical policies and procedures approved by the Institutional Animal Care and Use Committee of China Agricultural University (approval number: 20185001–3).
Materials and animals
Neoagarotetraose (NAT) was prepared by enzymatic hydrolysis of agarose using β-agarase [26]. Its content was more than 93.2 % (w/w) as determined using high-performance liquid chromatography (HPLC). Metformin (MET) was obtained from Sigma Aldrich (St. Louis, USA). All chemicals and reagents used in this study were of analytical grade.
C57BL/6J mice (male, 8 months old) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). APP/PS1 double transgenic (APPswe/PSEN1dE9) mice with five familial Alzheimer's diseases (5 × FAD) and littermate wild-type C57BL/6J mice (male, 8 months old) were provided by Zhishan Institute of Health Medicine Co., Ltd (Beijing, China). Mice were housed in cages with 40–60 % humidity, a light/dark (12 h/12 h) cycle, and a temperature of 23–25 °C. All mice had free access to drinking water, and were fed a standard diet for one week after arrival. After adaptation, drinking water was spiked with NAT or MET. The dose was determined on the basis of the daily water intake and body weight of mice.
Animal experiment design
Male C57BL/6J mice were divided into 3 groups (n = 20/group). Natural aging (NA) mice were administered in normal drinking water; mice from the MET group based on the previous studies [27], [28] were supplied with MET at a dosage of 100 mg/kg·bw/d; mice from the NAT group were supplied with NAT at a dosage of 1,000 mg/kg·bw/d for 12 months, according to our preliminary experiments and related studies [29], [30]. Both MET and NAT were dissolved in drinking water.
Male APP/PS1 mice were divided into the AD model group (AD group) and NAT-treated AD group (NAT group); littermate wild-type C57BL/6J male mice served as the control group (WT group) (n = 20/group). NAT was administered in drinking water at 1000 mg/kg·bw/d, based on the previously measured water consumption. Mice of WT group and AD group received normal water. The administration began at 8 months old of mice and continued for 3 months. The behavioral tests on mice were performed after 3-month intervention.
During the intervention period, the aging scores of mice were monitored every week. Detailed scoring standards for aging scores were previously described [31]. At the end of these experiments, all mice were completely anesthetized; blood samples were then collected and centrifuged (3,000 rpm, 4 °C for 10 min) for separating serum following sacrifice. The brain, heart, liver, spleen, kidney, colon, small intestine, epididymal fat, subcutaneous fat, and cecal contents were collected, and stored at −80 °C or by immersion in 4 % paraformaldehyde phosphate-buffered solution.
Behavioral tests
Morris water maze (MWM) test was performed according to the previous method with slight modification [32]. Briefly, the mice were subjected to 4-day navigation trial, with each mouse performing 5 trials per day. Mice were placed in the water at the desired starting position, and the latency to find the platform was timed. On day 5, the platform was removed and the mice were allowed to swim for 60 s. A video camera was used to record the trace and the number of crossing platform.
Step-through passive avoidance behavior was measured in accordance with the previous method [33]. Briefly, after mice entering the dark room, the door was closed and foot shock (1.0 mA, 3 s) was given until they entered the light box. After the learning trial, the mice were immediately removed from the apparatus. The retention trial was performed after 24 h. Latency and the number of errors were measured during the 180-second testing period.
Measurement of biochemical indicators
Aging biomarkers including nitric oxide (NO), lactic acid, advanced glycation end products (AGEs), and telomerase in the serum were measured in accordance with the manufacturer's protocols (Beijing Biotechnology and Bioengineering Laboratory and Services Co. Ltd., China). Serum levels of total anti-oxygenic capacity (T-AOC), catalase (CAT), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), insulin (RI), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were determined as directed by the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Histology and immunohistochemistry staining analysis
Neuronal injury in brain tissues was detected using Nissl staining as previously described [34]. Image Pro Plus 6.0 software was used to digitize the number of neurons in per field of the cerebral cortex and hippocampus (400× magnification). The fresh brain, liver, kidney, colon, and small intestine tissues were fixed, embedded, sliced, and stained with H & E. The morphological features of all samples were observed by light microscopy.
The expression level of amyloid β peptide 1–42 (Aβ1-42) in brain tissues was determined using immunohistochemistry. Briefly, slides were deparaffinized, and then blocked with 10 % normal goat serum for 1 h. After incubation with the primary antibody [anti-Aβ1-42 antibody (Abcam, cat. number ab201061); diluted 1: 100] on the sections, the secondary antibody (1: 500) was applied. Then, 3,30-diaminobenzidine (DAB) was used for staining. Images were then observed using a fluorescence microscope (BX60, Olympus, Japan).
Immunofluorescence analysis
Mouse brain tissues were permeabilized and then blocked with 1 % bovine serum albumin (BSA) and 2 % fetal bovine serum (FBS) following deparaffinization and citrate antigen retrieval. After blocking, the primary antibodies including anti-neuronal nuclei (NeuN; Abcam, cat. number ab177487), anti-brain-derived neurotrophic factor (BDNF; Abcam, cat. number ab108319), and anti-phospho-Tau (Thr231; Abcam, cat. number ab151559) were applied overnight. An immunofluorescence assay using an antibody against p53-binding protein 1 (53BP1, Abcam, cat. number ab175933) was used to mark DNA damage foci to determine DNA damage. All antibodies were diluted 1: 200 for the experiment. A confocal ultraspectral microscope (FV3000, Olympus, Japan) was used to obtain the immunofluorescence images.
Real-time qPCR analysis
Total RNA was extracted from the brain and colon tissues in mice using RNA extraction kit, and then reverse-transcribed into cDNA using PrimeScript™ RT reagent kit (Takara, Japan). The primers for quantitative real-time PCR (qPCR) were designed and synthesized (Sangon Biotech Co. Ltd., Shanghai, China). The forward and reverse primer sequences are shown in supplementary Table S1. β-actin was used as the reference for normalization. The cycle threshold (CT) was employed for target gene quantification.
Western blotting analysis
Total proteins from mouse brain and colon tissues were extracted using RIPA extraction buffer. The protein concentrations were measured by BCA protein assay kit (Beyotime Technology, Shanghai, China). The detailed procedure of Western blotting was performed according to the previous method [35]. Primary antibodies (1: 1000) against β-actin (Abcam, cat. number ab8226), p53 (Abcam, cat. number ab26), Caspase-3 (Abcam, cat. number ab184787), PTEN (Proteintech, cat. number 22034-1-AP), PI3K (p110 β; Proteintech, cat. number 20584-1-AP), phosphorylated PI3K (Tyr458, p85; CST, cat. number 4228T), AKT (Proteintech, cat. number 10176-2-AP), phosphorylated AKT (Thr308, p-AKT; CST, cat. number 13038 T), mTOR (Proteintech, cat. number 20657-1-AP), phosphorylated mTOR (Ser2448, p-mTOR; CST, cat. number 5536T), p21 (Abcam, cat. number ab188224), p16 (Abcam, cat. number ab51243), ZO-1 (Abcam, cat. number ab216880), Occludin (Abcam, cat. number ab216327), p38 MAPK (CST, cat. number 8690T), phosphorylated p38 MAPK (Thr180/Tyr182, p-p38; CST, cat. number 4511T), GPR43 (Proteintech, cat. Number 19952-1-AP), ERK1/2 (ZEN BIO, cat. number 201245-4A4) and phosphorylated ERK1/2 [(Thr202/Tyr204)/(Thr185/Tyr187), p-ERK; ZEN BIO, cat. number 301245] were respectively used to probe their target proteins. Protein bands were captured using an automated chemiluminescence imaging system (Tanon 4800, Tanon Science and Technology Co., Ltd, China) and densitometry analysis was performed using Image J software; the values were normalized against the loading control.
Gut microbiota and short chain fatty acids (SCFAs) analysis
Extraction of the total genomic DNA from cecal contents of natural aging mice was under aseptic conditions. PCR amplification of the bacterial 16S rRNA gene V3–V4 regions was performed using the forward primer (341F 5′- CCT AYG GGR BGC ASC AG-3′) and the reverse primer (806R 5′-GGA CTA CNN GGG TAT CTA AT-3′). Sequencing was performed on the Illumina MiSeq sequencing platform with a PE250 strategy. All bioinformatics amplicon read analyses were performed in amplicon sequence variants (ASV). Principle coordinates analysis (PCoA) plots based on Jaccard distance were generated using the q2-diversity plugin and visualized using Emperor. The functional profiles of non-redundant genes were obtained by annotation against MetaCyc pathway database using the DIAMOND alignment algorithm.
Total genomic DNA extracted from cecal contents of AD mice was fragmented to an average size of about 400 bp using Covaris M220 (Gene Company Limited, China), followed by sequencing on an Illumina Novaseq 6000 platform (Illumina, San Diego, CA, USA) at Majorbio Bio-Pharm Technology (Shanghai, China) according to the manufacturer’s protocols. The data of metagenomic sequencing were analyzed on the free online platform of Majorbio Cloud Platform.
The concentrations of SCFAs in cecal contents of mice were measured as previously described [36]. HPLC was performed on an Agilent Hi-Plex system at a flow rate of 0.5 mL/min and a temperature of 55 °C. Acetic acid, propionic acid, and butyric acid were used as standards.
Statistical analyses
Data were expressed as the mean ± standard deviation (SD). All bar plots were generated using GraphPad Prism 8.0 (GraphPad Software, San Diego, USA). Statistically significant differences between the model group and the other groups were determined using either Student’s t-test or Mann-Whitney’s U non-parametric test with SPSS 20.0 (IBM SPSS, USA); statistical significance was set at *P-value < 0.05 and **P-value < 0.01.
Results
NAT extended lifespan and ameliorated metabolic aging in mice
Natural aging mice at 8 months of age were treated with NAT or metformin for 12 months (Fig. 1A). Compared with the natural aging (NA) group, the body weight of mice in the NAT and MET groups had no significant differences (Fig. 1B). After NAT treatment, mice showed significantly decreased organ indices of subcutaneous and epididymal fat tissues (P < 0.01; Fig. 1C). Strikingly, the NAT group showed a significant increase in maximum longevity (33.3 %) compared with that of the NA group, similar to the effect of MET treatment (41.7 %; Fig. 1D).
Fig. 1.
Effects of neoagarotetraose treatment on the general status of natural aging mice. (A) Schematic of the experimental proposition; (B) body weights of mice in each group during the 0–12-month intervention process; (C) organ indices of mice; (D) survival rate of mice; (E) morphology of mouse tissues stained with hematoxylin and eosin (H & E; 200 × ). Brain: pyramidal cells are indicated by black arrows; liver: the hepatic portal vein is indicated by a black arrow, the cell nucleus is indicated by a red arrow, and lipid droplets are indicated by green arrows; kidney: the glomerulus is indicated by a blue arrow, the balloon cavity is indicated by a black arrow, and epithelial cells are indicated by a red arrow; intestine: the mucosa is indicated by a blue arrow, the villus by a black arrow, and muscle by a red arrow. NA: natural aging group; MET: metformin group; NAT: neoagarotetraose group. Data are expressed as the mean ± standard deviation (SD). *P < 0.05, **P < 0.01 compared with the natural aging group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Histological staining was performed for the brain, liver, kidney, and small intestine tissues (Fig. 1E). Compared with the NA group, the CA1 pyramidal cells in the brain tissue of NAT group mice significantly increased (P < 0.01; Supplementary Table S2) and showed dense organization with a restored structure. The liver tissue of NAT group mice showed a neatly arranged hepatic cord, few inflammatory infiltrations, and limited binucleation of hepatocytes, along with a significantly reduced degree of hepatic steatosis (P < 0.01; Supplementary Table S2). The glomerulus showed obvious atrophy in NA group mice (Supplementary Table S2), with a balloon-widened cavity and decreased epithelial cells, along with extensive renal edema. Notably, NAT intervention showed obvious attenuation of the unhealthy pathological changes in the kidney and small intestine tissues displayed significantly increased villus height and muscle thickness (P < 0.01; Supplementary Table S2), manifesting protective activities. Mice after NAT treatment showed less pronounced lordokyphosis and periophthalmic lesions, faster reactivity, and more grooming than that in natural aging mice (Table 1).
Table 1.
Effects of neoagarotetraose treatment on aging score of natural aging mice.
| 3 months | ||||||
|---|---|---|---|---|---|---|
| Group | Reactivity | Coarseness | Hair loss | Ulcer | Periophthalmic lesion | Lordokyphosis |
| NA | × | × | 1.81 ± 1.68 | 0.38 ± 1.02 | × | 0.81 ± 1.80 |
| MET | × | × | 0.75 ± 1.34 | 0.13 ± 0.34 | × | 0.25 ± 0.58 |
| NAT | × | × | 1.75 ± 1.91 | × | × | 0.44 ± 1.09 |
| 6 months | ||||||
| NA | 0.47 ± 1.30 | 0.40 ± 1.12 | 2.67 ± 0.82 | 0.53 ± 1.30 | 0.60 ± 1.18 | 1.67 ± 0.90 |
| MET | × | × | 1.53 ± 0.52** | 0.20 ± 0.41 | × | 0.47 ± 1.06** |
| NAT | × | × | 2.07 ± 0.59* | 0.73 ± 0.88 | × | 1.53 ± 0.74 |
| 9 months | ||||||
| NA | 2.67 ± 0.65 | 1.75 ± 0.97 | 3.92 ± 1.08 | 2.08 ± 0.79 | 3.17 ± 0.58 | 3.08 ± 0.79 |
| MET | 1.33 ± 0.78** | 1.00 ± 0.85 | 1.58 ± 1.16** | 0.42 ± 0.51** | 1.42 ± 0.79** | 0.83 ± 0.58** |
| NAT | 1.08 ± 0.67** | 1.50 ± 0.67 | 2.17 ± 0.58** | 0.75 ± 0.62** | 1.83 ± 0.72** | 2.08 ± 1.16* |
| 12 months | ||||||
| NA | 5.25 ± 0.87 | 5.42 ± 1.56 | 5.25 ± 1.36 | 2.83 ± 0.72 | 5.42 ± 1.56 | 4.67 ± 1.07 |
| MET | 1.42 ± 0.51** | 2.25 ± 0.62** | 2.17 ± 0.74** | 1.25 ± 0.62** | 2.08 ± 0.51** | 1.67 ± 0.65** |
| NAT | 2.42 ± 0.90** | 2.75 ± 1.29** | 2.33 ± 0.78** | 1.42 ± 0.51** | 2.42 ± 1.08** | 2.58 ± 0.67** |
NA: natural aging group; MET: metformin group; NAT: neoagarotetraose group. × indicates that the phenomenon is not observed. Results are expressed as mean ± SD (n = 20 mice/group). *P < 0.05, **P < 0.01 compared with the natural aging group.
We further investigated aging and metabolism biomarkers in the serum (Table 2). Compared with NA group mice, the levels of NO, AGEs, and lactic acid in the NAT group were reduced by 62.3 %, 30.8 % and 40.1 %, respectively. Interestingly, significant increases were observed in the telomerase levels of the NAT (by 82.5 %) and MET (by 174.0 %) groups. The levels of T-AOC, CAT, and GSH-Px in the NAT group were improved by 174.2 %, 45.0 %, and 1.9 %, respectively, whereas MDA decreased by 49.4 % compared with that in the NA group mice. Furthermore, the concentrations of RI, TC, TG and LDL-C of the NAT group mice were decreased by 13.7 %, 23.0 %, 17.6 % and 36.8 %, respectively, compared with the NA group, while HDL-C was increased by 38.6 %.
Table 2.
Effects of neoagarotetraose treatment on serum indexes of natural aging mice.
| Aging biomarkers | ||||
|---|---|---|---|---|
| Group | NO (nmol/mL) | Lactic acid (mmol/L) | AGEs (pg/mL) | Telomerase (U/L) |
| NA | 9.05 ± 0.76 | 1.52 ± 0.41 | 53.33 ± 8.58 | 2.23 ± 0.68 |
| MET | 3.94 ± 1.01** | 0.69 ± 0.31** | 29.99 ± 8.31** | 6.11 ± 2.54** |
| NAT | 3.41 ± 0.53** | 0.91 ± 0.30* | 36.92 ± 10.50* | 4.07 ± 0.78 |
| Antioxidant activity | ||||
| Group | T-AOC (U/mL) | CAT (U/mL) | GSH-Px (U/mL) | MDA (nmol/mL) |
| NA | 9.56 ± 1.31 | 7.02 ± 0.64 | 778.51 ± 7.05 | 11.51 ± 0.72 |
| MET | 20.31 ± 2.46** | 9.05 ± 1.28 | 979.75 ± 30.86** | 6.94 ± 1.40** |
| NAT | 26.21 ± 2.44** | 10.18 ± 1.69** | 793.39 ± 14.07 | 5.82 ± 1.34** |
| Metabolism biomarkers | |||||
| Group | RI (ng/mL) | TC (mmol/L) | TG (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) |
| NA | 1.90 ± 0.36 | 2.52 ± 0.30 | 0.85 ± 0.12 | 0.38 ± 0.16 | 1.27 ± 0.36 |
| MET | 1.36 ± 0.22** | 1.92 ± 0.51* | 0.67 ±0.07** | 0.20 ± 0.05** | 1.82 ± 0.10** |
| NAT | 1.64 ± 0.31 | 1.94 ± 0.41* | 0.70 ± 0.09* | 0.24 ± 0.02** | 1.76 ±0.30** |
NO, nitric oxide; AGEs, advanced glycation end products; T-AOC, total anti-oxygenic capacity; CAT, catalase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; RI, insulin; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; NA: natural aging group; MET: metformin group; NAT: neoagarotetraose group. Results are expressed as mean ± SD (n = 20 mice/group). *P < 0.05, **P < 0.01 compared with the natural aging group.
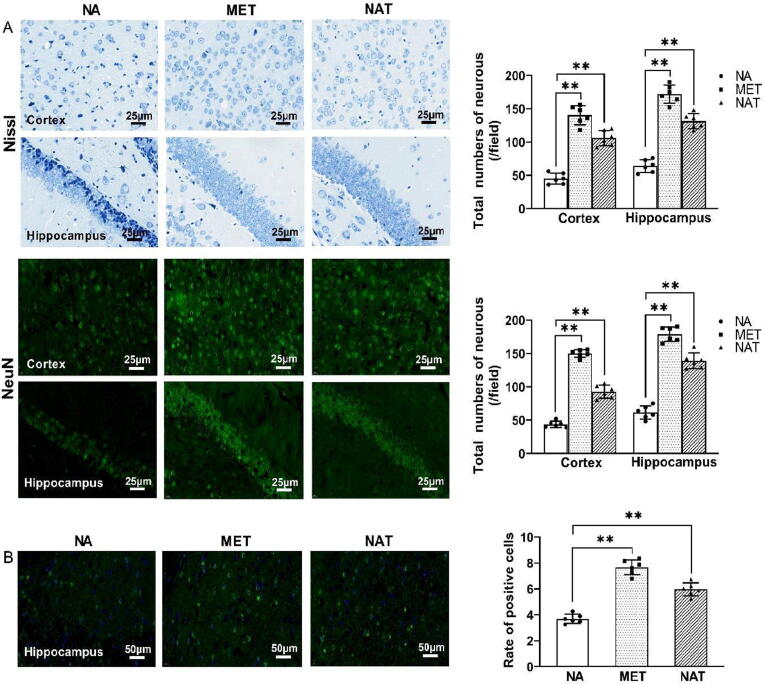
NAT ameliorated neuronal injuries and DNA damage in natural aging mice
Significant reduction in the number of neurons in the cerebral cortex and hippocampal CA1 areas of natural aging mice was observed (Fig. 2A). NAT treatment significantly preserved neuronal survival in these areas compared with the NA group mice (P < 0.01). The level of BDNF in different mice groups was examined using an immunofluorescence assay (Fig. 2B). Compared with the NA group, the expression level of BDNF was increased by 61.8 % and 107.9 %, respectively, after NAT and MET treatments. These results suggest that NAT treatment could ameliorate neuronal injury in natural aging mice.
Fig. 2.
Effects of neoagarotetraose treatment on cerebral injury in natural aging mice. (A) Representative photomicrographs and quantitative analysis of the number of neurons per field in the cerebral cortex and hippocampal CA1 areas, analyzed by Nissl staining and NeuN immunostaining; (B) immunofluorescence analysis of BDNF in the hippocampus. NA: natural aging group; MET: metformin group; NAT: neoagarotetraose group. Data are expressed as the mean ± standard deviation (SD). *P < 0.05, **P < 0.01 compared with the natural aging group.
To determine whether neuronal injury was caused by apoptosis, the expression levels of apoptosis-related genes and proteins in brain tissue were measured. NAT group mice showed significantly inhibited mRNA expression of pro-apoptotic genes, including p53, Bax, and Caspase-3, as well as the increased expression of Bcl-2 (all P < 0.01; Fig. 3A). Simultaneously, the expression levels of p53 and Caspase-3 were evaluated (Fig. 3B). Compared with the NA group mice, NAT treatment reduced the p53 and Caspase-3 levels by 60.0 % and 32.9 %, respectively. To further assess the cell apoptosis effects on neuronal injury, the influence of NAT treatment on the PI3K/AKT/mTOR pathway was investigated. Compared with the NA group, the protein phosphorylation levels of PI3K, AKT, and mTOR in the NAT group were increased by 456.8 %, 12.5 %, and 94.6 %, respectively (Fig. 3C). PTEN protein expression was also reduced by the NAT and MET treatments.
Fig. 3.
Effects of neoagarotetraose treatment on cell apoptosis and DNA damage in the brain tissue of natural aging mice. (A) mRNA expression levels of p53, Bax, Bcl-2, and Caspase-3; (B) protein expression ratio of p53 and Caspase-3 to β-actin; (C) protein expression levels of the PTEN/PI3K/AKT/mTOR signal pathway; (D) immunofluorescence analysis of the DNA damage marker 53BP1. NA: natural aging group; MET: metformin group; NAT: neoagarotetraose group. Data are expressed as the mean ± standard deviation (SD). *P < 0.05, **P < 0.01 compared with the natural aging group.
We further addressed the impact of NAT treatment on age-associated DNA damage in brain tissue. Immunofluorescence assays using an antibody against 53BP1 to mark DNA damage, showed that the NAT and MET treatments decreased the 53BP1 expression levels by 50.9 % and 85.1 %, respectively (all P < 0.01; Fig. 3D). In comparison with the NA group, significant decreases in p21 and p16 expression were observed in the NAT and MET groups, indicating that NAT treatment reduced DNA damage in aging mice (Supplementary Fig. 1).
NAT decreased intestinal barrier injury and inflammation in natural aging mice
The gut-brain axis in brain injury involves the intestinal barrier [37]. NAT treatment most likely affected intestinal physiological changes and the gut microbiota during aging. Therefore, we analyzed gut barrier injury and inflammatory responses in all mice groups. HE staining in NA group mice showed mucous layer atrophy, crypt loss, and obvious inflammatory infiltration (Fig. 4A), suggesting that aging mice exhibited obvious defects in gut barrier integrity; however, NAT and MET treatments attenuated the magnitude of histological damage in the colon. Furthermore, significant increases were observed in the mRNA levels of intestinal permeability-related genes, namely ZO-1 (by 66.4 %), Occludin (by 30.7 %), and Claudin-1 (by 39.0 %) in NAT group mice in comparison with those in the NA group (Fig. 4B). Concurrently, the intestinal mRNA levels of Cox2, Tnfa and Nos2 were significantly decreased in NAT group mice (all P < 0.01; Fig. 4C). As illustrated in Fig. 4D, the protein levels of ZO-1 and Occludin were also significantly increased in the NAT and MET groups (all P < 0.01). Compared with the NA group mice, the level of phosphorylated p38 was decreased by NAT treatment, which was indicative of gut barrier reconditioning.
Fig. 4.
Effects of neoagarotetraose treatment on the intestinal permeability and inflammatory responses of natural aging mice. (A) Morphology of colon tissue stained with hematoxylin and eosin (H & E; 100 × ). Villus heights are indicated by a red arrow, depths of crypts are indicated by a black arrow, muscle thickness is indicated by a blue arrow, and inflammatory infiltration is indicated by a green circle; (B) mRNA expression levels of ZO-1, Occludin, and Claudin-1; (C) mRNA expression levels of Cox2, Tnfα, and Nos2; (D) protein expression levels of intestinal integrity-related proteins. NA: natural aging group; MET: metformin group; NAT: neoagarotetraose group. Data are expressed as the mean ± standard deviation (SD). *P < 0.05, **P < 0.01 compared with the natural aging group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
NAT modified gut microbiota composition in natural aging mice
The impact of NAT treatment on gut microbiota was also investigated. The Faith PD index of the NAT and MET groups was decreased in comparison with the NA group mice (Supplementary Fig. 2A). PCoA based on Jaccard distance indicated that the gut microbiota structure in the NA group showed significant difference compared with the NAT and MET groups (Supplementary Fig. 2B). Compared with the NA group, an increased abundance of phylum Actinobacteria as well as decreased abundance of phylum Deferribacteres were observed in both the NAT and MET groups (Fig. 5A). The NAT group mice exhibited higher abundance of the genera Lactobacillus, Butyricimonas, Odoribacter, and Akkermansia than those in the NA group (P < 0.05 or P < 0.01). Furthermore, the abundance of Prevotella, Bilophila, and Clostridium was significantly reduced after NAT treatment (all P < 0.01; Fig. 5B and Fig. 5C). Linear discriminant analysis Effect Size (LEfSe) also showed that NAT group mice had less Deferribacteres and more Actinobacteria than those in the NA group (Supplementary Fig. 3). At the taxonomic level, NAT group mice were characterized by a higher abundance of beneficial bacteria like Lactobacillus than those in the NA group mice (Supplementary Fig. 3). SCFAs play an important role in anti-inflammation, immunomodulation, intestinal mucosal barrier protection and health maintenance in the host [38]. In comparison with the NA group, the concentrations of acetic acid, propionic acid, and butyric acid in the cecal contents of the NAT group were increased by 65.0 %, 132.5 %, and 49.2 %, respectively (Fig. 5D). Meanwhile, the mRNA expression levels of Gpr41, Gpr43, and Gpr109a genes, which are important as SCFAs receptors, in the NAT group of brain tissue were increased by 101.6 %, 94.0 %, and 38.7 %, respectively, in comparison with those in the NA group (Fig. 5E).
Fig. 5.
Effects of neoagarotetraose treatment on the gut microbiota composition using 16S rDNA sequencing and short chain fatty acids (SCFAs) contents of natural aging mice. (A) Microbiota bacterial changes at the phylum level for each group; (B) microbiota relative reference (%) at the genus taxonomy level for each fecal sample; (C) bacterial population differences at the phylum and genus level based on amplicon sequence variants (ASV) analysis; (D) the contents of short chain fatty acids (SCFAs); (E) the mRNA expression levels of Gpr41, Gpr43 and Gpr109a genes in brain tissue. NA: natural aging group; MET: metformin group; NAT: neoagarotetraose group. Data are expressed as the mean ± standard deviation (SD). *P < 0.05, **P < 0.01 compared with the natural aging group.
The relationship between key aging indexes and bacterial abundance was further analyzed using the Spearman’s correlation coefficient (Supplementary Fig. 4A and 4B). Among bacteria at the genus level, Lactobacillus, Akkermansia, and Butyricimonas were positively correlated with the contents of acetic, propionic, and butyric acids, whereas they were negatively associated with the expression of p53, p21, p16, and inflammation-related factors (Cox2, Tnfa, and Nos2). In contrast, Deferribacteres and Prevotella showed a positive correlation with the levels of p53/p21/p16 and inflammation-related factors, and a negative correlation with SCFAs contents.
NAT modified metabolic pathways of gut microbiota in natural aging mice
Compared with the NA group, the number of predicted functional genes in the NAT and MET groups was increased (Fig. 6A). Clustering analysis of metagenomic functions showed an increased abundance of metabolic pathways in the NAT group compared with those in the MET and NA groups (Fig. 6B). As shown in Fig. 6C, the Metacyc indexes of sucrose biosynthesis I, allantoin degradation IV (anaerobic), and gondoate biosynthesis (anaerobic) pathways, which are related to glucose and lipid metabolism, were significantly lower in the NAT group than those in the NA group (P < 0.05 or P < 0.01). Furthermore, the Metacyc index of the fatty acid biosynthesis superpathways in the NAT group was significantly increased (P < 0.05). Meanwhile, the L-glutamate degradation V and L-glutamate degradation VIII pathways in the NAT group tended to show lower Metacyc indexes than those in the NA group (P < 0.01).
Fig. 6.
Effects of neoagarotetraose treatment on the metabolic pathways of the gut microbiota based on functional enrichment analysis. (A) Venn diagram showing the numbers of predicted functional genes; (B) clustering analysis of relative abundance based on PICRUSt function; (C) functional prediction of altered gut microbiota based on the Metacyc metabolic pathways: PWY-3801 (sucrose degradation II), PWY0-41 (allantoin degradation IV (anaerobic)), PWY-6285 (superpathway of fatty acids biosynthesis), PWY-7663 (gondoate biosynthesis (aerobic)), P162-PWY (L-glutamate degradation V), PWY-5088 (L-glutamate degradation VIII). NA: natural aging group; MET: metformin group; NAT: neoagarotetraose group. Data are expressed as the mean ± standard deviation (SD). *P < 0.05, **P < 0.01 compared with the natural aging group.
NAT ameliorated cognitive impairment in APP/PS1 mice
The essential role of the gut microbiota in brain aging revealed above indicates the potential to regulate the gut microbiota for therapeutic implications in AD. To confirm this hypothesis, APP/PS1 mice were treated with NAT for 3 months (Fig. 7A). Oral administration of NAT did not largely affect the body weight and food consumption of AD mice (Fig. 7B; Supplementary Fig. 5A). Interestingly, NAT treatment caused AD mice to manifest smoother and thicker hair, and faster reactivity (Fig. 7C; Supplementary Fig. 5B). Four-day navigation trials were then performed (Supplementary Fig. 6A and 6B). On the first day, there was no significant difference in escape latency and swimming distance between the groups. As training progressed, the escape latency and swimming distance of the NAT group significantly decreased compared with that of the AD group. On the fourth day, NAT treatment reduced the escape latency and swimming distance by 30.1 % and 26.8 %, respectively. On the fifth day, the escape latency and swimming distance of AD group were significantly increased, and the numbers of crossing platform were significantly decreased when compared with those of the WT group (Fig. 7D; all p < 0.01). In contrast, compared with the AD group, mice in NAT group showed significantly increased swimming distance (by 40.5 %) and time (by 40.0 %) in the platform quadrant, as well as increased entry times onto the platform (by 108.3 %). In the passive avoidance test (Fig. 7E), treatment with NAT increased the escape latency by 84.8 % and reduced the number of errors by 61.0 %, showing a significant difference compared with those in the AD mice (P < 0.01 for both).
Fig. 7.
Effects of neoagarotetraose treatment on the AD parameters of APP/PS1 mice. (A) Schematic of experimental proposition; (B) body weight changes; (C) general status; (D) Morris water maze (MWM) test, and (E) step-through passive avoidance test. WT: wild-type group; AD: Alzheimer’s disease model group; NAT: neoagarotetraose group. Data are expressed as the mean ± standard deviation (SD). *P < 0.05, **P < 0.01 compared with the WT group; #P < 0.05, ##P < 0.01 compared with the AD group.
NAT alleviated amyloid-β (Aβ) and Tau pathology in APP/PS1 mice
Compared with age-paired WT mice, notable histopathological injury, structural degeneration of layered pyramidal neurons, and neuronal loss were observed in the brain tissue of AD group mice (Fig. 8A). NAT treatment ameliorated these alterations, as indicated by the better morphology in HE staining and the increased number of neurons in the cerebral cortex (by 84.9 %) and hippocampus (by 17.7 %) as determined using Nissl staining. High accumulation of Aβ1-42 plaque deposition was observed in the cerebral cortex and hippocampus of APP/PS1 mice compared with that in the WT group mice (Fig. 8B). Conversely, NAT treatment significantly reduced Aβ1-42 plaque deposition in the cerebral cortex (by 53.8 %) and hippocampus (by 44.3 %) compared with that in AD group mice. Tau phosphorylation (Thr231) in the cerebral cortex and hippocampus of AD group mice was significantly increased compared with that in WT mice (P < 0.01), whereas NAT treatment showed a decline by 57.0 % and 42.4 %, respectively (Fig. 8C).
Fig. 8.
Effects of neoagarotetraose treatment on the amyloid-β (Aβ) and Tau pathology of APP/PS1 mice. (A) Morphology of mouse brain tissues subjected to H & E and Nissl staining; changes in the (B) Aβ1-42 positive area and (C) phosphorylated Tau (p-Tau) positive area in the cerebral cortex and hippocampus of APP/PS1 mice. WT: wild-type group; AD: Alzheimer’s disease model group; NAT: neoagarotetraose group. Data are expressed as the mean ± standard deviation (SD). *P < 0.05, **P < 0.01 compared with the WT group; #P < 0.05, ##P < 0.01 compared with the AD group.
NAT modulated gut microbiota composition and SCFAs contents of APP/PS1 mice
In order to further explore the effects of NAT on gut microbiota community, metagenomic sequencing in the cecal contents of APP/PS1 mice was performed. Compared with the WT group, the increased abundance of the phylum Firmicutes and Actinobacteria as well as decreased abundance of the phylum Bacteroidetes and Verrucomicrobia were observed in AD group mice. On the contrary, NAT treatment remarkably suppressed Firmicutes and increased Bacteroidetes and Proteobacteria in APP/PS1 mice. Among the bacteria at genus level, the abundance of Akkermansia and Bacteroides was increased as well as the decreased Clostridium and Prevotella after NAT treatment. At the species level, the abundance of Bacteroides sp. was increased, while Firmicutes_bacterium_M10-2, Dorea_sp._5 and Prevotella_sp._PINT were decreased after NAT treatment (Fig. 9A, 9B and 9C). Furthermore, compared with the AD group, the concentrations of acetic acid and propionic acid in the cecal contents of the NAT group mice were increased by 100.1 % and 92.6 %, respectively (Fig. 9D). The protein expression levels of GPR43 and ERK signal pathway were measured (Fig. 9E). Compared with the AD group, the protein expression level of GPR43 in the NAT group was increased by 512.3 %. The protein phosphorylation level of ERK was reduced by 39.4 % after NAT treatment.
Fig. 9.
Effects of neoagarotetraose treatment on the gut microbiota composition using metagenomic sequencing and short chain fatty acids (SCFAs) contents of APP/PS1 mice. (A) Histograms of relative species abundance at the phylum level; (B) histograms of relative species abundance at the genus level; (C) heatmap analysis of relative species abundance at the species level; (D) the contents of SCFAs; (E) the protein expression of GPR43 and associated pathway. WT: wild-type group; AD: Alzheimer’s disease model group; NAT: neoagarotetraose group. Data are expressed as the mean ± standard deviation (SD). *P < 0.05, **P < 0.01 compared with the WT group; #P < 0.05, ##P < 0.01 compared with the AD group.
Discussion
The adverse effects of aging including both diseases and death pose numerous challenges in humans. Conceiving effective strategies to mitigate these challenges is essential for healthy aging [39]. Nutraceutical interventions using prebiotics aim to regulate signal pathways or replenish the gut with advantageous commensal bacteria; these interventions have emerged as potential strategies to alleviate age-related diseases [40], [41], [42]. Some studies have shown that functional oligosaccharides play an essential role in anti-aging [43], [44], [45]. However, the effect of functional oligosaccharides on lifespan in mice remains unclear. In this study, long-term treatment using NAT, a novel tetraoligosaccharide of NAOS, was found to extend lifespan by up to 33.3 % (Fig. 1D), and significantly increased the number of neurons in natural aging mice (Fig. 2A). These results, together with other results shown in Fig. 2, Fig. 3, suggest that NAT can restore distinct age-associated changes in neurons, further mediating a pro-longevity effect. Importantly, this neuronal activation is often sufficient to improve health and longevity [46].
Neuronal injury due to aging is closely associated with inflammation status via the microbiota-gut-brain axis [37]. The gut barrier fragility and consequent susceptibility to the gut microbiota community are accentuated at the end of life [47]. Aging alters the balance between inflammatory and anti-inflammatory cytokines, which directly affects intestinal permeability [48]. A previous study established the protective effect of mannose on the integrity of gut barrier [49]. Notably, NAT treatment enhanced the integrity of the gut epithelial barrier by upregulating tight junction protein expression, including ZO-1 and Occludin, as well as inhibited gut barrier disruption and inflammatory responses by decreasing p38 phosphorylation level and the mRNA levels of inflammation-related genes (Fig. 4). NAT treatment was suggested to exert a therapeutic effect on the intestinal barrier injury and inflammatory, further ameliorating the aging phenotype in natural aging mice.
Aging is usually accompanied by a reduction in SCFAs-producing bacteria, and an increase in pathogenic bacteria that are tightly associated with age-related diseases [50]. NAT treatment effectively elevated the relative abundance of beneficial gut microbiota, especially Lactobacillus, Butyricimonas, and Odoribacter (Fig. 5C), which are important microbes for maintaining intestinal homeostasis. Additional striking observations regarding the gut microbiota at the genus level indicated that NAT treatment promoted Akkermansia abundance (Fig. 5C). As previously described [51], a high level of Akkermansia in the intestine may lead to mucosa thickening, which can strengthen intestinal barrier function, and significantly delay age-associated diseases. The levels of SCFAs and SCFAs receptors genes were markedly increased after NAT treatment (Fig. 5D and 5E). SCFAs can send signals to the host via G protein-coupled receptors (GPCRs) to influence microbiota-gut-brain interactions, thereby regulating brain and neuronal function [38]. Therefore, NAT supplementation might attenuate neuronal injury during natural aging through modulation of SCFAs production by the gut microbiota. Furthermore, NAT treatment significantly changed the relative abundances of genes in different metabolic pathways (Fig. 6B). Gut microbiota-host interactions have a key role in controlling glucose and lipid metabolism [52], [53]. Prediction of functional changes based on the Metacyc metabolic pathways has been a helpful tool to explore the impact of changes in the body’s environment on the gut microbiota. NAT inhibits the allantoin degradation pathway which is positively associated with fasting glucose level [54], which are indicative of gut metabolism improvement. NAT administration influences the L-glutamate degradation pathways, which are related to other important pathways such as biosynthesis of amino acids [54] and bile acids [55]. Thus, owing to the modulation of gut microbiota components, NAT treatment can affect gut metabolism and physiology, which can impact neural health through the gut-brain axis.
Neuronal degeneration can result in the development of AD characterized by progressive memory loss and cognitive dysfunction [56]. One striking finding of this study was that NAT exhibited therapeutic effects on reversing cognitive disorder, as shown by the improved spatial learning and passive memory performance of APP/PS1 mice (Fig. 7D and Fig. 7E). This effect might result from the significant ameliorative effect of NAT on cerebral neuronal injuries (Fig. 8A). Indeed, NAT treatment decreased cerebral neuronal injuries in natural aging mice. The major markers of AD are the formation of aggregates from Aβ and Tau protein [57]. NAT also improved behavioral phenotypes, attenuated Aβ1-42 plaque accumulation, and reduced the expression level of P-Thr231 Tau in APP/PS1 mice (Fig. 8B and 8C). Thus, this study provides vital proof showing the therapeutic impact of NAT on alleviating AD parameters. NAT treatment may thus be an appealing neuron-centric anti-AD strategy, which warrants further investigation. Interestingly, the altered gut microbial composition and SCFAs contents in the cecal contents of AD mice under NAT treatment (Fig. 9A-9D) were generally consistent with Fig. 5. Furthermore, NAT exerted the neuronal protective functions via the upregulation of GPR43 and suppression of the ERK pathway (Fig. 9E). Our results agreed with the previous study [58], which demonstrated that the coupling of GPR43 and acetoacetate ameliorated the hippocampal neurons in the aging mice by inhibition of GPR43-pERK pathway.
Conclusion
In conclusion, the novel tetraoligosaccharide, NAT, could reshape the gut microbiota community and restore the metabolic homeostasis of gut bacteria in aging mice, which can affect gut physiology, enhance intestinal integrity, and suppress inflammation. These positive impacts mitigated age-associated cerebral injury in natural aging mice and cognitive impairment in AD model mice through gut-brain axis. It will be interesting to further investigate the impact of NAT treatment in different disease models and humans.
Compliance with Ethics Requirements
All Institutional and National Guidelines for the care and use of animals (fisheries) were followed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project was supported by the National Key Research and Development Program of China (Grant number: 2021YFC2100302).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.04.014.
Contributor Information
Zhenglong Gu, Email: zg27@cornell.edu.
Zhengqiang Jiang, Email: zhqjiang@cau.edu.cn.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Ximerakis M., Lipnick S.L., Innes B.T., Simmons S.K., Adiconis X., Dionne D., et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat Neurosci. 2019;22(10):1696–1707. doi: 10.1038/s41593-019-0491-3. [DOI] [PubMed] [Google Scholar]
- 2.Li X.A., Khan I., Xia W.R., Huang G.X., Liu L., Law B.Y.K., et al. Icariin enhances youth-like features by attenuating the declined gut microbiota in the aged mice. Pharmacol Res. 2021;168 doi: 10.1016/j.phrs.2021.105587. [DOI] [PubMed] [Google Scholar]
- 3.Fournie C., Chouchou F., Dalleau G., Caderby T., Cabrera Q., Verkindt C. Heart rate variability biofeedback in chronic disease management: A systematic review. Complement Ther Med. 2021;60 doi: 10.1016/j.ctim.2021.102750. [DOI] [PubMed] [Google Scholar]
- 4.Gong J.B., Yu X.W., Yi X.R., Wang C.H., Tuo X.P. Epidemiology of chronic noncommunicable diseases and evaluation of life quality in elderly. Aging Med. 2018;1(1):64–66. doi: 10.1002/agm2.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J.G., Wang Z.H., Du W.W., Huang F.F., Jiang H.R., Bai J., et al. Twenty-five-year trends in dietary patterns among Chinese adults from 1991 to 2015. Nutrients. 2021;13(4):1327. doi: 10.3390/nu13041327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J., Chen Z.W., Yu H.X., Lu Y.W., Yu W.N., Miao M.Y., et al. Anti-aging effects of a functional food via the action of gut microbiota and metabolites in aging mice. Aging-US. 2021;13(13):17880–17900. doi: 10.18632/aging.202873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535(7610):65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 8.Barcena C., Valdes-Mas R., Mayoral P., Garabaya C., Durand S., Rodriguez F., et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med. 2019;25(8):1234–1242. doi: 10.1038/s41591-019-0504-5. [DOI] [PubMed] [Google Scholar]
- 9.Ding J.H., Jin Z., Yang X.X., Lou J., Shan W.X., Hu Y.X., et al. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J Gastroentero. 2020;26(40):6141–6162. doi: 10.3748/wjg.v26.i40.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burokas A., Arboleya S., Moloney R.D., Peterson V.L., Murphy K., Clarke G., et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiat. 2017;82(7):472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Neri-numa I.A., Pastore G.M. Novel insights into prebiotic properties on human health: A review. Food Res Int. 2020;131 doi: 10.1016/j.foodres.2019.108973. [DOI] [PubMed] [Google Scholar]
- 12.Zhang N., Mao X.Z., Li R.W., Hou E.L., Wang Y.M., Xue C.H., et al. Neoagarotetraose protects mice against intense exercise-induced fatigue damage by modulating gut microbial composition and function. Mol Nutr Food Res. 2017;61(8):1600585. doi: 10.1002/mnfr.201600585. [DOI] [PubMed] [Google Scholar]
- 13.Hong S.J., Lee J.H., Kim E.J., Yang H.J., Park J.S., Hong S.K. Toxicological evaluation of neoagarooligosaccharides prepared by enzymatic hydrolysis of agar. Regul Toxicol Pharm. 2017;90:9–21. doi: 10.1016/j.yrtph.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Zhang N., Hou E.L., Song J., Li J., Tang Q.J., Mao X.Z. Neoagarotetraose-modulated gut microbiota and alleviated gut inflammation in antibiotic treatment mice. Food Agr Immunol. 2017;28(6):1408–1423. [Google Scholar]
- 15.Xu X.Q., Su B.M., Xie J.S., Li R.K., Yang J., Lin J., et al. Preparation of bioactive neoagaroligosaccharides through hydrolysis of Gracilaria lemaneiformis agar: A comparative study. Food Chem. 2017;240:330–337. doi: 10.1016/j.foodchem.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Xu S.Y., Kan J., Hu Z., Liu Y., Du H., Pang G.C., et al. Quantification of neoagaro-oligosaccharide production through enzymatic hydrolysis and its anti-oxidant activities. Molecules. 2018;23(6):1354. doi: 10.3390/molecules23061354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong S.J., Lee J.H., Kim E.J., Yang H.J., Chang Y.K., Park J.S., et al. In vitro and in vivo investigation for biological activities of neoagarooligosaccharides prepared by hydrolyzing agar with β-agarase. Biotechnol Bioproc E. 2017;22(4):489–496. [Google Scholar]
- 18.Wang W., Liu P., Hao C., Wu L.J., Wan W.J., Mao X.Z. Neoagaro-oligosaccharide monomers inhibit inflammation in LPS-stimulated macrophages through suppression of MAPK and NF-kappa B pathways. Sci Rep. 2017;7:44252. doi: 10.1038/srep44252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang D.R., Yoon G.Y., Cho J., Lee S.J., Lee S.J., Park H.J., et al. Neoagarooligosaccharides prevent septic shock by modulating A20-and cyclooxygenase-2-mediated interleukin-10 secretion in a septic-shock mouse model. Biochem Bioph Res Co. 2017;486(4):998–1004. doi: 10.1016/j.bbrc.2017.03.152. [DOI] [PubMed] [Google Scholar]
- 20.Park S.H., Lee C.R., Hong S.K. Implications of agar and agarase in industrial applications of sustainable marine biomass. Appl Microbiol Biot. 2020;104(7):2815–2832. doi: 10.1007/s00253-020-10412-6. [DOI] [PubMed] [Google Scholar]
- 21.Hong S.J., Lee J.H., Kim E.J., Yang H.J., Park J.S., Hong S.K. Anti-obesity and anti-diabetic effect of neoagarooligosaccharides on high-fat diet-induced obesity in mice. Mar Drugs. 2017;15(4):90. doi: 10.3390/md15040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin F.D., Yang D.D., Huang Y.Y., Zhao Y., Ye J., Xiao M.T. The potential of neoagaro-oligosaccharides as a treatment of Type II diabetes in mice. Mar Drugs. 2019;17(10):541. doi: 10.3390/md17100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C.X., Liu J., Ma J.W., Yan Q.J., Jiang Z.Q. Neoagarotetraose extends the lifespan of Caenorhabditis elegans through AMPK mediated signaling pathways and activation of autophagy. J Funct Foods. 2021;77 [Google Scholar]
- 24.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azcarate-Peril M.A., Ritter A.J., Savaiano D., Monteagudo-Mera A., Anderson C., Magness S.T., et al. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc Natl Acad Sci USA. 2017;114(3):E367–E375. doi: 10.1073/pnas.1606722113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J.W., Yan Q.J., Yi P., Yang S.Q., Liu H.J., Jiang Z.Q. Biochemical characterization of a truncated beta-agarase from Microbulbifer sp. suitable for efficient production of neoagarotetraose. Process Biochem. 2019;87:119–127. [Google Scholar]
- 27.Kodali M., Attaluri S., Madhu L.N., Shuai B., Upadhya R., Gonzalez J.J., et al. Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus. Aging Cell. 2021;20(2):e13277. doi: 10.1111/acel.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin X., Du D.F., Chen Q., Wu M., Wu T., Wen J.Y., et al. Metformin prevents murine ovarian aging. Aging-US. 2019;11(11):3785–3794. doi: 10.18632/aging.102016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang N., Wang Q., Lin F.D., Zheng B.D., Huang Y.Y., Yang Y.C., et al. Neoagarotetraose alleviates high fat diet induced obesity via white adipocytes browning and regulation of gut microbiota. Carbohydr Polym. 2022;296 doi: 10.1016/j.carbpol.2022.119903. [DOI] [PubMed] [Google Scholar]
- 30.Liu X.Y., Wu C.X., Han D., Liu J., Liu H.J., Jiang Z.Q. Partially hydrolyzed guar gum attenuates d-galactose-induced oxidative stress and restores gut microbiota in rats. Int J Mol Sci. 2019;20(19):4861. doi: 10.3390/ijms20194861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosokawa M., Kasai R., Higuchi K., Takeshita S., Shimizu K., Hamamoto H., et al. Grading score system: a method for evaluation of the degree of senescence in senescence accelerated mouse (SAM) Mech Ageing Dev. 1984;26(1):91–102. doi: 10.1016/0047-6374(84)90168-4. [DOI] [PubMed] [Google Scholar]
- 32.Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riekkinen P., Schmidt B., Stefanski R., Kuitunen J., Riekkinen M. Metrifonate improves spatial navigation and avoidance behavior in scopolamine-treated, medial septum-lesioned and aged rats. Eur J Pharmacol. 1996;309(2):121–130. doi: 10.1016/0014-2999(96)00336-6. [DOI] [PubMed] [Google Scholar]
- 34.Miller J.A., Ding S.L., Sunkin S.M., Smith K.A., Ng L., Szafer A., et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muñoz-Lorente M.A., Cano-Martin A.C., Blasco M.A. Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat Commun. 2019;10:4723. doi: 10.1038/s41467-019-12664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dostal A., Baumgartner J., Riesen N., Chassard C., Smuts C.M., Zimmermann M.B., et al. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: a randomised, placebo-controlled intervention trial in South African children. Brit J Nutr. 2014;112(4):547–556. doi: 10.1017/S0007114514001160. [DOI] [PubMed] [Google Scholar]
- 37.Yang X.Q., Yu D.K., Xue L., Li H., Du J.R. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm Sin B. 2020;10(3):475–487. doi: 10.1016/j.apsb.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastro Hepat. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 39.Bufenstein R., Ruby J.G. Opportunities for new insight into aging from the naked mole-rat and other non-traditional models. Nat Aging. 2021;1:3–4. doi: 10.1038/s43587-020-00012-4. [DOI] [PubMed] [Google Scholar]
- 40.Zmora N., Suez J., Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastro Hepat. 2019;16(1):35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 41.Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastro Hepat. 2019;16(10):605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 42.Zhu D., Yan Q.J., Li Y.X., Liu J., Liu H.J., Jiang Z.Q. Effect of Konjac mannan oligosaccharides on glucose homeostasis via the improvement of insulin and leptin resistance in vitro and in vivo. Nutrients. 2019;11(8):1705. doi: 10.3390/nu11081705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X.Y., Sun G.Q., Feng T., Zhang J., Huang X., Wang T., et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Res. 2019;29(10):787–803. doi: 10.1038/s41422-019-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouyang Y.Z., Liu D., Zhang L.Z., Li X.Q., Chen X.H., Zhao C. Green Alga enteromorpha prolifera oligosaccharide ameliorates ageing and hyperglycemia through gut-brain axis in age-matched diabetic mice. Mol Nutr Food Res. 2022;66(4):2100564. doi: 10.1002/mnfr.202100564. [DOI] [PubMed] [Google Scholar]
- 45.Tusi S.K., Khalaj L., Ashabi G., Kiaei M., Khodagholi F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials. 2011;32(23):5438–5458. doi: 10.1016/j.biomaterials.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Miller H.A., Huang S.J., Dean E.S., Schaller M.L., Tuckowski A.M., Munneke A.S., et al. Serotonin and dopamine modulate aging in response to food odor and availability. Nat Commun. 2022;13(1):3271. doi: 10.1038/s41467-022-30869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnold J.W., Roach J., Fabela S., Moorfield E., Ding S.L., Blue E., et al. The pleiotropic effects of prebiotic galacto-oligosaccharides on the aging gut. Microbiome. 2021;9(1):31. doi: 10.1186/s40168-020-00980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thevaranjan N., Puchta A., Schulz C., Naidoo A., Szamosi J.C., Verschoor C.P., et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455–466. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong L.J., Xie J.W., Wang Y.Y., Jiang H.L., Chen K., Li D.T., et al. Mannose ameliorates experimental colitis by protecting intestinal barrier integrity. Nat Commun. 2022;13(1):4804. doi: 10.1038/s41467-022-32505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z.Y., He S.D., Cao X.D., Ye Y.K., Yang L., Wang J.H., et al. Potential prebiotic activities of soybean peptides Maillard reaction products on modulating gut microbiota to alleviate aging-related disorders in D-galactose-induced ICR mice. J Funct Foods. 2020;65 [Google Scholar]
- 51.Luo D.S., Chen K.C., Li J.B., Fang Z.Y., Pang H.T., Yin Y.F., et al. Gut microbiota combined with metabolomics reveals the metabolic profile of the normal aging process and the anti-aging effect of FuFang Zhenshu TiaoZhi (FTZ) in mice. Biomed Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109550. [DOI] [PubMed] [Google Scholar]
- 52.Bonfili L., Cecarini V., Gogoi O., Berardi S., Scarpona S., Angeletti M., et al. Gut microbiota manipulation through probiotics oral administration restores glucose homeostasis in a mouse model of Alzheimer's disease. Neurobiol Aging. 2020;87:35–43. doi: 10.1016/j.neurobiolaging.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Bonfili L., Cuccioloni M., Gong C.M., Cecarini V., Spina M., Zheng Y.D., et al. Gut microbiota modulation in Alzheimer’s disease: Focus on lipid metabolism. Clin Nutr. 2022;41(3):698–708. doi: 10.1016/j.clnu.2022.01.025. [DOI] [PubMed] [Google Scholar]
- 54.Chen L.M., Collij V., Jaeger M., van den Munckhof I.C.L., Vila A.V., Kurilshikov A., et al. Gut microbial co-abundance networks show specificity in inflammatory bowel disease and obesity. Nat Commun. 2020;11(1):4018. doi: 10.1038/s41467-020-17840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renga B., Mencarelli A., Cipriani S., D’Amore C., Zampella A., Monti M.C., et al. The nuclear receptor FXR regulates hepatic transport and metabolism of glutamine and glutamate. BBA-Mol Basis Dis. 2011;1812(11):1522–1531. doi: 10.1016/j.bbadis.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Vickers J.C., Dickson T.C., Adlard P.A., Saunders H.L., King C.E., McCormack G. The cause of neuronal degeneration in Alzheimer's disease. Prog Neurobiol. 2000;60(2):139–165. doi: 10.1016/s0301-0082(99)00023-4. [DOI] [PubMed] [Google Scholar]
- 57.Lee W.J., Brown J.A., Kim H.R., La Joie R., Cho H., Lyoo C.H., et al. Regional Aβ-tau interactions promote onset and acceleration of Alzheimer’s disease tau spreading. Neuron. 2022;110(12):1932–1943. doi: 10.1016/j.neuron.2022.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X.J., Shu Q.Q., Wang B., Dong L., Hao B. Acetoacetate improves memory in Alzheimer's mice via promoting brain-derived neurotrophic factor and inhibiting inflammation. Am J Alzheimers Dis. 2022;37 doi: 10.1177/15333175221124949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.