Abstract
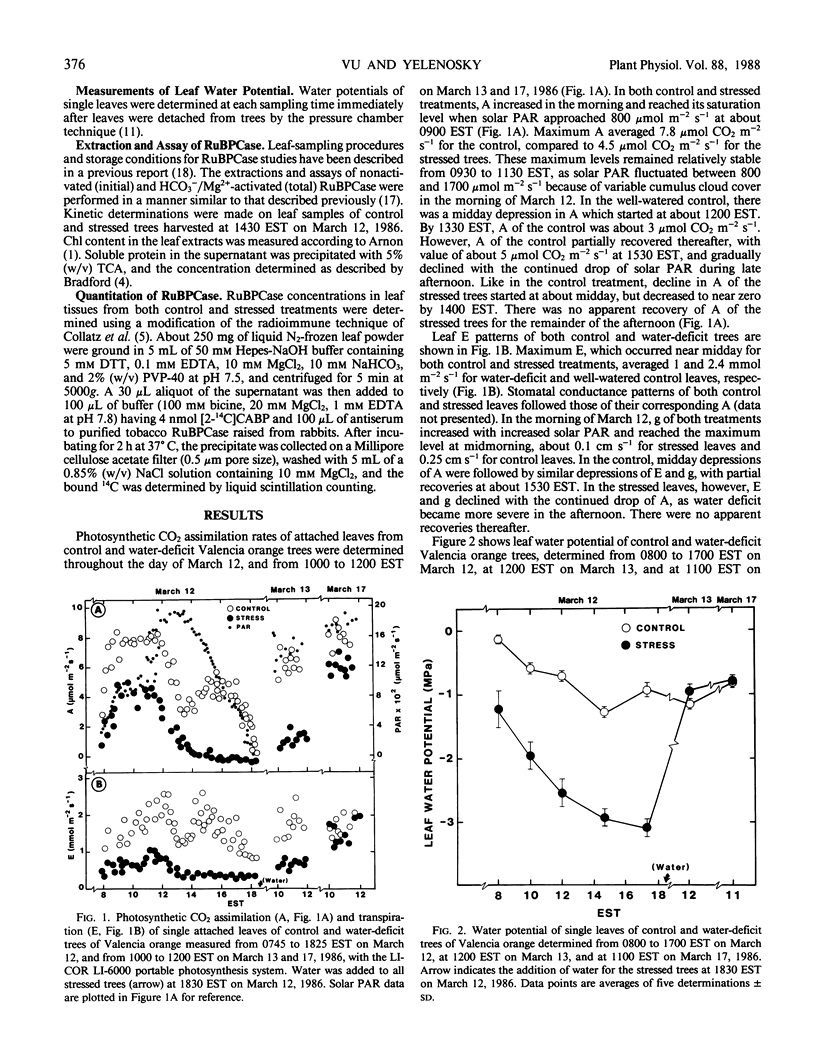
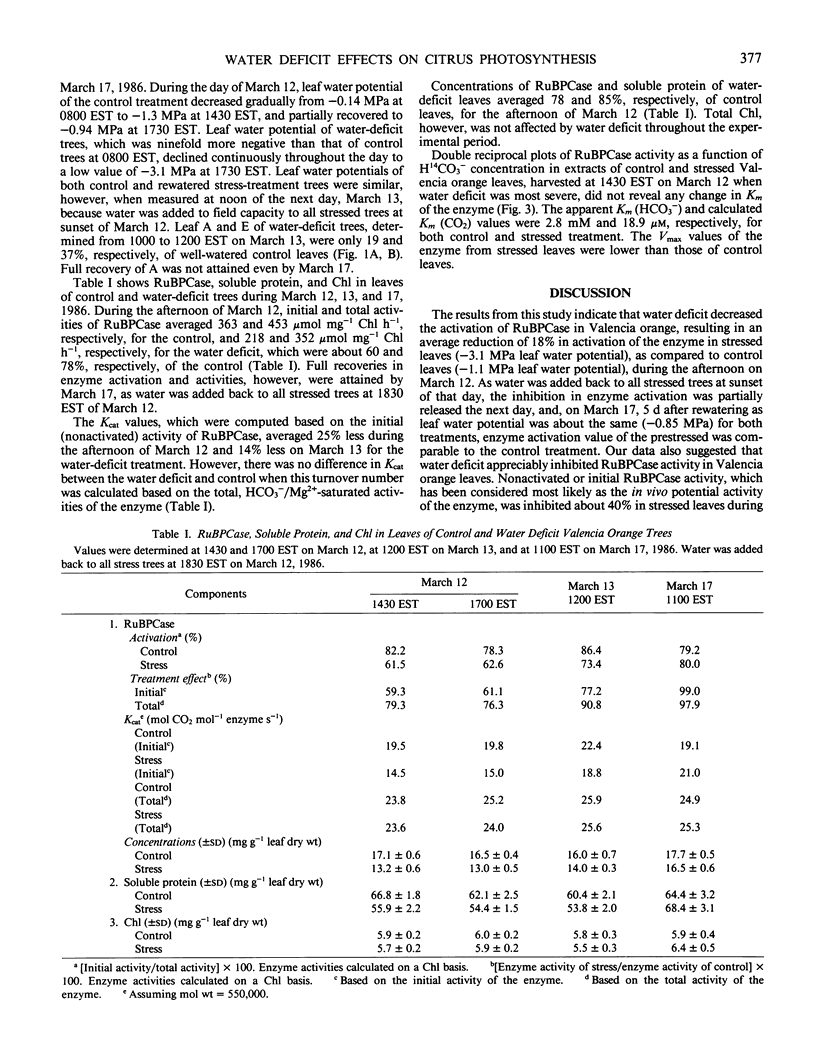
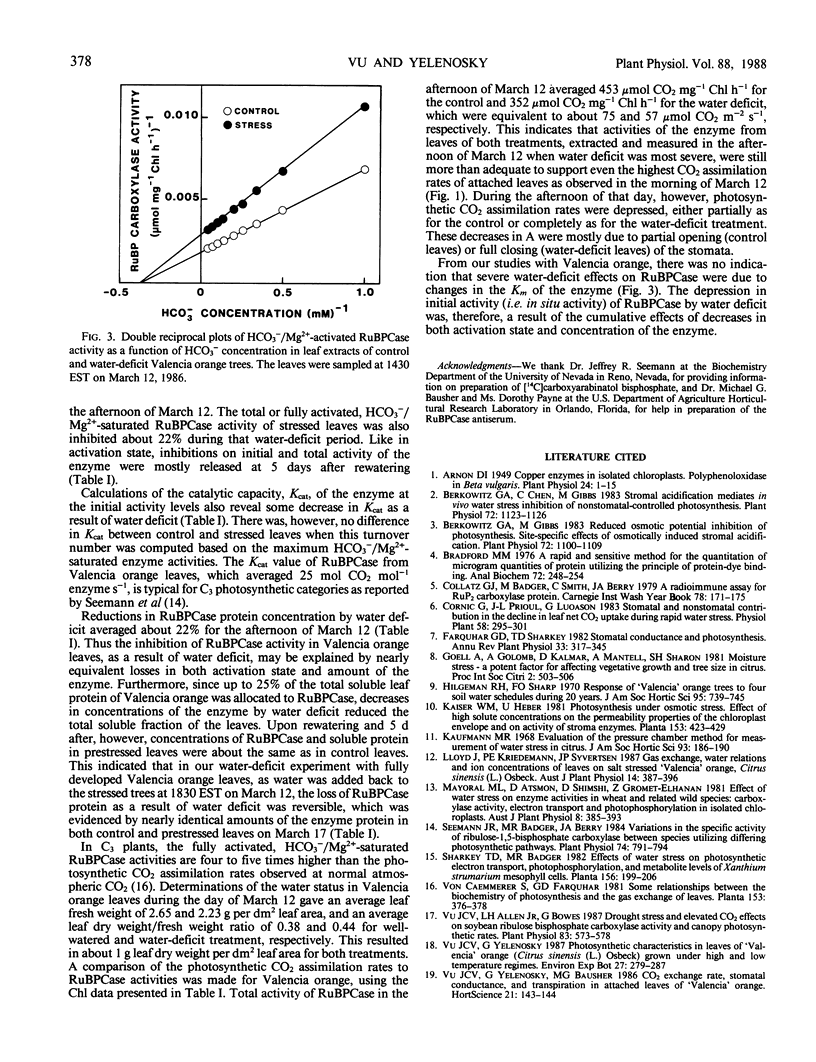
Photosynthetic CO2 assimilation, transpiration, ribulose-1,5-bisphosphate carboxylase (RuBPCase), and soluble protein were reduced in leaves of water-deficit (stress) `Valencia' orange (Citrus sinensis [L.] Osbeck). Maximum photosynthetic CO2 assimilation and transpiration, which occurred before midday for both control and stressed plants, was 58 and 40%, respectively, for the stress (−2.0 megapascals leaf water potential) as compared to the control (−0.6 megapascals leaf water potential). As water deficit became more severe in the afternoon, with water potential of −3.1 megapascals for the stressed leaves vs. −1.1 megapascals for control leaves, stressed-leaf transpiration declined and photosynthetic CO2 assimilation rapidly dropped to zero. Water deficit decreased both activation and total activity of RuBPCase. Activation of the enzyme was about 62% (of fully activated enzyme in vitro) for the stress, compared to 80% for the control. Water deficit reduced RuBPCase initial activity by 40% and HCO3−/Mg2+-saturated activity by 22%. However, RuBPCase for both stressed and control leaves were similar in Kcat (25 moles CO2 per mole enzyme per second) and Km for CO2 (18.9 micromolar). Concentrations of RuBPCase and soluble protein of stressed leaves averaged 80 and 85%, respectively, of control leaves. Thus, reductions in activation and concentration of RuBPCase in Valencia orange leaves contributed to reductions in enzyme activities during water-deficit periods. Declines in leaf photosynthesis, soluble protein, and RuBPCase activation and concentration due to water deficit were, however, recoverable at 5 days after rewatering.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. A., Chen C., Gibbs M. Stromal acidification mediates in vivo water stress inhibition of nonstomatal-controlled photosynthesis. Plant Physiol. 1983 Aug;72(4):1123–1126. doi: 10.1104/pp.72.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. A., Gibbs M. Reduced osmotic potential inhibition of photosynthesis : site-specific effects of osmotically induced stromal acidification. Plant Physiol. 1983 Aug;72(4):1100–1109. doi: 10.1104/pp.72.4.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Seemann J. R., Badger M. R., Berry J. A. Variations in the Specific Activity of Ribulose-1,5-bisphosphate Carboxylase between Species Utilizing Differing Photosynthetic Pathways. Plant Physiol. 1984 Apr;74(4):791–794. doi: 10.1104/pp.74.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu J. C., Allen L. H., Bowes G. Drought Stress and Elevated CO(2) Effects on Soybean Ribulose Bisphosphate Carboxylase Activity and Canopy Photosynthetic Rates. Plant Physiol. 1987 Mar;83(3):573–578. doi: 10.1104/pp.83.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]


