Abstract
LY303366 is a novel antifungal echinocandin with excellent in vitro activity against Aspergillus spp. We compared four doses (1, 2.5, 10, and 25 mg/kg of body weight) of LY303366 with amphotericin B (0.5 to 5 mg/kg) in a temporarily neutropenic murine model of invasive aspergillosis against an amphotericin B-susceptible (AF210) and an amphotericin B-resistant (AF65) Aspergillus fumigatus isolate based on in vivo response. Mice were immunosuppressed with cyclophosphamide (200 mg/kg) and infected 3 days later. Treatment started 18 h after infection and lasted for 10 days. LY303366 was given once daily intravenously for 10 days, and amphotericin B (at 0.5, 2, and 5 mg/kg) was given once daily intraperitoneally for 10 days, or only on days 1, 2, 4, and 7 (at 5 mg/kg). Kidneys and lungs from survivors were cultured on day 11. Control mice in both experiments had 90 to 100% mortality. Amphotericin B at 0.5 mg/kg and LY303366 at 1 mg/kg yielded 10 to 20% survival rates for mice infected with either AF210 or AF65. Amphotericin B at 2 and 5 (both regimens) mg/kg yielded a 70 to 100% survival rate for mice infected with AF210 but a 10 to 30% survival rate for mice infected with AF65 (P = 0.01 to 0.04 compared with AF210). Against AF210 and AF65, LY303366 at 2.5, 10, and 25 mg/kg produced a survival rate of 70 to 80%, which was as effective as amphotericin B for AF210, but superior to amphotericin B for AF65 (P < 0.03 to 0.0006). For AF65, LY303366 at 10 and 25 mg/kg/day was superior to amphotericin B at 2 and 5 mg/kg/day in reducing tissue colony counts (P = 0.01 to 0.003), and for AF210, amphotericin B at 5 mg/kg/day and at 5 mg/kg in four doses was more effective than all four regimens of LY303366 in reducing renal culture counts (P = 0.01 to 0.0001). The present study shows, for the first time, that in vivo resistance of A. fumigatus to amphotericin B exists, although this could not be detected by in vitro susceptibility assays. Furthermore, LY303366 appears to be effective against amphotericin B-susceptible and -resistant A. fumigatus infection in this model and should be further evaluated clinically.
Aspergillus fumigatus may cause life-threatening infections in immunocompromised patients, especially in those who receive treatment for hematological malignancies. Although amphotericin B remains the standard therapy, a successful outcome is achieved in only 34% of patients (7). The success of treatment depends on many factors, most importantly on the response of the underlying disease to therapy (7). The majority of patients with invasive aspergillosis who die during treatment with amphotericin B are persistently granulocytopenic due to refractory underlying disease or receive a suboptimal dose due to amphotericin B-related nephrotoxicity (11). Nevertheless, some patients who are treated with an adequate dose of amphotericin B and whose underlying immune deficiency is resolved fail to respond to therapy. In these patients, treatment failure could be due to decreased susceptibility of the fungus to amphotericin B. Since at present there is no reproducible and meaningful in vitro assay for measuring the susceptibility of A. fumigatus to amphotericin B, we compared the in vivo susceptibilities of A. fumigatus isolates cultured from patients with a clinically documented favorable response or failure to respond to treatment with amphotericin B desoxycholate. In addition, the efficacy of a new antifungal echinochandin, LY303366, against these isolates was determined and compared to that of amphotericin B. Echinocandins and pneumocandins are a new class of drugs with antifungal activity. The drugs specifically inhibit (1,3)-β-d-glucan synthetase, an enzyme complex that forms glucan polymers in the fungal cell wall (5). Fungi with (1,3)-β-d-glucans in their cell walls, including Candida species, Aspergillus species, and Pneumocystis carinii, are susceptible to echinocandins, although the full spectrum of activity has not yet been established. In the present study the efficacy of LY303366 was compared with that of amphotericin B in a temporarily neutropenic murine model of invasive aspergillosis which has been used previously to evaluate the in vivo efficacies of antifungal agents (8, 9, 13, 18).
MATERIALS AND METHODS
Aspergillus isolates.
Both isolates used for the studies were typical A. fumigatus isolates from clinical sources. Strain AF65 was cultured from a lung biopsy from a leukemic patient who showed no clinical improvement with therapy with amphotericin B and whose infection subsequently recurred. Strain AF210 was cultured from the abdominal viscera from a surgical patient who developed an Aspergillus wound infection following laparotomy and who responded to conventional amphotericin B treatment without change of immune status (3). These isolates have been deposited at the National Collection of Pathogenic Fungi (Bristol, United Kingdom) under no. NCPF 7097 (AF65) and NCPF 7101 (AF210).
In vitro studies.
A loopful of frozen stock was streaked on a Sabouraud glucose agar plate (Lab M, Bury, United Kingdom) and incubated at 35°C until sporulation occurred. The MIC and minimal fungicidal concentration (MFC) of amphotericin B were determined by a broth microdilution method using RPMI 1640 agar with 2% glucose as previously described (6). A typical MIC end point is not seen when LY303366 is tested against Aspergillus species. Instead, there is a transition from a homogeneous mat of long, thin hyphae to subspherical colonies in wells; most of the colonies are attached to the bottom of the microtiter well. Therefore, a minimal effective concentration (MEC), taken as the concentration in the first well to contain the small subspherical colonies with no hyphal growth, was used. The MEC and MFC of LY303366 were determined by the broth microdilution method using antibiotic medium 3 (Difco, Detroit, Mich.) and Casitone medium (Difco), since no reference medium has yet been established for the in vitro testing of echinocandins (5).
Preparation of inocula.
The inocula were prepared by culturing the organisms on potato glucose agar (Oxoid, Basingstoke, United Kingdom) for 10 days at 35°C. Conidia were collected in sterile 0.9% phosphate-buffered saline containing 0.01% Tween 80 (PBS-Tween) and were stored at 4°C. The viability of the conidial suspension was determined by serial dilutions in PBS-Tween, and the suspension was subcultured onto horse blood agar plates. The inoculum was stored at 4°C until the day of infection (always a Monday). Further adjustments to the inoculum, if necessary, were made just prior to infection of the mice.
Drugs and therapy regimens.
Amphotericin B desoxycholate (Fungizone; Squibb, Middlesex, United Kingdom), was given in 5% glucose intraperitoneally. For each isolate, groups of 10 mice were treated with 0.5, 2, or 5 mg of amphotericin B/kg of body weight/day by once-daily injection in 0.1 ml. One group was treated with 5 mg of amphotericin B/kg/day in four doses, one on each of days 1, 2, 4, and 7. Furthermore, groups of 10 mice were treated with 1, 2.5, 10, or 25 mg of LY303366 (Eli Lilly, Indianapolis, Ind.)/kg/day by once-daily injection in 0.1 ml in a lateral tail vein. A solution of 2.5% (wt/vol) Polysorbate 80, which is used as a solvent for LY303366, was used for the controls in a concentration equal to that found in the highest concentration of LY303366 used. Intraperitoneal injections with glucose served as controls for treatment with amphotericin B. Cages were randomly sorted after infection, and treatment groups were assigned by cage number. For all groups, treatment commenced 18 h postinfection and continued for 10 days.
Mice.
Virus-free male CD-1 mice (age, 5 weeks) were purchased from Charles River UK, Ltd. Mice were weighed on the day the experiment commenced (range, 20.3 to 26.9 g), reassorted, and grouped 10 to a cage. The mice were allowed food and water ad libitum.
Immunosuppression.
Mice received cyclophosphamide (C-7397; Sigma, Dorset, United Kingdom) at 200 mg/kg administered intravenously 3 days prior to challenge as described previously (8).
Infection.
A conidial suspension of 0.1 ml of 5 × 106 conidia of AF65/ml or 1.7 × 106 conidia of AF210/ml was injected into lateral tail veins. The 90% lethal doses (LD90) for these isolates had been determined previously in inoculum-finding studies (11a). Dilutions of this inoculum were streaked out on horse blood agar plates and counted over 48 h of incubation at 35°C. These showed that mice infected with AF65 had received 4.4 × 106 CFU, and those given AF210 were infected with 1.9 × 106 CFU.
Cultures.
Surviving mice were killed by cervical dislocation on day 11. Both lungs and kidneys were removed and placed into 5 ml of sterile PBS containing penicillin (100 IU/ml) and streptomycin (100 μg/ml) and were then homogenized in a tissue grinder (Kinematica, Lucerne, Switzerland) for 15 to 30 s. Three 10-fold serial dilutions were made, and 0.5 ml of each dilution was plated onto Sabouraud’s agar. The plates were incubated at 35°C, and colonies were counted daily for 7 days.
Statistics.
Survival and quantitative culture were compared by the Mann-Whitney rank sum test. Qualitative culture results were examined by Fisher’s exact test. Mice which died before day 11 were assumed to have quantitative counts in their organs at least as high as the highest counts in the organs of any surviving mice. All analyses were performed with the computer package Minitab (Minitab Data Analysis Software, Philadelphia, Pa.).
RESULTS
In vitro susceptibility.
The results of in vitro susceptibility testing, shown in Table 1, indicated that there was no significant difference between A. fumigatus isolates AF65 and AF210 in in vitro susceptibility to amphotericin B or LY303366.
TABLE 1.
In vitro susceptibilities of A. fumigatus AF210 and AF65 to amphotericin B and LY303366
| Isolate | ABa
|
LY303366b
|
||||
|---|---|---|---|---|---|---|
| In AM3
|
In CAS
|
|||||
| MIC (μg/ml) | MFC (μg/ml) | MEC (μg/ml) | MFC (μg/ml) | MEC (μg/ml) | MFC (μg/ml) | |
| AF210 | 2 | 8 | 0.003 | 0.03 | 0.075 | 0.06 |
| AF65 | 1 | 4 | 0.0018 | 0.0018 | 0.003 | >0.5 |
AB, amphotericin B.
AM3, antibiotic medium 3; CAS, Casitone medium.
Treatment efficacy against isolate AF210.
An LD90 to LD100 was achieved for both intravenously and intraperitoneally injected control mice infected with A. fumigatus isolate AF210, indicating that the model was reproducible. There was no difference in survival or in lung and renal colony counts between intravenously and intraperitoneally injected control mice (Tables 2 and 3). All treatment groups showed a significant increase in mouse survival (Table 2), although the lowest-dosage treatment groups for amphotericin B and LY303366, i.e., 0.5 and 1 mg/kg/day, respectively, showed no significant decrease in tissue colony counts compared to those of control mice (Table 3). Dosages of 2.5, 10, and 25 mg of LY303366/kg/day were as effective in improving survival as 2 and 5 mg of amphotericin B/kg/day and 5 mg of amphotericin B/kg in four doses (Table 2). For both drugs there was no apparent dose-response relationship. Although the three highest-dosage regimens of amphotericin B and LY303366 significantly reduced the tissue colony counts compared to controls, amphotericin B at 5 mg/kg in four doses and at 5 mg/kg/day was superior to all dosage regimens of LY303366 in reducing renal colony counts (Table 4). There was no significant difference between the different treatment groups in the number of infected organs (data not shown).
TABLE 2.
Survival times for mice infected with A. fumigatus AF210 or AF65 and treated with amphotericin B or LY303366
| Isolate | Median survival time (days) and range for mice receiving the following treatmenta:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| i.p. control | i.v. control | Amphotericin B
|
LY303366
|
|||||||
| 0.5 mg/kg/day | 2 mg/kg/day | 5 mg/kg in 4 doses | 5 mg/kg/day | 1 mg/kg/day | 2.5 mg/kg/day | 10 mg/kg/day | 25 mg/kg/day | |||
| AF210 | 3 (2–5) | 3 (2–3) | 5 (2–11)* | 11 (3–11)**,† | 11 (11)**,† | 11 (2–11)**,† | 5.5 (3–11)* | 11 (4–11)** | 11 (5–11)** | 11 (3–11)** |
| AF65 | 3.5 (3–11) | 3 (3–4) | 4 (3–11) | 4 (3–11) | 3 (3–11) | 3 (3–11) | 5 (3–11) | 11 (6–11)**,‡ | 11 (3–11)**,‡ | 11 (7–11)**,‡ |
i.p., intraperitoneal; i.v., intravenous; *, P < 0.05 (compared with controls); **, P < 0.01 (compared with controls); †, P < 0.05 (compared with AF65); ‡, P < 0.05 (compared with amphotericin B treatment of AF65).
TABLE 3.
Culture results for lungs and kidneys of mice infected with A. fumigatus AF210 and AF65
| Treatmenta | AF210
|
AF65
|
||||
|---|---|---|---|---|---|---|
| No. of survivors/total no. of mice | Mean CFU (102)b in:
|
No. of survivors/total no. of mice | Mean CFU (102)b in:
|
|||
| Lungs | Kidneys | Lungs | Kidneys | |||
| i.p. control | 0/10 | 3.1 (0) | 330 (0) | 1/10 | 16.1 (7.8) | 344 (171.8) |
| i.v. control | 0/10 | 3.1 (0) | 330 (0) | 0/10 | 20 (0) | 430 (0) |
| AB (mg/kg) | ||||||
| 0.5/day | 1/10 | 2.8 (0.9) | 297 (97.8) | 4/10 | 12.2 (9.5) | 269 (198.7) |
| 2/day | 7/10 | 0.9 (1.4)** | 101 (149.7)** | 1/10 | 18.0 (6.0) | 388 (126.0) |
| 5, in 4 doses | 10/10 | 0.6 (1.2)** | 3.5 (4.3)** | 3/10 | 14.1 (9.0) | 306 (189.3) |
| 5/day | 8/10 | 1.0 (1.3)** | 66 (131.6)** | 1/10 | 18.0 (5.9) | 419 (33.0) |
| LY (mg/kg/day) | ||||||
| 1 | 3/10 | 2.4 (1.2) | 271 (118.0) | 2/10 | 16.1 (7.7) | 365 (130.0) |
| 2.5 | 7/10 | 0.9 (1.4)** | 109 (144.7)** | 8/10 | 4.6 (7.7)** | 182 (177.3)* |
| 10 | 8/10 | 0.6 (1.2)** | 77 (126.4)** | 7/10 | 7.2 (8.8)** | 135 (192.6)* |
| 25 | 8/10 | 0.9 (1.4)** | 127 (139.3)** | 8/10 | 7.0 (8.9)** | 116 (164.1)* |
i.p., intraperitoneal; i.v., intravenous; AB, amphotericin B; LY, LY303366.
Numbers in parentheses represent standard deviations. *, P < 0.05 (comparison with controls); **, P < 0.01 (comparison with controls).
TABLE 4.
Efficacy of amphotericin B compared with that of LY303366 in reducing tissue colony counts for mice infected with A. fumigatus AF210 or AF65a
| Treatment |
P value for difference in reducing CFU ofb:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF210
|
AF65
|
|||||||||||||||
| LY 1
|
LY 2.5
|
LY 10
|
LY 25
|
LY 1
|
LY 2.5
|
LY 10
|
LY 25
|
|||||||||
| Lung | Kidney | Lung | Kidney | Lung | Kidney | Lung | Kidney | Lung | Kidney | Lung | Kidney | Lung | Kidney | Lung | Kidney | |
| AB 0.5 | 0.73 | 0.68 | 0.02* | 0.01* | 0.008* | 0.003* | 0.02* | 0.01* | 0.43 | 0.34 | 0.21 | 0.56 | 0.39 | 0.47 | 0.24 | 0.38 |
| AB 2 | 0.09 | 0.01# | 0.79 | 0.34 | 0.85 | 0.23 | 0.85 | 0.22 | 0.21 | 0.78 | 0.01* | 0.02* | 0.04* | 0.02* | 0.03* | 0.009* |
| AB 5/4 | 0.002# | 0.0001# | 0.21 | 0.01# | 0.09 | 0.003# | 0.21 | 0.004# | 0.39 | 0.55 | 0.08 | 0.14 | 0.21 | 0.11 | 0.12 | 0.11 |
| AB 5 | 0.03# | 0.004# | 0.97 | 0.03# | 0.97 | 0.02# | 0.97 | 0.01# | 0.24 | 0.66 | 0.08 | 0.01* | 0.02* | 0.01* | 0.02* | 0.003* |
LY 1, LY 2.5, LY 10, and LY 25, LY303366 at 1, 2.5, 10, and 25 mg/kg/day, respectively; AB 0.5, AB 2, and AB 5, amphotericin B at 0.5, 2, and 5 mg/kg/day, respectively; AB 5/4, amphotericin B at 5 mg/kg, given in four doses.
*, Treatment with LY303366 was superior; #, treatment with amphotericin B was superior.
Treatment efficacy against isolate AF65.
An LD90 to LD100 of A. fumigatus AF65 was achieved for control mice, and there was no difference in survival or in lung and renal colony counts between intravenously and intraperitoneally injected control mice (Tables 2 and 3). Mice treated with amphotericin B (all regimens) showed no improvement in survival compared to the controls (Table 2) and no significant reduction in tissue colony counts (Table 3). Mice treated with LY303366 at 2.5, 10, or 25 mg/kg/day showed a significant increase in survival (Table 2), and the tissue colony counts were significantly reduced compared to those of controls (Table 3). LY303366 at 2.5, 10, and 25 mg/kg/day was superior to amphotericin B therapy in improving survival (Table 2). Moreover, these dosage regimens of LY303366 reduced tissue colony counts significantly better than amphotericin B at 2 and 5 mg/kg/day (Table 4). There was no significant difference among the different treatment groups in the number of infected organs (data not shown).
Comparison of experiments.
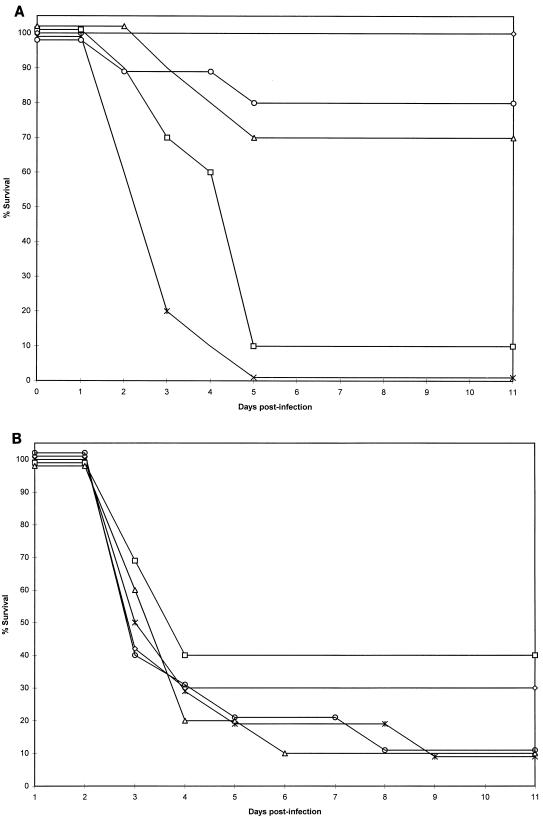
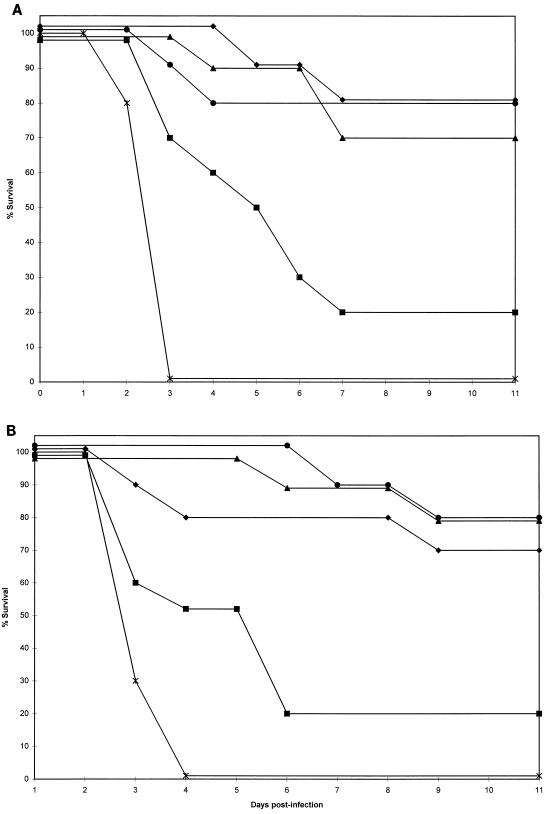
Median survival for control mice intravenously infected with AF210 was not significantly different from that of those intravenously infected with AF65 (Table 2). However, the median survival for control mice intraperitoneally infected with AF65 was longer than that for those intraperitoneally infected with AF210, and this difference just reached statistical significance (P < 0.05). For both groups of mice, those infected with A. fumigatus isolate AF210 and those infected with A. fumigatus isolate AF65, the efficacies of treatment with amphotericin B and with LY303366 are shown in Fig. 1 and 2, respectively. There was a statistically significant difference in median survival between mice infected with AF210 and those infected with AF65 for groups treated with amphotericin B at 2 and 5 mg/kg/day and at 5 mg/kg in four doses, but not for any groups treated with LY303366 (Table 2).
FIG. 1.
Survival for CD-1 mice infected with isolate AF210 (A) or AF65 (B) and treated with amphotericin B at 0.5 mg/kg/day (□), 2 mg/kg/day (▵), 5 mg/kg given on days 1, 2, 4, and 7 (◊), or 5 mg/kg/day (○), or given dextrose intraperitoneally (∗).
FIG. 2.
Survival for CD-1 mice infected with isolate AF210 (A) or AF65 (B) and treated with LY303366 at 1 mg/kg/day (▪), 2.5 mg/kg/day (▴), 10 mg/kg/day (⧫), or 25 mg/kg/day (•), or given 2.5% (wt/vol) Polysorbate 80 solvent intravenously (∗).
DISCUSSION
Although amphotericin B has been used for treating fungal infections in humans for more than 40 years, resistance to the drug has rarely been reported. Clinical resistance to amphotericin B has been documented for several Candida species, including Candida albicans (4, 14, 21), C. glabrata (21), C. tropicalis (21, 24, 30), C. lusitaniae (2, 19, 21), C. guilliermondii (10, 21), and C. krusei (24). In some cases, in vitro susceptibility testing results correlated with treatment outcome, but there is considerable variation in breakpoints, which range from 0.8 to >100 μg/ml (2, 10, 19, 21, 23). C. parapsilosis has been shown to be tolerant for amphotericin B, since the MFC was more than 32-fold higher than the MIC (26). In some cases, resistance to amphotericin B was shown to emerge during treatment with the drug (10, 19). The frequency of resistance is not well known, although a recent study found a prevalence of approximately 1% among more than 600 Candida isolates (16).
Amphotericin B resistance among fungi has been shown for clinically resistant organisms, such as Trichosporon beigelii (29), Fusarium species (1), zygomycetes (12), and Pseudallescheria boydii (17). Resistance among Aspergillus species is very rare, although resistance to polyenes has been reported in laboratory strains of A. nidulans (32) and A. fennelliae (15). Amphotericin B was found to have lost its in vitro activity against six A. fumigatus isolates after these isolates were exposed to subfungicidal concentrations of itraconazole (25), which may be due to antagonism between the two antifungal drugs. Our study provides, for the first time, evidence of differential activity of amphotericin B against two different A. fumigatus isolates examined under highly controlled, stringent therapeutic conditions. Our murine model, with some variations, has been used extensively to evaluate the efficacy of several novel antifungal compounds (8, 9, 13, 18) and for the development of meaningful and reproducible in vitro assays for susceptibility testing of antifungal azoles (6). The amphotericin B-resistant isolate AF65 may be less virulent than isolate AF210, since a threefold higher inoculum of AF65 was needed to achieve comparable mortality for both isolates. Furthermore, the median survival of intraperitoneally injected control mice infected with AF65 was longer than that for those infected with isolate AF210 (P = 0.04). Nevertheless, amphotericin B showed significantly reduced activity in mice infected with isolate AF65.
The low frequency of secondary resistance of fungi to amphotericin B has been thought to be due to the complex interaction between amphotericin B and the plasma membrane, disruption of which would require multiple changes (27, 28). Furthermore, membrane alterations that can lead to decreased sensitivity to amphotericin B may also reduce virulence (27, 28), which was also apparent in the resistant isolate we used. However, amphotericin B resistance among A. fumigatus isolates may be difficult to detect, since we were unable to confirm the observed in vivo resistance by use of standard in vitro susceptibility tests. Therefore, the frequency of resistance is unknown. It is clear that meaningful in vitro assays to detect amphotericin B resistance among A. fumigatus isolates need to be developed in the near future. The finding of in vivo resistance provides the first step in developing such an assay, an approach that has been followed successfully in developing an in vitro assay for antifungal azoles (6).
The demonstration of resistance to amphotericin B among A. fumigatus isolates underscores the need for the development and evaluation of novel compounds with antifungal activity. In the present study, we evaluated the efficacy of the echinocandin LY303366, which is a member of a new class of antifungal agents active against a wide range of fungal pathogens. The mode of action involves noncompetitive inhibition of (1,3)-β-d-glucan synthase, an enzyme complex that forms glucan polymers in the fungal cell wall (5). The compound is active against fungi which contain this enzyme complex in their cell walls, such as Candida species, P. carinii, and A. fumigatus, but not against Cryptococcus neoformans, as this pathogen has little or no (1,3)-β-d-glucan synthase enzyme (5). LY303366 is a compound which is derived from the echinocandin B nucleus and is active in vitro against Candida species including C. glabrata and C. krusei, and against azole-resistant C. albicans (20, 22, 31). The in vitro activity of LY303366 against A. fumigatus is difficult to access by the methods presently available, since end points are subjective and difficult to read (18a). The efficacy of LY303366 in increasing survival was comparable to that of amphotericin B for mice infected with the amphotericin B-susceptible A. fumigatus isolate AF210, although amphotericin B was superior in reducing the fungal burden in the kidneys. However, LY303366 was superior to amphotericin B in increasing survival and reducing tissue colony counts in mice infected with A. fumigatus AF65. The present study indicates that LY303366 has potentially useful activity against both amphotericin B-susceptible and -resistant A. fumigatus isolates in a murine model of invasive aspergillosis, and therefore further clinical evaluation of this compound is warranted.
REFERENCES
- 1.Anaissie E J, Hachem R, Legrand C, Legenne P, Nelson P, Bodey G. Lack of activity of amphotericin B in systemic murine fusarial infection. J Infect Dis. 1992;165:1155–1157. [PubMed] [Google Scholar]
- 2.Anaissie E J, Karyotakis N C, Hachem R, Dignani M C, Rex J H, Paetznick V. Correlation between in vitro and in vivo activity of antifungal agents against Candida species. J Infect Dis. 1994;170:384–389. doi: 10.1093/infdis/170.2.384. [DOI] [PubMed] [Google Scholar]
- 3.Carlson G L, Mughal M M, Birch M, Denning D W. Aspergillus wound infection following laparotomy. J Infect. 1996;33:119–121. doi: 10.1016/s0163-4453(96)93062-5. [DOI] [PubMed] [Google Scholar]
- 4.Conley J, Johnson J, Farah S, Hellman L. Disseminated candidiasis due to amphotericin B-resistant Candida albicans. J Infect Dis. 1992;165:761–764. doi: 10.1093/infdis/165.4.761. [DOI] [PubMed] [Google Scholar]
- 5.Denning D W. Echinocandins and pneumocandins—a new antifungal class with a novel model of action. J Antimicrob Chemother. 1997;40:611–614. doi: 10.1093/jac/40.5.611. [DOI] [PubMed] [Google Scholar]
- 6.Denning D W, Venkateswarlu K, Oakley K L, Anderson M J, Manning N J, Stevens D A, Warnock D W, Kelly S L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denning D W. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 8.Denning D W, Hall L, Jackson M, Hollis S. Efficacy of D0870 compared with those of itraconazole and amphotericin B in two murine models of invasive aspergillosis. Antimicrob Agents Chemother. 1995;39:1809–1814. doi: 10.1128/aac.39.8.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denning D W, Stevens D A. Efficacy of cilofungin alone and in combination with amphotericin B in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother. 1991;35:1329–1333. doi: 10.1128/aac.35.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dick J D, Rosengard B R, Merz W G, Stuart R K, Hutchins G M, Saral R. Fatal disseminated candidiasis due to amphotericin-B-resistant Candida guilliermondii. Ann Intern Med. 1985;102:67–68. doi: 10.7326/0003-4819-102-1-67. [DOI] [PubMed] [Google Scholar]
- 11.Gerson S, Talbot G, Hurwitz S, Strom B, Lusk E, Cassileth P. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345–351. doi: 10.7326/0003-4819-100-3-345. [DOI] [PubMed] [Google Scholar]
- 11a.Hall, L., and D. W. Denning. Unpublished data.
- 12.Hammer G S, Bottone E J, Hirschman S Z. Mucormycosis in a transplant recipient. Am J Clin Pathol. 1975;64:389–398. doi: 10.1093/ajcp/64.3.389. [DOI] [PubMed] [Google Scholar]
- 13.Hanson L H, Clemons K V, Denning D W, Stevens D A. Efficacy of oral saperconazole in systemic murine aspergillosis. J Med Vet Mycol. 1995;33:311–317. doi: 10.1080/02681219580000631. [DOI] [PubMed] [Google Scholar]
- 14.Kelly S L, Lamb D C, Kelly D E, Manning N J, Loeffler J, Hebart H, Schumacher U, Einsele H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol Δ5,6-desaturation. FEBS Lett. 1997;400:80–82. doi: 10.1016/s0014-5793(96)01360-9. [DOI] [PubMed] [Google Scholar]
- 15.Kim S J, Kwon-Chung K J, Milne G W A, Prescott B. Polyene-resistant mutants of Aspergillus fennelliae: identification of sterols. Antimicrob Agents Chemother. 1974;6:405–410. doi: 10.1128/aac.6.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law D, Moore C B, Denning D W. Amphotericin B resistance testing of Candida spp.: a comparison of methods. J Antimicrob Chemother. 1997;40:109–112. doi: 10.1093/jac/40.1.109. [DOI] [PubMed] [Google Scholar]
- 17.Lutwick L I, Galgiani J N, Johnson R H, Stevens D A. Visceral fungal infections due to Petriellidium boydii (allescheria boydii). In vitro drug sensitivity studies. Am J Med. 1976;61:632–640. doi: 10.1016/0002-9343(76)90141-8. [DOI] [PubMed] [Google Scholar]
- 18.Oakley K L, Morrissey G, Denning D W. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1504–1507. doi: 10.1128/aac.41.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Oakley, K. L., and D. W. Denning. Unpublished data.
- 19.Pappagianis D, Collins M S, Hector R, Remington J. Development of resistance to amphotericin B in Candida lusitaniae infecting a human. Antimicrob Agents Chemother. 1979;16:123–126. doi: 10.1128/aac.16.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller M A, Messer S A, Coffman S. In vitro susceptibilities of clinical yeast isolates to a new echinocandin derivative, LY303366, and other antifungal agents. Antimicrob Agents Chemother. 1997;41:763–766. doi: 10.1128/aac.41.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powderly W G, Kobayashi G S, Herzig G P, Medoff G. Amphotericin B-resistant yeast infection in severely immunocompromised patients. Am J Med. 1988;84:826–832. doi: 10.1016/0002-9343(88)90059-9. [DOI] [PubMed] [Google Scholar]
- 22.Rennie R, Sand C, Smith S. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro activity of antifungal agent LY303366 against Candida species, other yeasts, and Aspergillus species, abstr. F45; p. 107. [Google Scholar]
- 23.Rex J H, Cooper C R, Jr, Merz W G, Galgiani J N, Anaissie E J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995;39:906–909. doi: 10.1128/aac.39.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safe L M, Safe S H, Subden R E, Morris D C. Sterol content and polyene antibiotic resistance in isolates of Candida krusei, Candida parakrusei, and Candida tropicalis. Can J Microbiol. 1977;23:398–401. doi: 10.1139/m77-058. [DOI] [PubMed] [Google Scholar]
- 25.Schaffner A, Bohler A. Amphotericin B refractory aspergillosis after itraconazole: evidence for significant antagonism. Mycoses. 1993;36:421–424. doi: 10.1111/j.1439-0507.1993.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 26.Seidenfeld S M, Cooper B H, Smith J W, Luby J P, Mackowiak P A. Amphotericin B tolerance: a characteristic of Candida parapsilosis not shared by other Candida species. J Infect Dis. 1983;147:116–119. doi: 10.1093/infdis/147.1.116. [DOI] [PubMed] [Google Scholar]
- 27.Vanden Bossche, H., D. W. Warnock, B. Dupont, D. Kerridge, S. Sen Gupta, L. Improvisi, P. Marichal, F. C. Odds, F. Provost, and O. Ronin. 1994. Mechanisms and clinical impact of antifungal drug resistance. J. Med. Vet. Mycol. 32(Suppl. 1):189–202. [DOI] [PubMed]
- 28.Vanden Bossche H, Marichal P, Odds F C. Molecular mechanisms of drug resistance in fungi. Trends Microbiol. 1994;2:393–400. doi: 10.1016/0966-842x(94)90618-1. [DOI] [PubMed] [Google Scholar]
- 29.Walsh T J, Melcher G P, Rinaldi M G, Lecciones J, McGough D A, Kelly P, Lee J, Callender D, Rubin M, Pizzo P A. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J Clin Microbiol. 1990;28:1616–1622. doi: 10.1128/jcm.28.7.1616-1622.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woods R A, Bard M, Jackson I E, Drutz D J. Resistance to polyene antibiotics and correlated sterol changes in two isolates of Candida tropicalis from a patient with an amphotericin B resistant fungemia. J Infect Dis. 1974;129:53–58. doi: 10.1093/infdis/129.1.53. [DOI] [PubMed] [Google Scholar]
- 31.Zhanel G G, Karlowsky J A, Harding G A J, Balko T V, Zelenitsky S A, Friesen M, Kabani A, Turik M, Hoban D J. In vitro activity of a new semisynthetic echinocandin, LY-303366, against systemic isolates of Candida species, Cryptococcus neoformans, Blastomyces dermatitidis, and Aspergillus species. Antimicrob Agents Chemother. 1997;41:863–865. doi: 10.1128/aac.41.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziogas B N, Sisler H D, Lusby W R. Sterol content and other characteristics of pimaricin-resistant mutants of Aspergillus nidulans. Pestic Biochem Physiol. 1983;20:320–329. [Google Scholar]