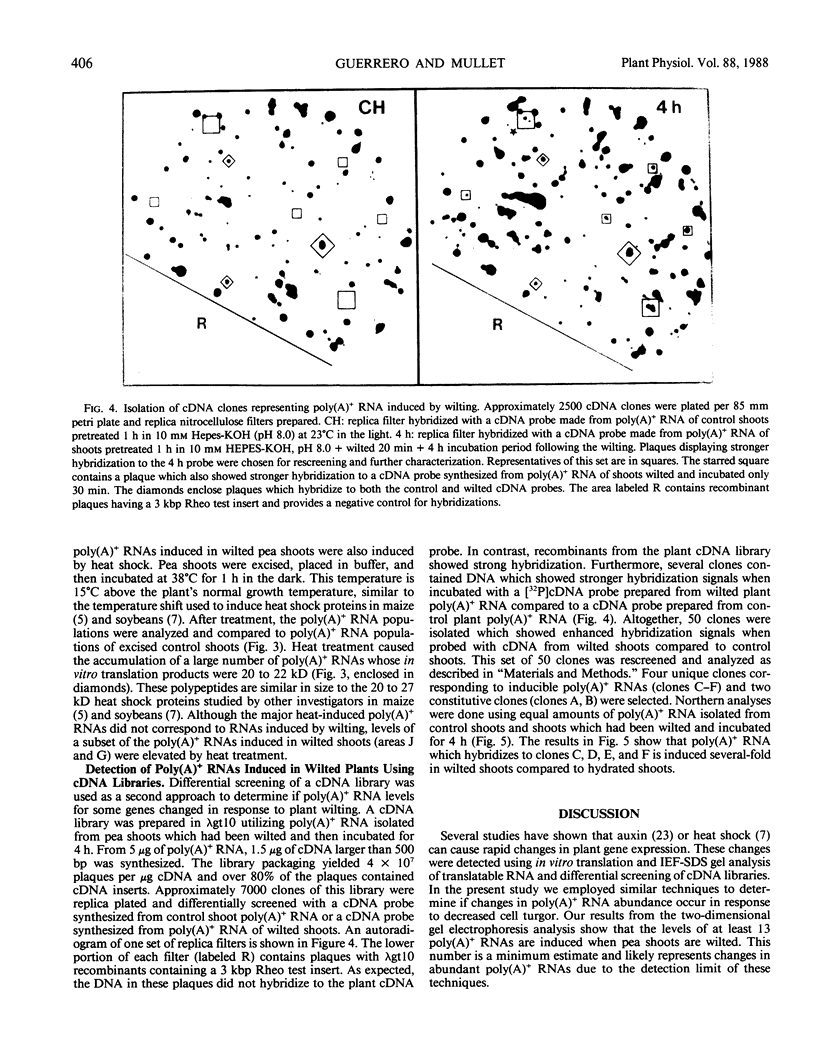
Abstract
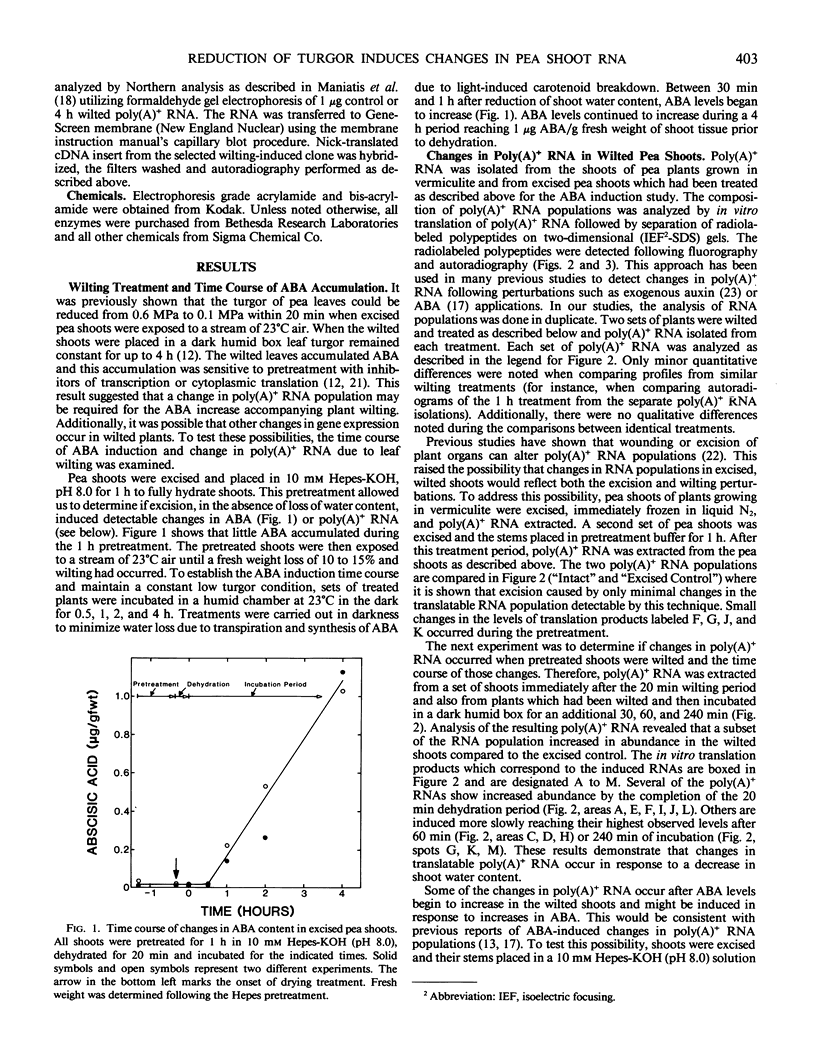
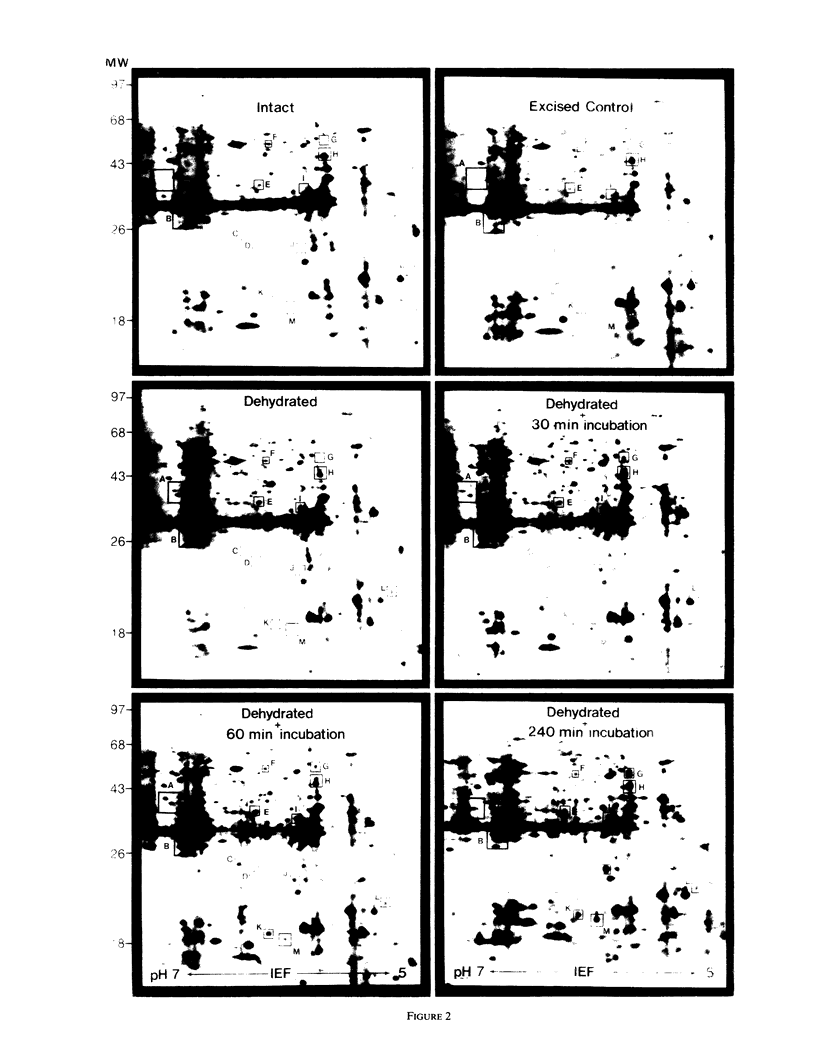
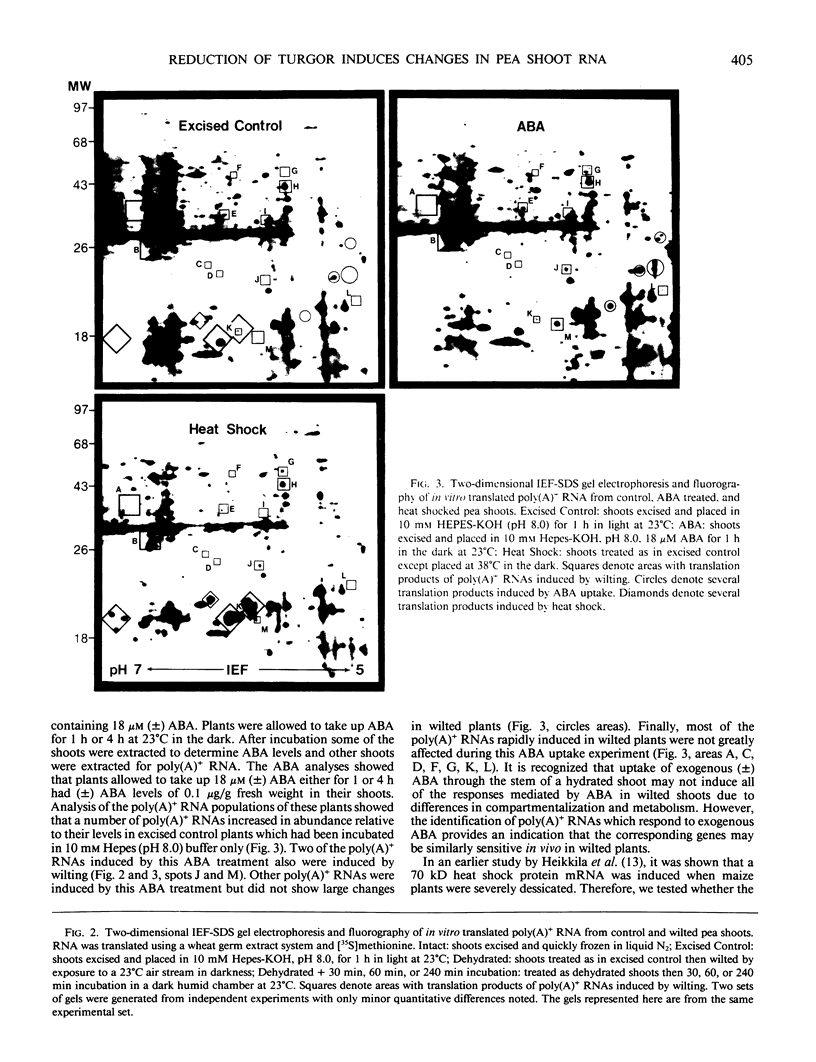
The turgor of pea (Pisum sativum) leaves was reduced by exposing excised pea shoots to a stream of 23°C air for 20 min. Poly(A)+ RNA was isolated from control and wilted shoots, translated in vitro and radiolabeled translation products separated by electrophoresis on two-dimensional (isoelectric focusing-sodium dodecyl sulfate) polyacrylamide gels. This analysis showed that the levels of several poly(A)+ RNAs increased in wilted plants. Most of the poly(A)+ RNAs induced in wilted plants did not accumulate in response to heat shock or exogenously applied ABA even though endogenous ABA levels were found to increase in shoots 30 min after wilting and by 4 h had increased 50-fold (1 versus 0.02 microgram per gram fresh weight). A λgt10 cDNA library was constructed using poly(A)+ RNA from wilted shoots which had been incubated for 4 hours. Differential screening of the library identified four clones corresponding to poly(A)+ RNAs which are induced in wilted shoots.
Full text
PDF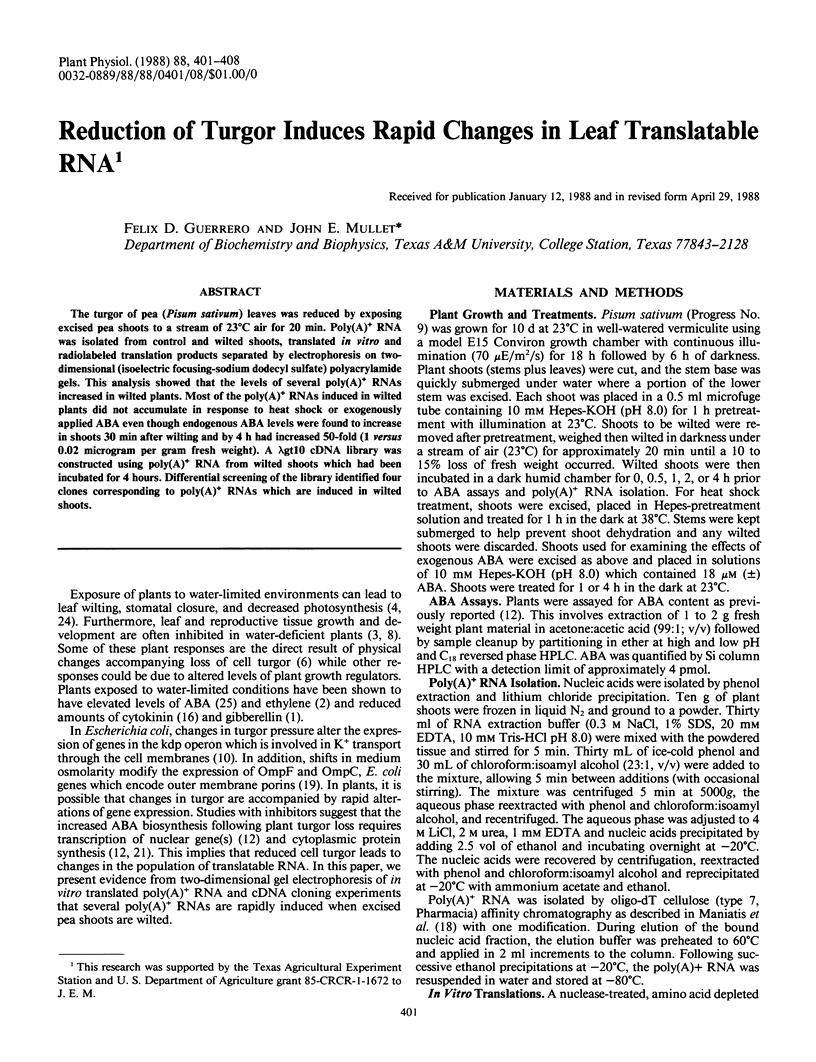



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N. Endogenous gibberellin and abscisic Acid content as related to senescence of detached lettuce leaves. Plant Physiol. 1978 Aug;62(2):224–228. doi: 10.1104/pp.62.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Yang S. F. Biosynthesis of stress ethylene induced by water deficit. Plant Physiol. 1981 Sep;68(3):594–596. doi: 10.1104/pp.68.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S., Bowen B. L. Inhibition of oxygen evolution in chloroplasts isolated from leaves with low water potentials. Plant Physiol. 1970 May;45(5):612–615. doi: 10.1104/pp.45.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Relationship of water potential to growth of leaves. Plant Physiol. 1968 Jul;43(7):1056–1062. doi: 10.1104/pp.43.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Heat shock proteins in maize. Plant Physiol. 1983 Feb;71(2):215–222. doi: 10.1104/pp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J., Van Volkenburgh E., Cleland R. E. Stress relaxation of cell walls and the yield threshold for growth: demonstration and measurement by micro-pressure probe and psychrometer techniques. Planta. 1984;162(1):46–54. doi: 10.1007/BF00397420. [DOI] [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Evaluation of isoelectric focusing running conditions during two-dimensional isoelectric focusing/sodium dodecyl sulfate-polyacrylamide gel electrophoresis: variation of gel patterns with changing conditions and optimized isoelectric focusing conditions. Anal Biochem. 1984 Apr;138(1):144–155. doi: 10.1016/0003-2697(84)90783-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guerrero F., Mullet J. E. Increased Abscisic Acid Biosynthesis during Plant Dehydration Requires Transcription. Plant Physiol. 1986 Feb;80(2):588–591. doi: 10.1104/pp.80.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila J. J., Papp J. E., Schultz G. A., Bewley J. D. Induction of heat shock protein messenger RNA in maize mesocotyls by water stress, abscisic Acid, and wounding. Plant Physiol. 1984 Sep;76(1):270–274. doi: 10.1104/pp.76.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986 Jul;81(3):802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itai C., Vaadia Y. Cytokinin Activity in Water-stressed Shoots. Plant Physiol. 1971 Jan;47(1):87–90. doi: 10.1104/pp.47.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. S., Ho T. H. Mode of action of abscisic Acid in barley aleurone layers : induction of new proteins by abscisic Acid. Plant Physiol. 1986 Sep;82(1):289–297. doi: 10.1104/pp.82.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Schuster A. M., Davies E. Ribonucleic Acid and Protein Metabolism in Pea Epicotyls : II. Response to Wounding in Aged Tissue. Plant Physiol. 1983 Nov;73(3):817–821. doi: 10.1104/pp.73.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Zeevaart J. A. Changes in the Levels of Abscisic Acid and Its Metabolites in Excised Leaf Blades of Xanthium strumarium during and after Water Stress. Plant Physiol. 1980 Oct;66(4):672–678. doi: 10.1104/pp.66.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]






