Abstract
Introduction:
Placental pathology is an important contributor to the understanding of preterm birth and reveals major differences between spontaneous preterm birth (SPTB) and iatrogenic preterm birth (IPTB). The aim of this study was to investigate these relationships.
Methods:
Research midwives collected placentas from 1101 women with singleton pregnancies who were enrolled in the Safe Passage Study. Trained pathology technologists prepared and processed placenta specimens for macroscopic and microscopic examination by designated pathologists. Statistical analyses were done with STATISTICA version 13.
Results:
In SPTB we found more cases of accelerated villous maturation; however, the other features of maternal vascular malperfusion (MVM) were not present. The prevalence rate of funisitis was also increased. In IPTB, multiple features of MVM - accelerated villous maturation, distal villous hypoplasia, decidual arteriopathy, increased syncytial knots, increased perivillous fibrin, and prominent extravillous trophoblast were increased, as were features of fetal vascular malperfusion (FVM) - umbilical cord vessel thrombosis, avascular villi, and fetal vascular thrombosis. Increased syncytial knots were found in 26% of preterm stillbirths and in 29% of preterm infant demises as compared to 81% of IPTB infants alive at one year.
Discussion:
SPTB and IPTB differ. The detected “abnormal” accelerated villous maturation pattern in SPTB and preterm demises, suggests an inability of the placenta to adapt and may be a trigger for SPTB. Funisitis was the only inflammatory response significant for SPTB. MVM and FVM are implicated in IPTB, but not an inflammatory process.
Keywords: Spontaneous preterm birth, Iatrogenic preterm birth, Placental pathology
1. Introduction
Preterm birth (PTB) has devastating maternal and neonatal consequences, accounting for 75% of perinatal mortalities and more than 50% of chronic infant morbidities [1]. Placental pathology associated with PTB may be useful in categorizing and determining its etiology.
Despite the attention to spontaneous preterm birth (SPTB) in medical research, the etiology remains largely unexplained. It is considered a syndrome caused by multiple pathologies such as infection, inflammation, malperfusion, haemorrhage, stress, uterine over-distention and other immunologically mediated processes [2].
Increasing evidence indicates that placental dysfunction, such as maternal or fetal vascular malperfusion, is an important risk factor for PTB [3,4], but information from low-income-countries is lacking. Macroscopic and microscopic placental features may illuminate the clinical understanding of preterm delivery, perhaps most demonstratable in communities with high prevalence rates [5-7]. The rate of PTB is 13.8% in a large low-to middle income community in Cape Town, South Africa [8] where this study was conducted to investigate the relationship between placental pathology and spontaneous or iatrogenic preterm birth (IPTB) and poor neonatal outcomes.
2. Methods
Women attending the antenatal clinics of Midwife Obstetric Units (MOUs) near Tygerberg Academic Hospital (TBH), Cape Town, were recruited (n = 7060) for the South African (SA) part of the Safe Passage Study (SPS) from August 2007 to January 2015 [9]. Forty percent, of the women enrolled before 24 weeks were randomly selected for an embedded study which included placental histology. The earliest ultrasound examination was used to calculate the gestational age (GA). Socio-economic data were obtained from a maternal history and demographics questionnaire. Maternal chart abstraction was done after delivery to establish pregnancy outcome. For this study we focused on women with singleton pregnancies who participated in the study for the first time, and who had placental histology available. Z-scores of the birthweights were calculated using INTERGROWTH-21st study references [10]. The study cohort was classified into SPTB (any spontaneous birth <37 weeks), IPTB and spontaneous term birth (STB).
After delivery, placentas were placed in a refrigerator at 4 °C. They were then transported to TBH and labelled with the participant’s study identity number. The only information the pathologist received was the GA at birth and whether it was a live or stillbirth.
The placentas were examined macroscopically, recorded on a standard template and the maternal and fetal surfaces photographed. Thereafter two sections of the umbilical cord (proximal and distal), a membrane roll and three full thickness sections of parenchyma (normal and lesions) were taken according to standardised protocols and processed into paraffin embedded wax blocks. Subsequently sections were cut, and haematoxylin and eosin-stained slides produced for histological examination by SA perinatal pathologists (CW/PS). For stillbirths, the slides were also examined by external perinatal pathologists. Quality control was ensured by checking inter-observer concordance between all pathologists with a standard of 90% for individual items.
The trimmed placental weight for gestation was considered small when <10th centile according to SPS centiles [11]. Macroscopic infarction was defined as firm solid area(s) extending from the basal plate with the apex towards the fetal surface. Any infarction in a preterm placenta and >5% infarction at term was considered pathological. Microscopic infarction was diagnosed as villi crowded together with trophoblastic smudging or complete necrosis. Distal villous hypoplasia was diagnosed when villi were small and stringy, elongated or appeared tiny, barely the size of a syncytial knot with increased intervillous space. Accelerated villous maturation indicates villi hyper-mature for GA and is usually associated with an increase in syncytial knots. Increased syncytial knots were reported if they were found in >33% of villi at term and >30% preterm [12]. Decidual arteriopathy included muscular hypertrophy and/or fibrinoid necrosis with or without atherosis. Increased perivillous fibrin (where the fibrin surrounds villi) and increased extravillous trophoblast (trophoblast fibrinoid islands) were also recorded.
Fetal vascular malperfusion (FVM) was defined per the Amsterdam International Consensus Criteria [13]. Avascular villi were defined as regional or diffuse and are terminal villi with loss of villous capillaries and hyalinized stroma. Villous stromal karyorrhexis was defined as regional or diffuse, demonstrating nuclear dust with extravasation of red cells into the villous stroma with preservation of the surrounding trophoblast. Chorionic plate/stem villus thrombosis was categorised into venous, arterial, both or indeterminate, focal or multifocal and included occlusive and non-occlusive thrombi. It also included intramural fibrin deposition. Microscopic thrombosis of the umbilical cord vessels was categorised into venous, arterial or both. Fetal vascular thrombosis was diagnosed when two or more of the four above-mentioned features (avascular villi, villous stromal karyorrhexis, chorionic plate/stem villus thrombosis or microscopic thrombosis of the cord) were present.
Macroscopic retroplacental haemorrhage (abruption) was defined as an indentation and/or haemorrhage involving >15% of the maternal surface of the placenta. The microscopic criteria for retroplacental haemorrhage were the presence of blood beneath the decidua and dissecting into the decidua and placental parenchyma, with congestion, and/or intravillous haemorrhage (haemorrhage into villous stroma) or associated dome-shaped infarction.
Vasculitis of the umbilical cord was recorded when one or more vessels had inflammation within the muscular wall, while funisitis of the umbilical cord was recorded when inflammation present was perivascular and constituted a fetal inflammatory response (FIR). These correspond to stage 1–2 or stage 3 per the Amsterdam Criteria [13] respectively.
Chorioamnionitis was defined when inflammatory cell types of membranes and chorionic plate was acute, chronic or eosinophillic and the location was subchorion, chorion or chorioamnion and constituted a maternal inflammatory response (MIR). Villitis was recorded when inflammatory cell types of parenchyma were chronic-specific (infective) or chronic-nonspecific (non-infective) also known as villitis of unknown etiology (VUE) where infectious causes were ruled out.
Statistical analyses utilised STATISTICA (Dell Inc. (2015). Dell Statistica (data analysis software system), version 13. software.dell.com). Continuous variables were compared versus the different groups of interest with either analysis of variance (ANOVA) or with repeated measures ANOVA with the assumption of compound symmetry i.e., equicorrelation among repetitions. Bonferroni corrections for multiple comparisons were used to identify significant differences among means. Maximum likelihood chi-square statistics were used to analyse associations between nominal variables in contingency tables. For 2x2 contingency tables odds ratios (ORs) with their 95% confidence intervals (CIs) were computed.
3. Results
This study (Fig. 1), consists of 1101 women of whom 99.8% were from mixed ancestry who had either a SPTB (n = 141 (12.8%)) or an IPTB (n = 84 (7.6%)) or a STB (n = 876 (79.6%)). The basic statistics of these three groups are summarised in Table 1.
Fig. 1.
Study profile.
Table 1.
Basic statistics of all groups.
| Variable | N | Mean | Std Dev |
Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Spontaneous preterm birth group (141) | ||||||
| Gestational age at enrolment (days) | 141 | 118 | 37 | 118 | 49 | 203 |
| Maternal age (years) | 141 | 25.0 | 5.8 | 24.0 | 16.0 | 42.0 |
| Maternal arm circumference | 138 | 268 | 44 | 259 | 194 | 429 |
| Body mass index (kg/m2) | 139 | 24.4 | 5.9 | 22.7 | 15.3 | 43.8 |
| Gravidity | 141 | 2.3 | 1.4 | 2.0 | 1.0 | 7.0 |
| Parity | 141 | 1.1 | 1.2 | 1.0 | 0.0 | 6.0 |
| Education (years) | 141 | 9.8 | 1.7 | 10.0 | 4.0 | 13.0 |
| People per room | 123 | 1.6 | 1.1 | 1.3 | 0.5 | 8.0 |
| Household income (ZAR per month) | 83 | 934 | 663 | 750 | 167 | 3000 |
| Gestational age at delivery (days) | 141 | 229 | 30 | 241 | 149 | 258 |
| Birthweight (grams) | 138 | 1923 | 772 | 2105 | 360 | 3500 |
| Birthweight Z-score | 129 | −0.3 | 1.0 | −0.3 | −6.3 | 1.8 |
| Trimmed placenta weight (grams) | 141 | 313 | 108 | 319 | 82 | 565 |
| Iatrogenic preterm birth group (84) | ||||||
| Gestational age at enrolment (days) | 84 | 121 | 39 | 125 | 53 | 217 |
| Maternal age (years) | 84 | 25.7 | 6.4 | 25.0 | 16.0 | 43.0 |
| Maternal arm circumference | 82 | 278 | 48 | 270 | 196 | 475 |
| Body mass index (kg/m2) | 81 | 25.7 | 6.1 | 24.7 | 15.4 | 45.7 |
| Gravidity | 84 | 2.1 | 1.3 | 2.0 | 1.0 | 6.0 |
| Parity | 84 | 0.9 | 1.2 | 0.0 | 0.0 | 5.0 |
| Education (years) | 83 | 10.0 | 1.8 | 10.0 | 6.0 | 13.0 |
| People per room | 72 | 1.5 | 0.8 | 1.3 | 0.3 | 5.0 |
| Household income (ZAR per month) | 56 | 992 | 638 | 929 | 100 | 3000 |
| Gestational age at delivery (days) | 84 | 217 | 33 | 222 | 146 | 258 |
| Birthweight (grams) | 81 | 1500 | 867 | 1440 | 190 | 4170 |
| Birthweight Z-score | 75 | −1.0 | 1.4 | −0.8 | −5.7 | 3.1 |
| Trimmed placenta weight (grams) | 84 | 253 | 132 | 237 | 50 | 740 |
| Spontaneous term birth group (876) | ||||||
| Gestational age at enrolment (days) | 876 | 119 | 32 | 121 | 42 | 256 |
| Maternal age (years) | 876 | 24.4 | 5.8 | 24.0 | 16.0 | 41.0 |
| Maternal arm circumference | 856 | 273 | 44 | 265 | 190 | 458 |
| Body mass index (kg/m2) | 853 | 24.8 | 5.5 | 23.4 | 15.8 | 52.3 |
| Gravidity | 875 | 2.0 | 1.2 | 2.0 | 1.0 | 8.0 |
| Parity | 875 | 0.9 | 1.1 | 1.0 | 0.0 | 6.0 |
| Education (years) | 875 | 10.1 | 1.7 | 10.0 | 3.0 | 13.0 |
| People per room | 866 | 1.6 | 0.9 | 1.4 | 0.3 | 8.0 |
| Household income (ZAR per month) | 655 | 896 | 612 | 750 | 50 | 6000 |
| Gestational age at delivery (days) | 876 | 276 | 8 | 276 | 259 | 301 |
| Birthweight (grams) | 876 | 3099 | 439 | 3100 | 1160 | 4600 |
| Birthweight Z-score | 875 | −0.4 | 1.0 | −0.3 | −4.3 | 2.8 |
| Trimmed placenta weight (grams) | 875 | 427 | 90 | 420 | 158 | 877 |
ZAR = South African Rand (1$ = 15.2 ZAR/1 EURO = 18.5 ZAR), Std Dev = Standard deviation.
Indications for IPTB were 31 (36.9%) for severe hypertension, pre-eclampsia, or eclampsia; 21 (25.0%) for an intrauterine demise (IUD); 13 (15.5%) for preterm premature/prelabour rupture of membranes (PPROM); 5 (6.0%) each for intrauterine growth restriction (IUGR) and congenital anomalies; 2 (2.4%) each for antepartum haemorrhage or placental abruption, placenta praevia, fetal distress, inevitable miscarriage; and 1 (1.2%) for nephrotic syndrome. The pathological causes of IUD were placental abruption 8 (38%), fetal flow restriction of the umbilical cord 4 (19%), uteroplacental malperfusion 3 (14%), undetermined 2 (10%), maternal systemic disease, fetal ascending infection, severe congenital anomalies, and severe maternal hypotension 1 (5%) each. We assessed different complications for IPTB in three GA categories. The three main indications for each group were: IUD (9, 39%), hypertension/pre-eclampsia (6, 26%) and congenital anomalies (5, 22%) for group GA 20w0 to 27w6 (23, 27%); hypertension/pre-eclampsia (13, 42%), IUD (11, 35%), and PPROM (3, 10%) for group GA 28w0 to 33w6 (31, 37%); and hypertension/pre-eclampsia (12, 40%), PPROM (9, 30%), and IUGR (3, 10%) for group GA 34w0 to 36w6 (30, 36%).
Decidual arteriopathy was identified in 100 (9.1%) placentas, thrombosis of cord vessels in 22 (2.0%), avascular villi in 237 (21.5%), chorionic plate/stem villus thrombosis in 110 (10.0%), villous-stromal karyorrhexis in 293 (26.6%), chorioamnionitis in 361 (32.8%), villitis in 249 (22.6%), funisitis in 56 (5.1%), and vasculitis of umbilical cord vessels in 75 (6.8%). (Table 2).
Table 2.
Placenta microscopic assessment in 1101 participants.
| Features of maternal vascular malperfusion | ||||
| Decidual arteriopathy | Muscular hypertrophy | Fibrinoid necrosis | Fibrinoid necrosis with atherosis | |
| 100 (9.1%) | 57 (57.0%) | 43 (43.0%) | 0 (0.0%) | |
| Features of fetal vascular malperfusion | ||||
| Thrombosis of cord vessels | Artery | Vein | Both | |
| 22 (2.0%) | 6 (27.3%) | 15 (68.2%) | 1 (4.5%) | |
| Avascular villi | Regional | Diffuse | ||
| 237 (21.5%) | 229 (96.6%) | 8 (3.4%) | ||
| Chorionic plate thrombosis | Artery | Vein | Both | Indeterminate |
| 110 (10.0%) | 3 (2.7%) | 20 (18.2%) | 29 (26.4%) | 58 (52.7%) |
| Villous-stromal karyorrhexis | Regional | Diffuse | ||
| 293 (26.6%) | 268 (91.5%) | 25 (8.5%) | ||
| Inflammatory response | ||||
| Chorioamnionitis | Acute | Chronic | Eosinophilic | |
| 361 (32.8%) | 333 (92.2%) | 28 (7.8%) | 0 (0.0%) | |
| Subchorion | Chorion | Chorioamnion | Indeterminate | |
| 232 (64.3%) | 68 (18.8%) | 60 (16.6%) | 1 (0.3%) | |
| Villitis | Acute | Chronic specific | VUE | |
| 249 (22.6%) | 2 (0.8%) | 7 (2.8%) | 240 (96.4%) | |
| Funisitis | Perivascular | Superficial | Both | |
| 56 (5.1%) | 54 (96.4%) | 2 (3.6%) | 0 (0.0%) | |
| Vasculitis | One vessel | Two vessels | Three vessels | |
| 75 (6.8%) | 46 (61.3%) | 9 (12.0%) | 20 (26.7%) | |
| Artery | One | Two | ||
| 33 (44.0%) | 13 (39.4%) | 20 (60.6%) |
VUE villitis of unknown etiology (chronic non-specific).
The ORs for SPTB and IPTB as compared to STB are given in Table 3. For SPTB the following features of maternal vascular malperfusion (MVM) were associated with an increased risk: accelerated villous maturation (OR 17.94), macroscopic pathological infarction (OR 3.35), and microscopic pathological infarction (OR 1.85). Reduced risks for SPTB were associated with increased syncytial knots (OR 0.37), increased perivillous fibrin (OR 0.42), and increased extravillous trophoblast/fibrinoid islands (OR 0.55). For IPTB features associated with an increased risk were accelerated villous maturation (OR 69.9), macroscopic pathological infarction (OR 5.80), distal villous hypoplasia (OR 5.55), microscopic pathological infarction (OR 5.01) and decidual arteriopathy (OR 4.66).
Table 3.
Risks of placental features for spontaneous preterm and iatrogenic preterm birth compared to spontaneous term birth.
| Pathology variable | OR SPTB vs STB |
CI lower SPTB vs STB |
CI upper SPTB vs STB |
SPTB (141) n (% YES) of variable |
STB (876) n (% YES) of variable |
IPTB (84) n (% YES) of variable |
OR IPTB vs STB |
CI lower IPTB vs STB |
CI upper IPTB vs STB |
|---|---|---|---|---|---|---|---|---|---|
| Features of maternal vascular malperfusion | |||||||||
| Small trimmed placenta for GA | 0.79 | 0.55 | 1.12 | 66 (47%) | 456 (53%) | 49 (59%) | 1.29 | 0.81 | 2.03 |
| Macroscopic pathological infarction | 3.25 | 1.43 | 7.39 | 9 (6%) | 18 (2%) | 9 (11%) | 5.80 | 2.52 | 13.36 |
| Microscopic pathological infarction | 1.85 | 1.09 | 3.14 | 20 (14%) | 72 (8%) | 26 (31%) | 5.01 | 2.97 | 8.43 |
| Decidual arteriopathy | 1.68 | 0.94 | 3.00 | 16 (11%) | 62 (7%) | 22 (26%) | 4.66 | 2.69 | 8.08 |
| Accelerated villous maturation | 17.94 | 9.09 | 35.42 | 30 (21%) | 13 (1%) | 43 (51%) | 69.62 | 34.75 | 139.50 |
| Increased syncytial knots | 0.37 | 0.25 | 0.55 | 42 (30%) | 466 (53%) | 52 (62%) | 1.43 | 0.90 | 2.26 |
| Distal villous hypoplasia | 0.98 | 0.52 | 1.85 | 12 (9%) | 76 (9%) | 29 (35%) | 5.55 | 3.34 | 9.22 |
| Increased extravillous trophoblast | 0.55 | 0.35 | 0.86 | 26 (18%) | 255 (29%) | 28 (33%) | 1.22 | 0.76 | 1.96 |
| Increased perivillous fibrin | 0.42 | 0.29 | 0.62 | 42 (30%) | 439 (50%) | 46 (55%) | 1.21 | 0.77 | 1.89 |
| Features of abruption | |||||||||
| Macroscopic abruption | 1.59 | 0.88 | 2.88 | 15 (11%) | 61 (7%) | 16 (19%) | 3.14 | 1.72 | 5.75 |
| Microscopic abruption | 2.19 | 1.37 | 3.50 | 28 (20%) | 89 (10%) | 20 (24%) | 2.76 | 1.60 | 4.78 |
| Acute placental abruption (both) | 2.08 | 1.00 | 4.34 | 10 (7%) | 31 (4%) | 11 (13%) | 4.11 | 1.98 | 8.51 |
| Features of fetal vascular malperfusion | |||||||||
| Thrombosis of cord vessels (microscopic) | 0.88 | 0.20 | 3.93 | 2 (1%) | 14 (2%) | 6 (7%) | 4.85 | 1.81 | 12.98 |
| Avascular villi | 0.77 | 0.48 | 1.22 | 24 (17%) | 185 (21%) | 28 (33%) | 1.87 | 1.15 | 3.02 |
| Chorionic plate/stem villus thrombosis | 0.75 | 0.39 | 1.44 | 11 (8%) | 89 (10%) | 10 (12%) | 1.19 | 0.60 | 2.40 |
| Villous-stromal karyorrhexis | 0.47 | 0.29 | 0.76 | 22 (16%) | 246 (28%) | 25 (30%) | 1.09 | 0.66 | 1.77 |
| Fetal vascular thrombosis | 0.57 | 0.31 | 1.04 | 13 (9%) | 132 (15%) | 24 (29%) | 2.25 | 1.35 | 3.74 |
| Vascular necrosis | 991.00 | 992.00 | 993.00 | 0 (0%) | 8 (1%) | 1 (1%) | 1.34 | 0.17 | 10.82 |
| Inflammatory response | |||||||||
| Chorioamnionitis (MIR) | 1.40 | 0.97 | 2.02 | 56 (40%) | 280 (32%) | 25 (30%) | 0.90 | 0.55 | 1.47 |
| Villitis (MIR) | 1.33 | 0.89 | 2.00 | 38 (27%) | 190 (22%) | 19 (23%) | 1.06 | 0.62 | 1.80 |
| Funisitis of cord (FIR) | 2.24 | 1.19 | 4.23 | 14 (10%) | 41 (5%) | 1 (1%) | 0.25 | 0.03 | 1.85 |
| Vasculitis of cord vessels (FIR) | 1.58 | 0.86 | 2.92 | 14 (10%) | 57(7%) | 4 (5%) | 0.74 | 0.26 | 2.08 |
| Outcome | |||||||||
| Intrauterine growth restriction | 0.45 | 0.24 | 0.83 | 12 (9%) | 163 (19%) | 25 (33%) | 2.18 | 1.31 | 3.63 |
| Low birthweight | 30.16 | 19.32 | 47.10 | 99 (72%) | 68 (8%) | 69 (85%) | 68.32 | 35.27 | 132.34 |
| Stillbirths | 20.51 | 9.68 | 43.48 | 27 (19%) | 10 (1%) | 26 (31%) | 38.82 | 17.86 | 84.37 |
| Infant demises before one year | 9.48 | 3.55 | 25.33 | 10 (7%) | 7 (1%) | 4 (5%) | 6.21 | 1.78 | 21.66 |
SPTB, spontaneous preterm birth; IPTB, iatrogenic preterm birth; STB, spontaneous term birth; OR, odds ratio; CI, confidence interval; Bold indicates significant OR; MIR, maternal inflammatory response; FIR, fetal inflammatory response; vs, versus.
For SPTB microscopic abruption was associated with an increased risk (OR 2.19), while for IPTB acute placental abruption (both macroscopic and microscopic) (OR 4.11), macroscopic abruption (OR 3.14), and microscopic abruption (OR 2.76) were associated with an increased risk.
Villous-stromal karyorrhexis was associated with a reduced risk for SPTB (OR 0.47), while the following features of FVM were associated with increased risk for IPTB: thrombosis of cord vessels (OR 4.85), fetal vascular thrombosis (OR 2.25), and avascular villi (OR 1.87).
For SPTB perivascular funisitis of the umbilical cord (OR 2.24) was associated with a greater risk. Inflammatory response was not associated with increased risks for IPTB.
In the SPTB group, IUGR (OR 0.45) occurred less frequently but low birthweight (OR 30.16), stillbirth (OR 20.15) and demise before the age of one year (OR 9.48) more frequently. In the IPTB group, IUGR (OR 2.18), low birthweight (OR 68.32), stillbirth (OR 38.82), and demise before the age of one year (OR 6.21) carried increased risks (Table 3).
When comparing the placental histopathologic features of SPTB directly to the features of IPTB, features of MVM and FVM were significantly greater in the IPTB group. Only perivascular funisitis of the umbilical cord (FIR stage 3) occurred significantly more often in the SPTB group (Table 4).
Table 4.
Placenta pathology and spontaneous preterm birth compared to iatrogenic preterm birth.
| Pathology variable | SPTB (141) n (% YES) of variable |
IPTB (84) n (% YES) of variable |
p- value |
|---|---|---|---|
| Features of maternal vascular malperfusion | |||
| Small trimmed placenta for GA | 66 (47%) | 49 (59%) | 0.229 |
| Macroscopic pathological infarction | 9 (6%) | 9 (11%) | 0.729 |
| Microscopic pathological infarction | 20 (14%) | 26 (31%) | 0.009 |
| Decidual arteriopathy | 16 (11%) | 22 (26%) | 0.014 |
| Accelerated villous maturation | 30 (21%) | 43 (51%) | 0.000 |
| Increased syncytial knots | 42 (30%) | 52 (62%) | 0.000 |
| Distal villous hypoplasia | 12 (9%) | 29 (35%) | 0.000 |
| Increased extravillous trophoblast/fibrinoid islands | 26 (18%) | 28 (33%) | 0.037 |
| Increased perivillous fibrin | 42 (30%) | 46 (55%) | 0.001 |
| Features of abruption | |||
| Macroscopic abruption | 15 (11%) | 16 (19%) | 0.243 |
| Microscopic abruption | 28 (20%) | 20 (24%) | 1.000 |
| Acute placental abruption (both macro and micro) | 10 (7%) | 11 (13%) | 0.422 |
| Features of fetal vascular malperfusion | |||
| Thrombosis of cord vessels (microscopic) | 2 (1%) | 6 (7%) | 0.075 |
| Avascular villi | 24 (17%) | 28 (33%) | 0.017 |
| Chorionic plate/stem villus thrombosis | 11 (8%) | 10 (12%) | 0.937 |
| Villous-stromal karyorrhexis | 22 (16%) | 25 (30%) | 0.038 |
| Fetal vascular thrombosis (microscopic only) | 13 (9%) | 24 (29%) | 0.001 |
| Vascular necrosis | 0 (0%) | 1 (1%) | 0.469 |
| Inflammatory response | |||
| Chorioamnionitis (MIR) | 56 (40%) | 25 (30%) | 0.390 |
| Villitis (MIR) | 38 (27%) | 19 (23%) | 1.000 |
| Funisitis of cord (FIR) | 14 (10%) | 1 (1%) | 0.015 |
| Vasculitis of cord vessels (FIR) | 14 (10%) | 4 (5%) | 0.500 |
| Outcome | |||
| Intrauterine growth restriction | 12 (9%) | 25 (33%) | 0.000 |
| Low birthweight | 99 (72%) | 69 (85%) | 0.059 |
| Stillbirths | 27 (19%) | 26 (31%) | 0.137 |
| Infant demise before 1 year | 10 (7%) | 4 (5%) | 1.000 |
SPTB, spontaneous preterm birth; IPTB, iatrogenic preterm birth; Bold indicates significant p-value; MIR, maternal inflammatory response; FIR, fetal inflammatory response.
Gestational hypertension was observed in 42% of IPTBs in comparison to 8% in SPTBs (p = 0.000) and 8% in STBs (p = 0.000). Pre-eclampsia was observed in 23% of IPTBs in comparison to 3% in SPTBs (p = 0.000) and 2% in STBs (p = 0.000). Fetal growth restriction occurred in 33% of IPTBs in comparison to 9% in SPTBs (p = 0.000) and 19% in STBs (p = 0.012). IUGR also occurred significantly less in SPTBs compared to STBs (p = 0.016).
Significant differences were found when preterm stillbirths (n = 53) were compared to SPTB where the infants were alive at one year (n = 102). Amongst stillbirths 98% (n = 48) vs 61% (n = 62) had low birthweight (p = 0.000), 35% (n = 13) vs 5% (n = 5) were growth restricted (p = 0.000), 21% (n = 11) vs 1% (n = 1) had acute placental abruption (p = 0.000), 28% (n = 15) vs 6% (n = 6) had fetal vascular thrombosis (p = 0.003), 30% (n = 16) vs 9% (n = 9) had decidual arteriopathy (p = 0.012), 30% (n = 16) vs 11% (n = 11) had microscopic pathological infarction (p = 0.048), 18% (n = 10) vs 4% (n = 4) had macroscopic pathological infarction (p = 0.042), 23% (n = 12) vs 5% (n = 5) had distal villous hypoplasia (p = 0.016), and 8% (n = 4) vs 0% (n = 0) had thrombosis of the umbilical cord vessels (p = 0.042).
When preterm stillbirths (n = 53) were compared to IPTB infants who were alive at one year (n = 42), significant differences were found. Amongst stillbirths, 26% (n = 14) vs 81% (n = 34) had increased syncytial knots (p = 0.000), 57% (n = 30) vs 17% (n = 7) had chorioamnionitis (p = 0.000), and 98% (n = 48) vs 76% (n = 32) had low birthweight (p = 0.012).
Low birthweight was observed in 100% (n = 14) of preterm born infants who had died in contrast to 61% (n = 62) of SPTB infants who were alive at one year (p = 0.006). This was the only difference between the two groups.
Infants who had died (n = 14), differed from IPTB infants who were alive at one year (n = 42) regarding increased syncytial knots 29% (n = 4) vs 81% (n = 34) (p = 0.006) and avascular villi, 0% (n = 0) vs 36% (n = 15) (p = 0.020). These were the only variables that differed significantly.
4. Discussion
Placental pathology has strong associations with PTB, both spontaneous and iatrogenic, and provides important insights into these subtypes. We found many significant differences between SPTB and IPTB which will be discussed separately.
4.1. SPTB
The pathologic conditions associated with disturbed maternal blood flow are currently known as MVM (see Fig. 2), as defined in the Amsterdam consensus [13], with villous maldevelopment being the most important histologic finding [14]. Accelerated villous maturation i. e. premature formation of terminal villi is diagnosed in placentas prior to mid third trimester and can be seen as an adaptation of the placenta to decreased perfusion or placental insufficiency [15,16]. In MVM, global or partial obstruction of blood flow from abnormal spiral arteries leads to accelerated villous maturation while segmental, complete occlusion causes a villous infarct [17]. The normal villous maturation pattern is characterized by decreasing villous size and diameter, increasing syncytial knots for gestational age accompanied by increased intervillous fibrin deposition alternating with areas of villous paucity (distal villous hypoplasia) in order to maximize oxygen diffusion exchange surface [14,16,18]. In our SPTB group we found more cases showing accelerated maturation of villi (21%) but combined with less proliferation of syncytial knots, perivillous fibrin, and extravillous trophoblast. There was no association with distal villous hypoplasia. These findings were significantly associated with SPTB, suggesting that placentas not adapting by the accelerated villous maturation pattern, are more likely to suffer from hypoxia, stillbirth, infant demises or SPTB.
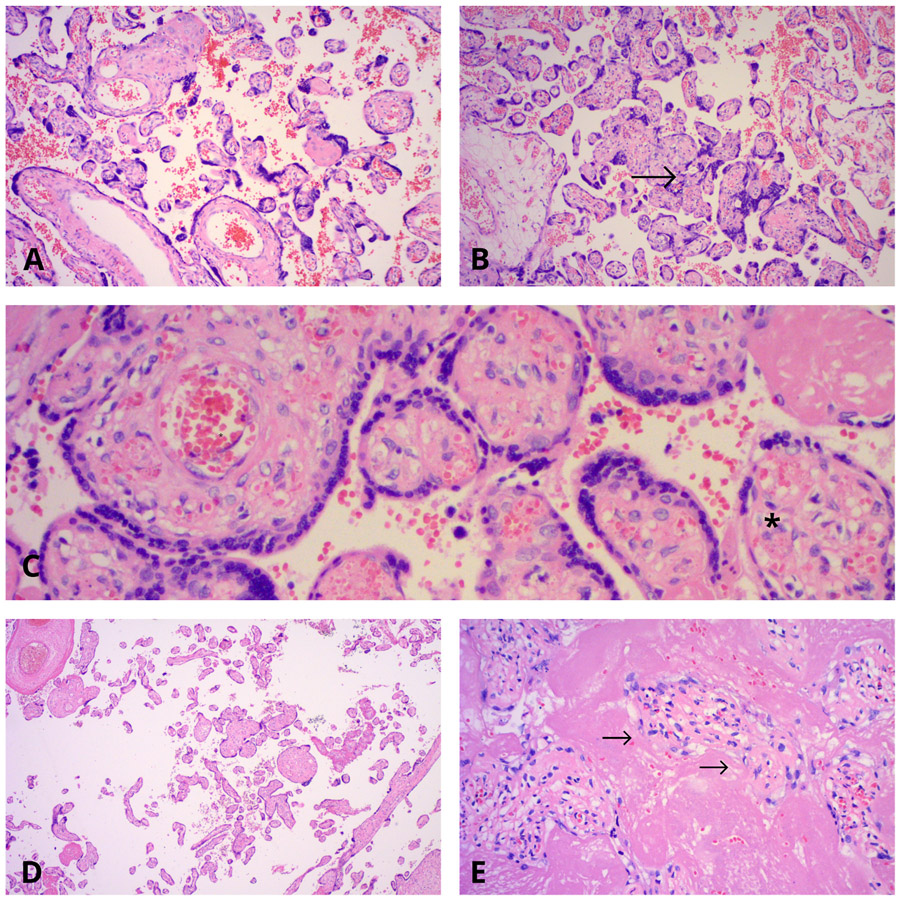
Fig. 2.
Microscopic lesions found in preterm births. A: Accelerated villous maturation at 31 weeks gestation. Haematoxylin and Eosin stain, 10x. B: Increased syncytial knots at 33 weeks gestation (indicated by ‘→‘). Haematoxylin and Eosin stain, 10x. C: Villous stromal-vascular karyorrhexis involving the villi with endothelial cell karyorrhexis and red blood cells extravasation and fragmentation (as indicated by the ‘*‘). Haematoxylin and Eosin stain, 20x. D: Distal villous hypoplasia at 36 weeks gestation. Note the large empty intervillous spaces in which very small villi are lying. Haematoxylin and Eosin stain, 4x. E: Increased perivillous fibrin encasing the villi. The villous lining trophoblast, in some of the villi, has already degenerated (as highlighted by ‘→‘). Other villi still have retained their syncytiotrophoblastic lining. Haematoxylin and Eosin stain, 40x.
A previous study found that MVM and placental ischemia were more prevalent than infectious findings [19], while others have shown that the incidence of MVM in PTB is higher than inflammation or infection [14], and among all PTBs, evidence of MVM was identified more often than infection [7]. These studies included both SPTB and IPTB, but we highlight the importance of separating PTBs as our research demonstrates different associations with placental pathology suggesting different mechanisms for PTB.
MVM and infection/inflammation are the most common placental findings associated with PTB [20,21]. We found MVM to be central for IPTB and funisitis (FIR) significant for SPTB. However, other studies found that a significant proportion of SPTBs were associated with MVM and accelerated villous maturation [2,7,14,22-24].
Funisitis (10% of SPTBs vs 1% of IPTBs or vs 5% of STBs) was the only significant inflammatory response finding. Weiner et al. [24] reported a high rate of FIR in SPTB, supporting our finding. Although they and Kovo et al. [25] also documented a high rate of MIR in SPTB, we found no significant association. Kim et al., found preterm infants to be more vulnerable and respond more vigorously to intrauterine infection than term fetuses constituting a trigger for preterm labour [26]. As funisitis was the only variable that was significantly more prevalent in SPTB than in IPTB, this might be linked to spontaneous initiation of preterm labour.
Our results did not support chorioamnionitis as a predominant cause of PTB [7,26-29]. Others, however, submit that chorioamnionitis is a consequence rather than a cause of PTB, as 10–15% of placentas at term have histological acute chorioamnionitis [6]. Given that 29% of our placentas at term had acute chorioamnionitis, we surmise that chorioamnionitis may not be a cause of PTB. In the literature, placentas from SPTB more commonly show acute chorioamnionitis with funisitis, vasculitis and chronic nonspecific villitis [6,29]. Van Vliet et al. and Goldstein et al. found chronic villitis associated with PTB [20,29], but we found no association with SPTB or IPTB.
Acute chorioamnionitis is more common than chronic chorioamnionitis. We found 34.2% and 29.2% of acute chorioamnionitis in our preterm and term birth groups respectively. Kim et al. reports that acute chorioamnionitis is present in 3–5% of placentas [30], while Roberts et al. reports a presence in 25% of term placentas [31], making our incidence higher. However, 64,3% of our chorioamnionitis overall was subchorionic, and only 18,8% chorionic and 16,6% chorioamniotic, respectively. The subchorionitis may be clinically insignificant and would now be diagnosed as maternal stage 0 (subchorionitis only) [31, 32].
Kim et al. reported chronic chorioamnionitis in preterm and term birth groups as 34–39% and 8–19%, respectively [33]. This is significantly higher than the 1.8% and 2.7% chronic chorioamnionitis found in our preterm and term groups. Infectious villitis, acute or chronic, is rare. Our prevalence of 0.2% and 0.6% respectively corresponds with the literature [29,34-37]. The overall frequency of VUE, has varied from 1% to 34%, depending on placental sampling, diagnostic criteria and the population group examined [34,35,38-45]. Our overall frequency was 22%, being higher (29%) in growth restricted cases.
Funisitis was found in 5.1% of our whole cohort and 6.7% of our PTB group, corresponding well with Jessop et al.‘s frequency of 5.7% [46]. The prevalence of 4.7% in our STB group was less than 6.7% reported by Lee et al. in term births [47], while higher than Liu et al. who reported an overall prevalence of 3.25%, 5.8% in the preterm and 2.8% in term group [48], but much less than the incidence of 44.4% in a preterm labour group reported by Pacora et al. [49].
Inflammation of umbilical vessels, vasculitis, can be seen in the umbilical vein and/or one or both umbilical arteries. Kim et al. found vasculitis in 17% of PTBs; 45% of vein and 55% of arteries [50]. We found vasculitis in only 8% of our PTB group. However, our findings of 44% with inflammation of vein and 56% of arteries, were similar.
4.2. IPTB
In IPTB we found features of MVM more commonly than in SPTB including microscopic pathological infarction (31%), decidual arteriopathy (26%), accelerated villous maturation (51%), increased syncytial knots (62%), distal villous hypoplasia (35%), increased extravillous trophoblast (33%) and increased perivillous fibrin (55%) (Table 4). Furthermore, we found high rates of obstetric complications such as gestational hypertension (42%), pre-eclampsia (23%) and fetal growth restriction (33%). This is not unexpected as other investigators have also found distal villous hypoplasia, increased syncytial knots and accelerated villous maturation were associated with MVM, fetal growth restriction, and pre-eclampsia [16,51]. MVM, is frequently cited as causing fetal growth restriction [28,52,53]. According to Visser et al., IUGR is associated with MVM and SPTB [2], but we found no evidence of this. Researchers have found pre-eclampsia [54], gestational hypertension, IUGR [55], abruption [2], and chronic villitis [23], to be commonly associated with placental insufficiency. Pregnancies complicated by MVM in our IPTB group, had a 4.5 times higher risk of developing pre-eclampsia or delivering a growth restricted baby according to Wright et al. [56].
Pre-eclampsia was observed in 23% (19) of IPTB women with only four having decidual arteriopathy (21%). The literature finds a higher prevalence of decidual arteriopathy at from 34 to 53% of women with pre-eclampsia [57,58], much more than our 21%. Possibly our pre-eclampsia was less severe, warranting other indications (see results) for induction.
In IPTB compared to STB we found no significant difference for chorioamnionitis, chronic or acute villitis, vasculitis of cord vessels or funisitis. Although Kovo et al. [25] discloses a significantly higher rate of inflammatory lesions for PPROM, we found no association in our IPTB group where nearly 16% were induced for PPROM.
FVM is a recently introduced term [13] to characterize a group of lesions previously described under the headings of fetal vascular obstructive lesions or thrombi, fetal thrombotic vasculopathy, and extensive avascular villi. FVM can be separated into partial or complete obstruction of fetoplacental blood flow [17]. Van Vliet et al. found fetal vascular thrombosis to be associated with PTB [20]. Our study revealed that fetal vascular thrombosis was associated with IPTB only, present in 29% vs 9% (SPTB) and 15% (STB). Those with fetal vascular thrombosis are more likely to have IUGR or oligohydramnios and necessitate delivery. This underscores the importance of differentiating between SPTB and IPTB rather than using the umbrella term: PTB. Our results only confirm a significant association between FVM and IPTB, but not with villitis as reported by Faye-Petersen [6].
Thrombosis of umbilical cord vessels is rare, reported at <0.1% of perinatal autopsies or high risk gestations [59,60]. We found umbilical cord vascular thrombosis present in 7.3% of our IPTB cases. Venous thrombosis occurred in 70%, arterial thrombosis (one or both umbilical arteries) in 10%, and both venous and arterial thrombosis in 20% of cases, according to literature reports [18,60]. Our venous thrombosis in 68% of overall cases, is consistent with the literature. Poor fetal outcome is more likely with arterial thrombosis [60]. We identified 27% of cases with arterial thrombosis in our cohort, (compared to 10% reported), which highlights the severity, especially in the IPTB group (33%). Furthermore Klaritsch et al. postulates that arterial thrombosis may cause severe IUGR [59], supporting the 33% growth restriction found in the IPTB group. Thrombosis of large fetal vessels in the placenta leads to regions of downstream avascular villi and have been associated with adverse outcomes and increased rates of IUGR [61]. Fox et al. who studied avascular villi, reported an overall incidence of 4.5% and a higher frequency of 14% in stillbirths [62], which is lower than the avascular villi incidence of 33% found in our IPTB group. Villous-stromal karyorrhexis (30% of our IPTB placentas), is deemed as a precursor of avascular villi [18]. Chorionic plate/stem villus thrombosis, found in 12% of our IPTB group, was mostly indeterminate. These features of FVM were all significantly increased and mostly associated with our IPTB group.
4.3. Demises
Preterm stillbirths, in comparison to SPTB infants alive at one year, had lower birthweights and more were more often growth restricted. They had more findings of placental abruption, fetal vascular thrombosis, and features of MVM (pathological infarction, decidual arteriopathy and/or distal villous hypoplasia). However, preterm stillbirths, compared to IPTB infants alive at one year, had significantly less proliferation of syncytial knots, increased chorioamnionitis and lower birthweights. Other researchers found infants delivered to mothers with acute chorioamnionitis were more preterm [5,63], which could explain our higher prevalence of low birthweight in stillbirths. Chorioamnionitis is frequently associated with perinatal death [26], which is consistent with our findings.
Preterm infants dying within one year of life, all had low birthweight compared to SPTB infants alive at one year, and, presented with less increased syncytial knots and no (0%) avascular villi in comparison to IPTB infants alive at one year.
The finding of less increased syncytial knots in preterm stillbirths, preterm infant demises and SPTBs differed significantly from increased syncytial knots found in IPTBs and STBs and is contrary to literature reports of extensive syncytial knotting with villous maturation. During pregnancy, syncytial knots are consistently present, increasing with gestational age, and utilised to evaluate villous maturity. Loukeris et al. found increased syncytial knots to be associated with conditions of MVM [12], supporting our findings regarding the IPTB group (significant MVM and increased syncytial knots) and the SPTB group (lack of both MVM and increased syncytial knots). Morgan et al. concluded that accelerated villous maturation with extensive syncytial knotting is a histological marker for placental insufficiency, and a common finding in idiopathic PTB [64]. Again, this was only true for our IPTB and not the SPTB group. According to Fogarty et al., the formation of increased syncytial knots with richly capillarised and highly branched terminal villi, in placentas from complicated pregnancies, can be attributed to premature aging of the placenta, providing a mechanism to enhance the placenta’s ability to transfer oxygen to the fetus [65]. But in cases where syncytial knots are not increased despite the accelerated villous maturation we propose that a “blocked” accelerated maturation pattern might be a trigger for SPTB and or result in a stillbirth or an infant demise. The lack of increased knotting in preterm stillbirths, in preterm born infants demised within one year of life, and in SPTB combined with a predominance of terminal villi, therefore demonstrating only partial accelerated maturation or abnormal/“blocked” maturation, needs to be investigated further.
5. Conclusion
SPTB and IPTB are pathologically different entities and should be investigated separately.
SPTB was not associated with features of maternal or fetal vascular malperfusion. Only funisitis occurred more frequently in this group.
We found significantly less increased syncytial knotting combined with a predominance of terminal villi in preterm demises and in SPTB, raising the question of whether abnormal villous maturation reflects a process that could trigger SPTB or result in a preterm demise?
IPTB was not associated with inflammation of the umbilical cord, membranes and chorionic plate, or parenchyma.
Acknowledgements
We wish to thank and acknowledge the following contributors: Andries Venter from the IT Department University of Stellenbosch for his great help with macros for Bonferroni comparisons and ML Chi-square statistics.
Pathology technologists Elaine Geldenhuys and Jean Coldrey from Tygerberg Hospital for their significant contributions to this study.
Clinical Coordinators of the South African leg of the Safe Passage Study for their outstanding job of collecting placentas.
We would like to dedicate this manuscript to our beloved colleague Elaine Geldenhuys who sadly passed away after losing her brave and long battle against cancer. This manuscript would have been incomplete without her dedicated and meticulous collection, preparation and processing of placenta specimens over many years.
Funding
The study was funded by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute on Deafness and Other Communication Disorders: U01 HD055154, U01 HD045935, U01 HD055155, U01 HD045991, and U01 AA016501. The funding body had no role in conducting the research or writing the paper.
Abbreviations:
- ANOVA
analysis of variance
- CI
confidence interval
- FIR
fetal inflammatory response
- FVM
fetal vascular malperfusion
- GA
gestational age
- IPTB
iatrogenic preterm birth
- IUD
intrauterine demise
- IUGR
intrauterine growth restriction
- MIR
maternal inflammatory response
- MOU
midwife obstetric unit
- MVM
maternal vascular malperfusion
- OR
odds ratio
- PPROM
preterm premature/prelabour rupture of membranes
- PTB
preterm birth
- SA
South Africa
- SPS
Safe Passage Study
- SPTB
spontaneous preterm birth
- STB
spontaneous term birth
- TBH
Tygerberg Academic Hospital
- VUE
villitis of unknown etiology
- ZAR
South African Rand
Footnotes
Disclosure of interests
The authors do not have any conflict of interest to refer to.
Details of ethics approval
Approval for the study was obtained from the Health Research Ethics Committee of Stellenbosch University (approval number: N06/10/210 and S19/07/119), as well as from the Western Cape Department of Health.
References
- [1].Goldenberg RL, Culhane JF, Iams JD, Romero R, Epidemiology and causes of preterm birth, Lancet 371 (2008) 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Visser L, van Buggenum H, van der Voorn JP, Heestermans LAPH, Hollander KWP, Wouters MGAJ, de Groot CJM, de Boer MA, Maternal vascular malperfusion in spontaneous preterm birth placentas related to clinical outcome of subsequent pregnancy, J. Matern. Neonatal Med (2019) 1–6, 10.1080/14767058.2019.1670811, 0. [DOI] [PubMed] [Google Scholar]
- [3].Hodyl NA, Aboustate N, Bianco-Miotto T, Roberts CT, Clifton VL, Stark MJ, Child neurodevelopmental outcomes following preterm and term birth: what can the placenta tell us? Placenta 57 (2017) 79–86, 10.1016/j.placenta.2017.06.009. [DOI] [PubMed] [Google Scholar]
- [4].Redline RW, Classification of placental lesions, Am. J. Obstet. Gynecol 213 (2015), 10.1016/j.ajog.2015.05.056. S21–S28. [DOI] [PubMed] [Google Scholar]
- [5].Çakir U, Yildiz D, Kahvecioğlu D, Okulu E, Alan S, Erdeve Ö, Okçu Heper A, Atasay B, Arsan S, Placenta, secret witness of infant morbidities: the relationship between placental histology and outcome of the premature infant, Turk. Patoloji Derg 35 (2019) 28–35, 10.5146/tjpath.2018.01443. [DOI] [PubMed] [Google Scholar]
- [6].Faye-Petersen OM, The placenta in preterm birth, J. Clin. Pathol 61 (2008) 1261–1275, 10.1136/jcp.2008.055244. [DOI] [PubMed] [Google Scholar]
- [7].Catov JM, Scifres CM, Caritis SN, Bertolet M, Larkin J, Parks WT, Neonatal outcomes following preterm birth classified according to placental features, Am. J. Obstet. Gynecol 216 (2017) 411, 10.1016/j.ajog.2016.12.022, e1–411.e14. [DOI] [PubMed] [Google Scholar]
- [8].Brink LT, Gebhardt GS, Mason D, Groenewald CA, Odendaal HJ, The association between preterm labour, perinatal mortality and infant death (during the first year) in Bishop Lavis, Cape Town, South Africa, S. Afr. Med. J 109 (2019) 102–106, 10.7196/samj.2019.v109i2.13438. [DOI] [PubMed] [Google Scholar]
- [9].Dukes KA, Burd L, Elliott AJ, Fifer WP, Folkerth RD, Hankins GDV, Hereld D, Hoffman HJ, Myers MM, Odendaal HJ, Signore C, Sullivan LM, Willinger M, Wright C, Kinney HC, The safe passage study: design, methods, recruitment, and follow-up approach, Paediatr. Perinat. Epidemiol 28 (2014) 455–465, 10.1111/ppe.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Papageorghiou AT, Ohuma EO, Altman DG, Todros T, Ismail LC, Lambert A, Jaffer YA, Bertino E, Gravett MG, Purwar M, Noble JA, Pang R, Victora CG, Barros FC, Carvalho M, Salomon LJ, Bhutta ZA, Kennedy SH, Villar J, International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project, Lancet 384 (2014) 869–879, 10.1016/S0140-6736(14)61490-2. [DOI] [PubMed] [Google Scholar]
- [11].Pinar H, Sung CJ, Oyer CE, Singer DB, Reference values for singleton and twin placental weights, Pediatr. Pathol. Lab. Med 16 (1996) 901–907, 10.1080/15513819609168713. [DOI] [PubMed] [Google Scholar]
- [12].Loukeris K, Sela R, Baergen RN, Syncytial knots as a reflection of placental maturity: reference values for 20 to 40 weeks’ gestational age, Pediatr. Dev. Pathol 13 (2010) 305–309, 10.2350/09-08-0692-OA.1. [DOI] [PubMed] [Google Scholar]
- [13].Khong TY, Mooney EE, Ariel I, Balmus NCM, Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye-Petersen OM, Gillan JE, Heazell AEP, Heller DS, Jacques SM, Keating S, Kelehan P, Maes A, McKay EM, Morgan TK, Nikkels PGJ, Parks WT, Redline RW, Scheimberg I, Schoots MH, Sebire NJ, Timmer A, Turowski G, Van Der Voorn JP, Van Lijnschoten I, Gordijn SJ, Sampling and definitions of placental lesions Amsterdam placental workshop group consensus statement, Arch. Pathol. Lab Med 140 (2016) 698–713, 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- [14].Ernst LM, Maternal vascular malperfusion of the placental bed, Apmis 126 (2018) 551–560, 10.1111/apm.12833. [DOI] [PubMed] [Google Scholar]
- [15].Nikkels PJG, Placenta pathology associated with maturation abnormalities and late intra uterine foetal death, Report/handout/workshop, 1996, pp. 1–9, http://www.forpath.org/workshops/minutes/0710/Handout.pdf. [Google Scholar]
- [16].Turowski G, Vogel M, Re-view and view on maturation disorders in the placenta, Apmis 126 (2018) 602–612, 10.1111/apm.12858. [DOI] [PubMed] [Google Scholar]
- [17].Redline RW, Ravishankar S, Fetal vascular malperfusion, an update, Apmis 126 (2018) 561–569, 10.1111/apm.12849. [DOI] [PubMed] [Google Scholar]
- [18].Roberts DJ, The placental Pathology Report, UpToDate, 2021, pp. 1–74. [Google Scholar]
- [19].Germain AM, Carvajal J, Sanchez M, Valenzuela GJ, Tsunekawa H, Chuaqui B, Preterm labor: placental pathology and clinical correlation, Obstet. Gynecol 94 (1999) 284–289, 10.1016/S0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- [20].Van Vliet EOG, De Kieviet JF, Van Der Voorn JP, Been JV, Oosterlaan J, Van Elburg RM, Placental pathology and long-term neurodevelopment of very preterm infants, Am. J. Obstet. Gynecol 206 (2012) 489, 10.1016/j.ajog.2012.03.024, e1-489.e7. [DOI] [PubMed] [Google Scholar]
- [21].Chisholm KM, Norton ME, Penn AA, Heerema-McKenney A, Classification of preterm birth with placental correlates, Pediatr. Dev. Pathol 21 (2018) 548–560, 10.1177/1093526618775958. [DOI] [PubMed] [Google Scholar]
- [22].Sakdapreecha L, Koonmee S, Triamwittayanon T, Kietpeerakool C, Kleebkaow P, Accelerated villous maturation of placentas in spontaneous preterm birth, J. Med. Assoc. Thail 100 (2017) 1145–1149. [Google Scholar]
- [23].Morgan TK, Role of the placenta in preterm birth: a review, Am. J. Perinatol 33 (2016) 258–266, 10.1055/s-0035-1570379. [DOI] [PubMed] [Google Scholar]
- [24].Weiner E, Dekalo A, Feldstein O, Barber E, Schreiber L, Bar J, Kovo M, The placental factor in spontaneous preterm birth in twin vs. singleton pregnancies, Eur. J. Obstet. Gynecol. Reprod. Biol 214 (2017) 1–5, 10.1016/j.ejogrb.2017.04.035. [DOI] [PubMed] [Google Scholar]
- [25].Kovo M, Schreiber L, Ben-Haroush A, Asalee L, Seadia S, Golan A, Bar J, The placental factor in spontaneous preterm labor with and without premature rupture of membranes, J. Perinat. Med 39 (2011) 423–429, 10.1515/JPM.2011.038. [DOI] [PubMed] [Google Scholar]
- [26].Kim CJ, Yoon BH, Park SS, Kim MH, Chi JG, Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response, Hum. Pathol 32 (2001) 623–629, 10.1053/hupa.2001.24992. [DOI] [PubMed] [Google Scholar]
- [27].Vangrieken P, Vanterpool SF, van Schooten FJ, Al-Nasiry S, Andriessen P, Degreef E, Alfer J, Kramer BW, von Rango U, Histological villous maturation in placentas of complicated pregnancies, Histol. Histopathol (2020), 18205, 10.14670/HH-18-205. [DOI] [PubMed] [Google Scholar]
- [28].Nakayama M, Significance of pathological examination of the placenta, with a focus on intrauterine infection and fetal growth restriction, J. Obstet. Gynaecol. Res 43 (2017) 1522–1535, 10.1111/jog.13430. [DOI] [PubMed] [Google Scholar]
- [29].Goldstein JA, Gallagher K, Beck C, Kumar R, Gernand AD, Maternal-fetal inflammation in the placenta and the developmental origins of Health and disease, Front. Immunol 11 (2020) 1–14, 10.3389/fimmu.2020.531543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM, Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance chong, Am J Obs. Gynecol 213 (2015) S29–S52, 10.1016/j.ajog.2015.08.040.Acute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roberts D, Polizzano C, Atlas of Placental Pathology, 2021. https://www.arppress.org/Atlas-of-Placental-Pathology-p/5f06.htm. [Google Scholar]
- [32].Roberts DJ, Celi AC, Riley LE, Onderdonk AB, Boyd TK, Johnson LC, Lieberman E, Acute histologic chorioamnionitis at term: nearly always noninfectious, PLoS One 7 (2012) 1–7, 10.1371/journal.pone.0031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG, Kim JS, The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth, Mod. Pathol 23 (2010) 1000–1011, 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Redline RW, Villitis of unknown etiology: noninfectious chronic villitis in the placenta, Hum. Pathol 38 (2007) 1439–1446, 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- [35].Nakamura Y, Sakuma S, Ohta Y, Kawano K, Hashimoto T, Detection of the human cytomegalovirus gene in placental chronic villitis by polymerase chain reaction, Hum. Pathol 25 (1994) 815–818, 10.1016/0046-8177(94)90252-6. [DOI] [PubMed] [Google Scholar]
- [36].Benirschke K, Mendoza GR, Bazeley PL, Placental and fetal manifestations of cytomegalovirus infection, Virchows Arch. B Cell Pathol 16 (1974) 121–139, 10.1007/BF02894070. [DOI] [PubMed] [Google Scholar]
- [37].Mortimer G, MacDonald DJ, Smeeth A, A pilot study of the frequency and significance of placental villitis, Br. J. Obstet. Gynaecol 92 (1985) 629–633, 10.1111/j.1471-0528.1985.tb01403.x. [DOI] [PubMed] [Google Scholar]
- [38].Gersell DJ, Chronic villitis, chronic chorioamnionitis, and maternal floor infarction, Semin. Diagn. Pathol 10 (1993) 251–266. [PubMed] [Google Scholar]
- [39].Knox WF, Fox H, Villitis of unknown aetiology: its incidence and significance in placentae from a british population, Obstet. Gynecol. Surv 40 (1985) 569–571, 10.1097/00006254-198509000-00004. [DOI] [PubMed] [Google Scholar]
- [40].Tamblyn JA, Lissauer DM, Powell R, Coxb P, Kilby MD, The immunological basis of villitis of unknown etiology - Review, Placenta 34 (2013) 846–855, 10.1016/j.placenta.2013.07.002. [DOI] [PubMed] [Google Scholar]
- [41].Russell P, Inflammatory lesions of the human placenta. III. The histopathology of villitis of unknown aetiology, Obstet. Gynecol. Surv 36 (1981) 362–363, 10.1097/00006254-198107000-00006. [DOI] [PubMed] [Google Scholar]
- [42].Becroft DM, Thompson JM, Mitchell EA, Placental villitis of unknown origin: epidemiologic associations, Am. J. Obstet. Gynecol 192 (2005) 264–271, 10.1016/j.ajog.2004.06.062. [DOI] [PubMed] [Google Scholar]
- [43].Altemani AM, Gonzatti AR, Villitis of unknown etiology in placentas of pregnancies with hypertensive disorders and of small-for-gestational-age infants, Rev. Assoc. Med. Bras 49 (2003), 10.1590/S0104-42302003000100036. [DOI] [PubMed] [Google Scholar]
- [44].Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee D-C, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim J-S, Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease, J. Immunol 182 (2009) 3919–3927, 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chan JSY, Villitis of unknown etiology and massive chronic intervillositis, Surg. Pathol. Clin 6 (2013) 115–126, 10.1016/j.path.2012.11.004. [DOI] [PubMed] [Google Scholar]
- [46].Jessop FA, Lees CC, Pathak S, Hook CE, Sebire NJ, Funisitis is associated with adverse neonatal outcome in low-risk unselected deliveries at or near term, Virchows Arch. 468 (2016) 503–507, 10.1007/s00428-015-1899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lee SM, Romero R, Lee KA, Yang HJ, Oh KJ, Park CW, Yoon BH, The frequency and risk factors of funisitis and histologic chorioamnionitis in pregnant women at term who delivered after the spontaneous onset of labor, J. Matern. Neonatal Med 24 (2011) 37–42, 10.3109/14767058.2010.482622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liu C, Chen Y, Zhao D, Zhang J, Zhang Y, Association between funisitis and childhood intellectual development: a prospective cohort study, Front. Neurol 10 (2019) 1–8, 10.3389/fneur.2019.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pacora P, Chaiworapongsa T, Maymon E, Kim Y, Gomez R, Yoon B, Ghezzi F, Berry S, Qureshi F, Jacques S, Kim J, Kadar N, Romero R, Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome, J. Matern. Fetal Neonatal Med 11 (2002) 18–25, 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- [50].Kim CJ, Yoon BH, Romero R, Bin Moon J, Kim M, Park SS, Chi JG, Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response, Am. J. Obstet. Gynecol 185 (2001) 496–500, 10.1067/mob.2001.116689. [DOI] [PubMed] [Google Scholar]
- [51].Dureau ZJ, Rogers BB, Placental pathology, Diagn. Histopathol 25 (2019) 341–349, 10.1016/j.mpdhp.2019.06.003. [DOI] [Google Scholar]
- [52].Woods DL, Malan AF, de H, Placental size of small-for-gestational-age infants at term, Early Hum. Dev 7 (1982) 11–15, 10.1016/0378-3782(82)90003-2. [DOI] [PubMed] [Google Scholar]
- [53].Hutcheon JA, McNamara H, Platt RW, Benjamin A, Kramer MS, Placental weight for gestational age and adverse perinatal outcomes, Obstet. Gynecol 119 (2012) 1251–1258, 10.1097/AOG.0b013e318253d3df. [DOI] [PubMed] [Google Scholar]
- [54].Redman CWG, Staff AC, Roberts JM, Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways, Am. J. Obstet. Gynecol (2021) 1–21, 10.1016/j.ajog.2020.09.047. [DOI] [PubMed] [Google Scholar]
- [55].Woods DL, Malan AF, de H, van Schalkwijk DJ, Infant size and relative placental weight at birth, Early Hum. Dev 4 (1980) 139–143, 10.1016/0378-3782(80)90017-1. [DOI] [PubMed] [Google Scholar]
- [56].Wright E, Audette MC, Ye XY, Keating S, Hoffman B, Lye SJ, Shah PS, Kingdom JC, Maternal vascular malperfusion and adverse perinatal outcomes in low-risk nulliparous women, Obstet. Gynecol 130 (2017) 1112–1120, 10.1097/AOG.0000000000002264. [DOI] [PubMed] [Google Scholar]
- [57].Kitzmiller JL, Watt N, Driscoll SG, Decidual arteriopathy in hypertension and diabetes in pregnancy: immunofluorescent studies, Am. J. Obstet. Gynecol 141 (1981) 773–779, 10.1016/0002-9378(81)90703-1. [DOI] [PubMed] [Google Scholar]
- [58].Mehrabian F, Mohammadizadeh F, Esfahani NM, Najafian A, Comparison of placental pathology between severe preeclampsia and HELLP syndrome, Arch. Gynecol. Obstet 285 (2011) 175–181, 10.1007/s00404-011-1948-0. [DOI] [PubMed] [Google Scholar]
- [59].Klaritsch P, Haeusler M, Karpf E, Schlembach D, Lang U, Spontaneous intrauterine umbilical artery thrombosis leading to severe fetal growth restriction, Placenta 29 (2008) 374–377, 10.1016/j.placenta.2008.01.004. [DOI] [PubMed] [Google Scholar]
- [60].Heifetz SA, Thrombosis of the umbilical cord: analysis of 52 cases and literature review, Pediatr. Pathol 8 (1988) 37–54, 10.3109/15513818809022278. [DOI] [PubMed] [Google Scholar]
- [61].Redline RW, Pappin A, Fetal thrombotic vasculopathy: the clinical significance of extensive avascular villi, Hum. Pathol 26 (1995) 80–85, 10.1016/0046-8177(95)90118-3. [DOI] [PubMed] [Google Scholar]
- [62].Fox H, Elston CW, Pathology of the placenta, Major Probl. Pathol 7 (1978) 1–491. [PubMed] [Google Scholar]
- [63].Nijman TAJ, van Vliet EOG, Benders MJN, Mol BWJ, Franx A, Nikkels PGJ, Oudijk MA, Placental histology in spontaneous and indicated preterm birth: a case control study, Placenta 48 (2016) 56–62, 10.1016/j.placenta.2016.10.006. [DOI] [PubMed] [Google Scholar]
- [64].Morgan TK, Tolosa JE, Mele L, Wapner RJ, Spong CY, Sorokin Y, Dudley DJ, Peaceman AM, Mercer BM, Thorp JM, O’sullivan MJ, Ramin SM, Rouse DJ, Sibai B, Placental villous hypermaturation is associated with idiopathic preterm birth, J. Matern. Neonatal Med 26 (2013) 647–653, 10.3109/14767058.2012.746297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fogarty NME, Ferguson-Smith AC, Burton GJ, Syncytial knots (Tenney-parker changes) in the human placenta: evidence of loss of transcriptional activity and oxidative damage, Am. J. Pathol 183 (2013) 144–152, 10.1016/j.ajpath.2013.03.016. [DOI] [PubMed] [Google Scholar]