Abstract
Plasmid-mediated mechanisms, comprising TEM hyperproduction, TEM derivative production, and OXA production, lead to amoxicillin-clavulanic acid resistance in enterobacteria. The ability of the single-strand conformation polymorphism (SSCP)-PCR method to differentiate the genes encoding inhibitor-resistant β-lactamases was evaluated with three blaTEM primer pairs. The blaTEM genes, which were known to be different on the basis of their nucleotide sequences (blaTEM-1A, blaTEM-1B, blaTEM-2, blaTEM-30, blaTEM-32, and blaTEM-35), were identified as different by their electrophoretic mobilities. The blaTEM-33, blaTEM-34, blaTEM-36, blaTEM-37, blaTEM-38, and blaTEM-39 genes, whose sequence differences have been established by oligotyping, displayed different SSCP profiles for different fragments, suggesting genetic differences in addition to those defined by oligotyping. Confirmed by sequencing, these additional genetic events concerned silent mutations at certain positions and, notably, a G→T transversion at position 1 of the −10 consensus sequence in blaTEM-34, blaTEM-36, blaTEM-37, and blaTEM-39. Applied to eight clinical isolates of Escherichia coli resistant to amoxicillin-clavulanic acid, the SSCP method detected TEM-1 in three strains and TEM-30, TEM-32, and TEM-35 in three other strains, respectively. A novel TEM derivative (TEM-58) was detected in another strain, and the deduced amino acid sequence showed two substitutions: Arg244Ser, which is known to confer amoxicillin-clavulanic acid resistance in TEM-30, and Val261Ile, which has not been described previously. The eighth strain produced an OXA β-lactamase. Given the discriminatory power and the applicability of SSCP-PCR, this method can be proposed as a means of following the evolution of the frequencies of the different inhibitor-resistant β-lactamases.
Resistance to amoxicillin-clavulanic acid appeared first in Escherichia coli isolates, then in other species of enterobacteria, and most recently in Haemophilus influenzae (7, 12, 13, 21, 22, 32). Four enzymatic mechanisms for this resistance have been described in E. coli: hyperproduction of class C chromosomal β-lactamase (cephalosporinase), hyperproduction of plasmid-mediated TEM-1 or TEM-2, production of inhibitor-resistant TEM (IRT), and production of a relatively inhibitor-resistant OXA-type β-lactamase (5, 8, 9, 29, 36). Epidemiological studies carried out in Europe have shown that the frequency of each mechanism varies in different countries (17, 33). The frequency of cephalosporinase hyperproduction was similar in France and England, but IRT production seemed to be much more frequent in France, whereas OXA production seemed to be more frequent in England. Such differences may be related to the different use of β-lactam antibiotics in the two countries, and the evolution of the incidence of the different mechanisms may influence the future use of different β-lactams in the hospital as well as in the community. Thus, it is important to define a strategy which allows continued observation of the frequency of the different mechanisms.
However, because only cephalosporinase hyperproduction can be indisputably detected by the classical antibiogram (resistance to both cephalothin and cefoxitin, in addition to amoxicillin-clavulanic acid resistance), other methods must be used to differentiate the mechanisms involving plasmid-encoded amoxicillin-clavulanic acid resistance. Such methods include the determination of the β-lactamase isoelectric point, determination of β-lactamase kinetic parameters, and/or oligotyping, but these methods are fastidious procedures and are too time-consuming for a national epidemiological survey of amoxicillin-clavulanic acid resistance. In the present study, the single-strand conformation polymorphism (SSCP)-PCR technique was evaluated to differentiate the plasmid-encoded enzymatic mechanisms of amoxicillin-clavulanic acid resistance (25). This technique, which is able to detect any genetic modification, i.e., a point mutation, deletion, or insertion, should be able to differentiate wild-type blaTEM genes from IRT-encoding genes derived from blaTEM genes by point mutations. Moreover, the use of specific primers should allow differentiation between blaTEM and blaOXA genes.
MATERIALS AND METHODS
Reference genes.
The genes blaTEM-1A, blaTEM-1B, and blaTEM-2, which were expressed by E. coli C600, were used as wild-type reference genes (15, 34). Previously described IRT-encoding genes were also included as reference genes in the study. These genes have been either sequenced (blaTEM-30 from E. coli E-GUER, blaTEM-32 from E. coli 1408, and blaTEM-35 from E. coli CF0042) or defined by oligotyping from clinical E. coli isolates (blaTEM-33, blaTEM-34, and from blaTEM-36 to blaTEM-39) (4, 6, 16, 31). The nucleotide differences which have been defined by the gene sequence and those which have been determined only by oligotyping are indicated in Table 1.
TABLE 1.
Nucleotide substitutions in blaTEM-1B, blaTEM-2, and IRT-encoding genes in comparison with the nucleotides of blaTEM-1A
| Gene | Nucleotide position (amino acid codon)a
|
Reference | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 32 | 175 | 226 (6) | 317 (39) | 346 (48) | 407 (69) | 409 (69) | 436 (78) | 604 (134) | 682 (160) | 695 (165) | 747 (182) | 925 (242) | 929 (244) | 1020 (275) | 1022 (276) | ||
| blaTEM-1A | C | A | C | C | A | A | G | C | G | T | T | T | G | C | G | A | 34 |
| Sequenced genes | |||||||||||||||||
| blaTEM-1B | G | T | T | T | 15 | ||||||||||||
| blaTEM-2 | T | A | G | T | C | A | 15 | ||||||||||
| blaTEM-30blaIRT-2 | G | T | C | A | A | 4 | |||||||||||
| blaTEM-32blaIRT-3 | A | C | 6 | ||||||||||||||
| blaTEM-35blaIRT-4 | T | G | C | T | G | 31 | |||||||||||
| Oligotyped genes | |||||||||||||||||
| blaTEM-33blaIRT-5 | C | 16 | |||||||||||||||
| blaTEM-34blaIRT-6 | G | 16 | |||||||||||||||
| blaTEM-36blaIRT-7 | G | G | 16 | ||||||||||||||
| blaTEM-37blaIRT-8 | A | G | 16 | ||||||||||||||
| blaTEM-38blaIRT-9 | G | T | 16 | ||||||||||||||
| blaTEM-39blaIRT-10 | C | C | G | 16 | |||||||||||||
Primers.
Three pairs of primers were designed from the nucleotide sequence of the blaTEM-1A gene and were used to amplify three overlapping fragments (Table 2). The first pair of primers allowed the amplification of a fragment including the whole-gene promoter. The positioning of each fragment in relation to the blaTEM gene is included in Table 1. One pair of primers was selected to amplify an internal 609-bp fragment of blaOXA-1 (Table 2).
TABLE 2.
Nucleotide sequences of the oligonucleotides used for blaTEM and blaOXA-1 amplification
| Gene and primer | Sequence | Positiona |
|---|---|---|
| blaTEM | ||
| A | 5′-ATAAAATTCTTGAAGAC-3′ | −7 |
| B | 5′-AAAACTCTCAAGGATCTT-3′ | 382 |
| C | 5′-AAAGATGCTGAAGATCA-3′ | 301 |
| D | 5′-TTTGGTATGGCTTCATTC-3′ | 726 |
| E | 5′-TTACCAATGCTTAATCA-3′ | 652 |
| F | 5′-TTTTTTGCACAACATGGG-3′ | 1069 |
| blaOXA-1 | ||
| G | 5′-TCAACTTTCAAGATCGCA-3′ | 211 |
| H | 5′-GTGTGTTTAGAATGGTGA-3′ | 820 |
SSCP-PCR.
Samples were prepared by suspending a freshly grown colony in 0.5 ml of lysis buffer (20 mM Tris HCl [pH 8.3], 50 mM KCl, 0.1% Tween 20) which was heated at 94°C for 10 min. Five microliters of this preparation was submitted to PCR in a final volume of 25 μl. To the PCR master mixture containing 20 mM Tris HCl (pH 8.0), 100 mM KCl, 3 mM MgCl2, 400 μM (each) deoxynucleotide triphosphate, 2.5 U of Taq DNA polymerase (Boehringer Mannheim GmbH, Mannheim, Germany), and 25 pmol of each primer, 0.25 μl (2.5 μCi) of radioactive [α-32P]dCTP was added. The amplification reaction consisted of 36 cycles of 30 s of denaturation at 94°C, 30 s of hybridization at 42°C, and 60 s of extension at 72°C, with a final extension step at 72°C for 10 min. The radioactive PCR product was diluted 1:2 with SSCP dilution buffer (2 mM EDTA, 0.1% sodium dodecyl sulfate), and 5 μl of the diluted product was mixed with 5 μl of loading buffer (95% formamide, 0.05% bromophenol blue, 0.05% xylene cyanole, 50 mM EDTA). Immediately prior to loading of the SSCP gel the samples were denatured for 15 min at 94°C, cooled on ice, and loaded onto a nondenaturating polyacrylamide gel. The nondenaturating polyacrylamide gel was prepared by mixing 20 ml of acrylamide-bisacrylamide (29:1) with 80 ml of 1× TBE (Tris-borate-EDTA). The gel was run for 4 h at 65 W with constant cooling. After termination of the run, the gel was transferred to a filter paper, dried, and exposed for 2 h to an X-ray film at −70°C with an intensifying screen.
Sequencing.
The PCR products for sequencing were prepared as indicated above but the radioactive nucleotide was omitted. The PCR products were purified with the QIAquick PCR Purification Kit (QIAGEN, Courtaboeuf, France) following the manufacturer’s recommendations. The nucleotide sequences of the purified PCR fragments were determined with the Sequenase PCR Product Sequencing Kit (Amersham, Les Ulis, France) and by following the manufacturer’s indications exactly. The sequencing reactions were run on a standard denaturating sequencing gel.
Clinical isolates.
Eight clinical isolates of E. coli (isolates AP1 to AP8), obtained from Ambroise-Paré Hospital between 1993 and 1994, were studied because they were resistant to amoxicillin and amoxicillin-clavulanic acid and susceptible to cefoxitin and broad-spectrum cephalosporins by the disk diffusion test according to the recommendations of the Antibiogram Committee of the French Microbiology Society (1).
Characterization of the amoxicillin-clavulanic acid resistance in the eight clinical isolates.
The MICs of amoxicillin (SmithKline Beecham, Nanterre, France), alone and associated with a fixed concentration of 2 μg of clavulanic acid (SmithKline Beecham) per ml, and piperacillin (Léderlé, St. Cloud, France), alone and associated with a fixed concentration of 4 μg of tazobactam (Léderlé) per ml, for the eight clinical isolates were measured by a dilution method on Mueller-Hinton agar with a Steers replicator device and an inoculum of 104 CFU per spot.
For each clinical E. coli isolate, crude extracts were submitted to isoelectric focusing as described previously (3), and their pIs were compared with the pI values of the following enzymes: RP4/TEM-1, pI 5.4; R111/TEM-2, pI 5.6; pUD101/TEM-30, pI 5.2 (4); and RGN238/OXA-1, pI 7.4 (26). The kinetic parameters for the enzymes, the β-lactamase specific activity (in milliunits per milligram of total protein), and Km values (in micromolar) were determined with crude extracts by computerized microacidimetry as described previously (20). All extracts were first studied at pH 7 and 37°C in the presence of NaCl. When an OXA-type β-lactamase was suspected, a complementary set of experiments was performed at pH 7 and 20°C and in the presence of Na2SO4 instead of the NaCl solution, since OXA enzymes are inhibited by chloride ions. After preincubation of the crude extracts for 10 min at 37°C with 100 μg of clavulanic acid per ml, the residual activity of the β-lactamases was determined in order to differentiate TEM enzymes which display a residual activity of ≤10% from IRT enzymes which display a residual activity of >20% (11). Under these experimental conditions, it has been previously observed that OXA enzymes are highly unstable (11).
RESULTS
SSCP-PCR of the wild-type reference genes.
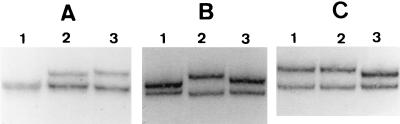
In accordance with the nucleotide sequences (Table 1), we obtained three different SSCP profiles for fragment 1 covering 389 bp starting at position −7 (34) for the three wild-type reference genes blaTEM-1A, blaTEM-1B, and blaTEM-2 (Fig. 1A). This was also the case for fragment 2, which covers 426 bp starting at position 301 (Fig. 1B). For fragment 3 we obtained, as expected (Table 1), two different SSCP migration profiles, one for both blaTEM-1 genes and one for blaTEM-2 (Fig. 1C).
FIG. 1.
SSCP-PCR of wild-type blaTEM genes. The SSCP profiles of amplified fragments 1 (A), 2 (B), and 3 (C) are shown. Lanes 1, blaTEM-1A; lanes 2, blaTEM-1B; lanes 3, blaTEM-2.
SSCP-PCR of sequenced IRT-encoding genes.
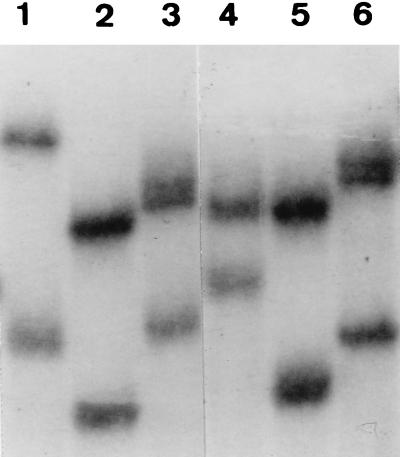
According to the nucleotide sequences of blaTEM-30, blaTEM-32, and blaTEM-35, we observed two different and specific SSCP profiles of fragments 1 of blaTEM-30 and blaTEM-35, whereas the profile for fragment 1 of blaTEM-32 was identical to that of fragment 1 of blaTEM-1A. For fragments 2 (Fig. 2) and 3, each of the IRT-encoding genes displayed a specific profile that was in accordance with the previously determined nucleotide sequence.
FIG. 2.
Comparative SSCP-PCR profiles of the amplified fragment 2 of wild-type blaTEM genes and previously sequenced IRT-encoding genes. Lane 1, blaTEM-35; lane 2, blaTEM-30; lane 3, blaTEM-1A; lane 4, blaTEM-32; lane 5, blaTEM-2; lane 6, blaTEM-1B.
SSCP-PCR of oligotyped IRT-encoding genes.
No data were available for the nucleotide sequences corresponding to fragment 1 of the oligotyped IRT encoding genes. By the SSCP method, we found that fragment 1 of blaTEM-33 was clearly different from the fragments 1 of all reference genes included in this study. For blaTEM-34, blaTEM-36, blaTEM-37, and blaTEM-39, the SSCP migration profile of fragment 1 differed slightly from that of fragment 1 of blaTEM-30, whereas the profile of fragment 1 of blaTEM-38 differed slightly from that of fragment 1 of blaTEM-1B.
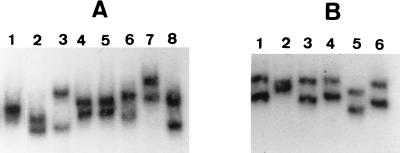
According to oligotyping analysis, at least two SSCP profiles different from those of the three blaTEM genes and the three sequenced IRT-encoding genes could be expected for fragment 2: one for blaTEM-34, blaTEM-36, and blaTEM-38 and one for blaTEM-39. On the other hand, the SSCP profile of fragment 2 of blaTEM-33 could be expected to be identical to that of fragment 2 of blaTEM-35, and that of fragment 2 of blaTEM-37 could be expected to be identical to that of fragment 2 of blaTEM-32. In fact, we obtained specific fragment 2 profiles for five of the six oligotyped IRT-encoding genes because it was shown that blaTEM-33 differed from blaTEM-35, blaTEM-37 differed from blaTEM-32, and blaTEM-38 differed from both blaTEM-34 and blaTEM-36. The fifth specific profile corresponded to blaTEM-39, as expected (Fig. 3A).
FIG. 3.
SSCP-PCR profiles of fragments 2 and 3 of oligotyped IRT encoding genes. (A) Fragment 2. Lane 1, blaTEM-32; lane 2, blaTEM-35; lane 3, blaTEM-33; lane 4, blaTEM-34; lane 5, blaTEM-36; lane 6, blaTEM-37; lane 7, blaTEM-38; lane 8, blaTEM-39. (B) Fragment 3. Lane 1, blaTEM-33; lane 2, blaTEM-34; lane 3, blaTEM-36; lane 4, blaTEM-38; lane 5, blaTEM-39; lane 6, blaTEM-37.
For fragment 3, the oligotype and sequence comparison could suggest a migration profile identity between fragments 3 of blaTEM-1, blaTEM-33, and blaTEM-34 on the one hand and between fragments 3 of blaTEM-35, blaTEM-36, and blaTEM-37 on the other hand. Inversely, a migration specificity could be expected for blaTEM-38 and blaTEM-39. These two IRT-encoding genes effectively displayed specific fragment 3 SSCP profiles, but such was also the case for blaTEM-34, which differed from blaTEM-33, which displayed the same profile as blaTEM-1 (Fig. 3B), and for blaTEM-36 and blaTEM-37, which differed from each other (Fig. 3B) and which both differed from blaTEM-35.
Characterization of the amoxicillin-clavulanic acid resistance in the eight clinical isolates. (i) Kinetic parameters of the β-lactamases.
The amoxicillin-clavulanic acid resistance was evaluated first by the disk diffusion test for the eight clinical isolates and was confirmed, as indicated in Table 3, by the MICs (MICs, >16 μg/ml). Compared to the MICs of amoxicillin, those of piperacillin were lower, and in association with 4 μg of tazobactam per ml, piperacillin was active against six of the eight clinical isolates (MIC, <8 μg/ml) (Table 3). The β-lactamases of strains AP1, AP2, AP3, and AP5 comigrated with TEM-1 (pI 5.4) and that of strain AP4 comigrated with OXA-1 (pI 7.4). The three remaining strains, AP6 to AP8, produced a β-lactamase with a pI of 5.2 (Table 3). According to the Km values for penicillin G, the clinical isolates could be separated into three groups: Km of <10 for strains AP4 and AP5, Km of ∼25 for strains AP1 to AP3, and Km of >100 for strains AP6 to AP8. Among the β-lactamases which displayed a pI of 5.4, three (those from strains AP1 to AP3) showed a very low residual activity (<10%) in the presence of clavulanic acid, suggesting the production of TEM enzymes, whereas one (that from strain AP5) kept a relatively high residual activity (56%), similar to those which were measured for the three β-lactamases having pIs of 5.2 (Table 3). These findings suggested that the clinical isolates AP6 to AP8 and AP5 produced IRT β-lactamases. The necessity of using Na2SO4 instead of NaCl solution to measure the β-lactamase activity of the enzyme produced by strain AP4 and the fact that this enzyme was highly unstable under the experimental conditions established for measurement of the residual activity suggested the presence of an OXA β-lactamase in this strain.
TABLE 3.
MICs and enzymatic kinetic parameters for the clinical E. coli isolates
| Clinical isolate | pI | MIC (μg/ml)
|
Penicillin G
|
Residual activity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Amoxicillin
|
Piperacillin
|
|||||||
| Alone | With Caa | Alone | With Tazb | Km (μM) | Sp act (mU/mg) | |||
| AP1 | 5.4 | 2,048 | 32 | 64 | 1 | 25 | 335 | 9.5 |
| AP2 | 5.4 | >4,096 | 256 | 1,024 | 32 | 25 | 1,786 | 9.3 |
| AP3 | 5.4 | 2,048 | 32 | 64 | 1 | 27 | 586 | 10 |
| AP4 | 7.4 | 4,096 | 128 | 256 | 32 | 5 | 61 | NDc |
| AP5 | 5.4 | 2,048 | 512 | 32 | 4 | 7 | 144 | 56 |
| AP6 | 5.2 | 4,096 | 2,048 | 128 | 4 | 138 | 3,200 | 46.5 |
| AP7 | 5.2 | 1,024 | 256 | 8 | 1 | 216 | 925 | 33 |
| AP8 | 5.2 | 2,048 | 1,024 | 32 | 2 | 232 | 660 | 32.5 |
Ca, clavulanic acid at 2 μg/ml.
Taz, tazobactam at 4 μg/ml.
ND, not determined (the enzyme was unstable).
(ii) SSCP-PCR.
Strain AP4, which was expected to produce an OXA-1 β-lactamase, did not yield an amplification product with the TEM-derived primers in repetitive experiments but yielded products with the specific primers of blaOXA-1 (data not shown). As indicated in Table 4, strains AP1 to AP3, whose β-lactamases displayed a very low residual activity in the presence of clavulanic acid, were shown to produce TEM enzymes by the SSCP method. The bla gene of strain AP1 could be identified as blaTEM-1B, and that of strain AP3 could be identified as blaTEM-1A. An unusual situation was observed with the bla gene of strain AP2 because the SSCP profiles of its fragments 1 and 2 corresponded to those of blaTEM-1A and blaTEM-1B, respectively. The four clinical strains which showed an important residual β-lactamase activity in the presence of clavulanic acid could be expected to produce IRT enzymes according to the SSCP results, namely, TEM-32 for strain AP5, TEM-35 for strain AP6, and TEM-30 for strain AP8. The amplification product obtained for fragment 3 of the blaTEM gene expressed by strain AP7 showed a particular migration profile that did not correspond to the profiles of any of the reference genes, while those of fragments 1 and 2 showed the same electrophoretic mobility as fragment 1 of blaTEM-33 and fragment 2 of blaTEM-1B, respectively.
TABLE 4.
SSCP-PCR profiles of the clinical E. coli isolates in comparison with the SSCP-PCR profiles of the reference blaTEM genes
| Fragment | Profile
|
||||||
|---|---|---|---|---|---|---|---|
| AP1 | AP2 | AP3 | AP5 | AP6 | AP7 | AP8 | |
| 1 | blaTEM-1B | blaTEM-1A | blaTEM-1A | blaTEM-1A | blaTEM-1B | blaTEM-33 | blaTEM-1B |
| 2 | blaTEM-1B | blaTEM-1B | blaTEM-1A | blaTEM-32 | blaTEM-35 | blaTEM-1B | blaTEM-30 |
| 3 | blaTEM-1A/B | blaTEM-1A/B | blaTEM-1A/B | blaTEM-32 | blaTEM-35 | Novel | blaTEM-30 |
Nucleotide sequencing.
Given the great diversity of SSCP profiles of oligotyped IRT-encoding genes, sequencing of amplified fragments 1 and 2 and some of fragment 3 was carried out and showed that different silent point mutations occurred. As indicated in Table 5 and in comparison with the blaTEM-1A gene sequence, these mutations consisted of a C→T transition at position 32 for blaTEM-38, a G→T transversion at position 162 for blaTEM-34, blaTEM-36, blaTEM-37, and blaTEM-39, an A→G transition at position 175 for blaTEM-33 and blaTEM-38, a C→T transition at position 226 for blaTEM-38, an A→G transition at position 346 for blaTEM-34, blaTEM-36, blaTEM-37, and blaTEM-39, a C→T transition at position 436 for all the blaTEM-derived genes, a G→T transversion at position 604 for blaTEM-33, a T→C transition at position 682 for blaTEM-34, blaTEM-36, and blaTEM-37, and a G→A transition at position 925 for blaTEM-36. By sequencing the blaTEM gene of clinical isolate AP7, for which the SSCP analysis was not able to define the type of IRT produced, three nucleotide changes were observed (Table 5). The first one consisted of an A→G transition at position 175, as was found in blaTEM-33 and blaTEM-38. The second one concerned a C→A transversion at position 929, leading to the amino acid substitution arginine→serine at position 244, while the third one was a G→A transition at position 980, leading to the amino acid replacement valine→isoleucine at position 261 (numbering of Ambler et al. [2]).
TABLE 5.
Nucleotide substitutions determined by sequencing of the previously oligotyped IRT-encoding genes and the blaTEM gene of strain AP7 compared with the sequence of blaTEM-1A
DISCUSSION
The amoxicillin-clavulanic acid resistance which appeared for the first time about 10 years ago in E. coli has now been observed in Klebsiella pneumoniae, Proteus mirabilis, Salmonella typhimurium, Shigella flexneri, and H. influenzae (7, 12, 13, 21, 22, 32). Contrary to E. coli, the recently involved species do not possess a chromosomal cephalosporinase whose hyperproduction results in amoxicillin-clavulanic acid resistance. Subsequently, strains of these species have acquired plasmid-encoded mechanisms, comprising TEM hyperproduction and IRT production, which were first identified in E. coli (7, 13, 21, 32). OXA production, which is the third plasmid-encoded mechanism, seems to be limited to E. coli. The reasons why bacteria, particularly E. coli, have developed so many mechanisms are unknown. However, we can observe differences in the incidences of the mechanisms in individual countries and differences in the spectrum of β-lactam resistance according to the amoxicillin-clavulanic acid resistance mechanism (17, 28, 30, 33).
Because plasmid-encoded mechanisms involved either completely different genes (blaTEM, blaOXA) or blaTEM-derived genes (IRT-encoding genes), the PCR-based SSCP method seemed suitable to us for the rapid identification of the genes responsible for amoxicillin-clavulanic acid-resistant phenotypes. In fact, using this method we were able to differentiate blaTEM genes which were known to be different on the basis of their nucleotide sequences. SSCP-PCR was able to indicate the occurrence of further genetic events in the genes for which only short stretches were oligotyped, and these were confirmed by sequencing. Thus, a G→T transversion at position 1 of the −10 consensus sequence was identified in blaTEM-34, blaTEM-36, blaTEM-37, and blaTEM-39. Such a transversion, which is known to be at the origin of a higher level of enzyme production, was recently described in the blaTEM-1 gene expressed by an S. flexneri isolate resistant to amoxicillin-clavulanic acid and in IRT-encoding genes, notably, blaTEM-45 (10, 32). Our study shows, as has already been described by Caniça et al. (10), that there is a great molecular diversity in the blaTEM genes encoding IRT but that some mutations are very frequent in such genes, notably, A346G, C436T, and T682C transitions. Although we have observed the G925A transition only in blaTEM-36, this mutation also seems to be frequent in IRT-encoding genes according to the study of Caniça et al. (10). Inversely, the C32T and G604T transitions which we identified in blaTEM-38 and blaTEM-33, respectively, seem to be rare in IRT-encoding genes (10).
The eight amoxicillin-clavulanic acid-resistant clinical isolates were studied by both enzymatic methods and SSCP-PCR. The results obtained by each method were in perfect concordance. Enzymes determined by the SSCP-PCR technique to be TEM enzymes showed by enzymatic analysis the typical Km values and residual activity of TEM enzymes (9, 11). Those enzymes determined by the SSCP-PCR method to be IRT enzymes displayed kinetic parameters which have previously been published for such enzymes (10). Nevertheless, the SSCP-PCR method, which is more rapid and whose performance is less fastidious, allowed us to characterize precisely the type of the IRT enzymes. The presence of TEM-32 in strain AP5, suggested by the Km value, which is known to be particularly low for this enzyme, was definitely proven by SSCP-PCR (11). Moreover, SSCP-PCR allowed us to detect a novel blaTEM gene (blaTEM-58) in clinical isolate AP7. The deduced amino acid sequence showed a previously unknown amino acid substitution, Val261Ile, in addition to the already known amino acid substitution Arg244Ser, which confers amoxicillin-clavulanic acid resistance in TEM-30 (8). By the two crystal structures of TEM-1 β-lactamase which are available (entries 1BTL and 1XPB; Protein Data Bank, Brookhaven National Laboratory, Brookhaven, N.Y. [14, 19]), Val261 was shown to be located on sheet s-5 in a group of four hydrophobic residues: Ile, Val, Val, and Ile, starting at position 260. Moreover, the side chain of Val261 is in the close vicinity of at least Leu221 (helix h10), Leu250, and Leu286 (helix h11), which form a highly hydrophobic pocket. The role of the substitution Val261Ile on the enzymatic activity of this TEM-derived enzyme should be determined in further studies.
We can also note that the blaTEM gene of strain AP7 showed the same migration profile as fragment 1 of blaTEM-33, for which we demonstrated a guanine at position 175. Such a mutation is extremely rare, because it is apparently present only in blaTEM-1B, blaTEM-5, blaTEM-6b, and blaTEM-38; however, it is present in association with a thymine at position 216 in these four genes (15, 18, 27).
Three clinical isolates (isolates AP1 to AP3) were shown by the SSCP-PCR method and kinetic parameters to produce TEM enzymes. Three molecular mechanisms have previously been described to explain the amoxicillin-clavulanic acid-resistant phenotype related to wild-type blaTEM genes: up-mutation in the regulatory region of the gene, the presence of multiple copies of the plasmid, and a gene dose effect (23, 29, 32, 35). Because we did not observe for the TEM enzymes produced by strains AP1 to AP3 specific SSCP-PCR profiles of fragment 1 covering the promoter region, an up-mutation cannot be assumed to be responsible for the amoxicillin-clavulanic acid resistance. However, AP2 has been shown by determination of kinetic parameters and SSCP-PCR to yield a higher level of β-lactamase activity, and its β-lactamase gene has been shown to have an unusual structure.
In conclusion, this study shows that the SSCP-PCR method is a suitable tool for rapidly screening for the different plasmid-encoded mechanisms which are known so far to confer amoxicillin-clavulanic acid resistance in enterobacteria. We have shown that this method can also be used to identify the different IRT enzymes and detect novel enzymes of this type. However, because it is a comparative technique and because IRT-encoding genes have a great molecular diversity, a large number of reference genes must be used. To decrease the number of reference genes the amplified fragments could be shortened and concentrated on mutations leading to relevant amino acid substitutions, as was shown for SHV derivatives (24).
ACKNOWLEDGMENTS
This work received financial support from the Beecham Institute, Paris, France.
We thank Danielle Sirot for providing us with strains producing the oligotyped IRT enzymes and Patrice Courvalin for providing us with blaTEM-1b and blaTEM-2.
REFERENCES
- 1.Acar J, Bergogne-Bérézin E, Brognard J M, Chabbert Y, Cluzel R, Courvalin P. Communiqué 1997 du Comité de l’Antibiogramme de la Société Francaise de Microbiologie. Pathol Biol. 1997;45:I–XII. [PubMed] [Google Scholar]
- 2.Ambler R P, Coulson A F N, Frere J M, Ghuysen J M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–272. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthélémy M, Guionie M, Labia R. Beta-lactamases: determination of their isoelectric points. Antimicrob Agents Chemother. 1978;13:695–698. doi: 10.1128/aac.13.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belaaouaj A, Lapoumeroulie C, Caniça M M, Vedel G, Névot P, Krishnamoorthy R, Paul G. Nucleotide sequences of the genes coding for the TEM-like β-lactamases IRT-1 and IRT-2 (formerly called TRI-1 and TRI-2) FEMS Microbiol Lett. 1994;120:75–80. doi: 10.1111/j.1574-6968.1994.tb07010.x. [DOI] [PubMed] [Google Scholar]
- 5.Bergström S, Normark S. β-Lactam resistance in clinical isolates of Escherichia coli caused by elevated production of the ampC-mediated chromosomal β-lactamase. Antimicrob Agents Chemother. 1979;16:427–433. doi: 10.1128/aac.16.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blazquez J, Baquero M R, Canton R, Alos I, Baquero F. Characterization of a new TEM-type β-lactamase resistant to clavulanate, sulbactam, and tazobactam in a clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1993;37:2059–2063. doi: 10.1128/aac.37.10.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bret L, Chanal C, Sirot D, Labia R, Sirot J. Characterization of an inhibitor-resistant enzyme IRT-2 derived from TEM-2 β-lactamase produced by Proteus mirabilis strains. J Antimicrob Chemother. 1996;38:183–191. doi: 10.1093/jac/38.2.183. [DOI] [PubMed] [Google Scholar]
- 8.Bush K, Jacoby G. Nomenclature of TEM β-lactamases. J Antimicrob Chemother. 1997;39:1–3. doi: 10.1093/jac/39.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caniça M M, Lu C Y, Krishnamoorthy R, Paul G C. Molecular diversity and evolution of blaTEM genes encoding β-lactamases resistant to clavulanic acid in clinical E. coli. J Mol Evol. 1997;44:57–65. doi: 10.1007/pl00006121. [DOI] [PubMed] [Google Scholar]
- 11.Chardon H, Farzaneh S, Labia R, Jarlier V, Nicolas M H, Paul G, Poyart C, Sirot D, Sirot J. Analysis of β-lactamases produced by cephalothin-susceptible Escherichia coli clinical isolates resistant to co-amoxiclav and ticarcillin-clavulanic acid. J Antimicrob Chemother. 1995;36:267–269. doi: 10.1093/jac/36.1.267. [DOI] [PubMed] [Google Scholar]
- 12.Doern G V, Brueggemann A B, Pierce G, Holley H P, Jr, Rauch A. Antibiotic resistance among clinical isolates of Haemophilus influenzae in the United States in 1994 and 1995 and detection of β-lactamase-positive strains resistant to amoxicillin-clavulanate: results of a national multicenter surveillance study. Antimicrob Agents Chemother. 1997;41:292–297. doi: 10.1128/aac.41.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinasse F, Gheorghiu R, Poiata A, Labia R, Nicolas-Chanoine M-H. Reduced susceptibility to co-amoxiclav in Escherichia coli, Salmonella typhimurium and Klebsiella pneumoniae isolated in Romania between 1985 and 1993. J Antimicrob Chemother. 1997;39:103–106. doi: 10.1093/jac/39.1.103. [DOI] [PubMed] [Google Scholar]
- 14.Fonzé E, Charlier P, To’th Y, Vermeire M, Raquet X, Dubus A, Frère J M. TEM-1 β-lactamase structure solved by molecular replacement and refined structure of the S235A mutant. Acta Crystallogr D. 1995;51:682–694. doi: 10.1107/S0907444994014496. [DOI] [PubMed] [Google Scholar]
- 15.Goussard S, Courvalin P. Sequence of the genes blaT-1B and blaT-2. Gene. 1991;102:71–73. doi: 10.1016/0378-1119(91)90540-r. [DOI] [PubMed] [Google Scholar]
- 16.Henquell C, Chanal C, Sirot D, Labia R, Sirot J. Molecular characterization of nine different types of mutants among 107 inhibitor-resistant TEM β-lactamases from clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1995;39:427–430. doi: 10.1128/aac.39.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henquell C, Sirot D, Chanal C, De C C, Chatron P, Lafeuille B, Texier P, Sirot J, Cluzel R. Frequency of inhibitor-resistant TEM beta-lactamases in Escherichia coli isolates from urinary tract infections in France. J Antimicrob Chemother. 1994;34:707–714. doi: 10.1093/jac/34.5.707. [DOI] [PubMed] [Google Scholar]
- 18.Hibbert-Rogers L C F, Heritage J, Todd N, Hawkey P M. Convergent evolution of TEM-26, a β-lactamase with extended-spectrum activity. J Antimicrob Chemother. 1994;33:707–720. doi: 10.1093/jac/33.4.707. [DOI] [PubMed] [Google Scholar]
- 19.Jelsch C, Mourey L, Masson J M, Samama J P. Crystal structure of Escherichia coli TEM-1 β-lactamase at 1.8 Å resolution. Protein Struct Funct Genet. 1993;16:364–383. doi: 10.1002/prot.340160406. [DOI] [PubMed] [Google Scholar]
- 20.Labia R, Andrillon J, Le Goffic F. Computerized microacidimetric determination of beta lactamase Michaelis-Menten constants. FEBS Lett. 1973;33:42–44. doi: 10.1016/0014-5793(73)80154-1. [DOI] [PubMed] [Google Scholar]
- 21.Lemozy J, Sirot D, Chanal C, Huc C, Labia R, Dabernat H, Sirot J. First characterization of inhibitor-resistant TEM (IRT) β-lactamases in Klebsiella pneumoniae strains. Antimicrob Agents Chemother. 1995;39:2580–2582. doi: 10.1128/aac.39.11.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez J L, Cercenado E, Rodriguez-Creixems M, Vicente-Perez M F, Delgado-Iribarren A, Baquero F. Resistance to beta-lactam/clavulanate. Lancet. 1987;ii:1473. doi: 10.1016/s0140-6736(87)91180-9. [DOI] [PubMed] [Google Scholar]
- 23.Martinez J L, Vicente M F, Delgado-Iribarren A, Perez-Diaz J C, Baquero F. Small plasmids are involved in amoxicillin-clavulanate resistance in Escherichia coli. Antimicrob Agents Chemother. 1989;33:595. doi: 10.1128/aac.33.4.595-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.M’Zali F-H, Gascoyne-Binzi D M, Heritage J, Hawkey P M. Detection of mutations conferring extended-spectrum activity on SHV β-lactamases using polymerase chain reaction single strand conformational polymorphism (PCR-SSCP) J Antimicrob Chemother. 1996;37:797–802. doi: 10.1093/jac/37.4.797. [DOI] [PubMed] [Google Scholar]
- 25.Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci USA. 1989;86:2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouellette M, Bissonnette L, Roy P H. Precise insertion of antibiotic resistance determinants into Tn21-like transposons: nucleotide sequence of the OXA-1 beta-lactamase gene. Proc Natl Acad Sci USA. 1987;84:7378–7382. doi: 10.1073/pnas.84.21.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peixe L V, Sousa J C, Perez D J, Baquero F. A blaTEM-1b-derived TEM-6 β-lactamase: a case of convergent evolution. Antimicrob Agents Chemother. 1997;41:1206. doi: 10.1128/aac.41.5.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prinarakis E E, Miriagou V, Tzelepi E, Gazouli M, Tzouvelekis L S. Emergence of an inhibitor-resistant β-lactamase (SHV-10) derived from an SHV-5 variant. Antimicrob Agents Chemother. 1997;41:838–840. doi: 10.1128/aac.41.4.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shannon K, Williams H, King A, Philipps I. Hyperproduction of TEM-1 beta-lactamase in clinical isolates of Escherichia coli serotype O15. FEMS Microbiol Lett. 1990;67:319–324. doi: 10.1016/0378-1097(90)90016-j. [DOI] [PubMed] [Google Scholar]
- 30.Sirot D, Chanal C, Henquell C, Labia R, Sirot J, Cluzel R. Clinical isolates of Escherichia coli producing multiple TEM mutants resistant to β-lactamase inhibitors. J Antimicrob Chemother. 1994;33:1117–1126. doi: 10.1093/jac/33.6.1117. [DOI] [PubMed] [Google Scholar]
- 31.Sirot D, Recule C, Chaibi E B, Bret L, Croize J, Chanal-Claris C, Labia R, Sirot J. A complex mutant of TEM-1 β-lactamase with mutations encountered in both IRT-4 and extended-spectrum TEM-15, produced by an Escherichia coli clinical isolate. Antimicrob Agents Chemother. 1997;41:1322–1325. doi: 10.1128/aac.41.6.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siu L K, Ho P L, Yuen K Y, Wong S S Y, Chau P Y. Transferable hyperproduction of TEM-1 β-lactamase in Shigella flexneri due to a point mutation in the Pribnow box. Antimicrob Agents Chemother. 1997;41:468–470. doi: 10.1128/aac.41.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stapleton P, Wu P J, King A, Shannon K, French G, Phillips I. Incidence and mechanisms of resistance to the combination of amoxicillin and clavulanic acid in Escherichia coli. Antimicrob Agents Chemother. 1995;39:2478–2483. doi: 10.1128/aac.39.11.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Togna A P, Shuler M L, Wilson D B. Effects of plasmid copy number and runaway plasmid replication on overproduction and excretion of β-lactamase from Escherichia coli. Biotechnol Prog. 1993;9:31–39. doi: 10.1021/bp00019a005. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X Y, Bordon F, Sirot D, Kitzis M D, Gutmann L. Emergence of clinical isolates of Escherichia coli producing TEM-1 derivatives or an OXA-1 β-lactamase conferring resistance to β-lactamase inhibitors. Antimicrob Agents Chemother. 1994;38:1085–1089. doi: 10.1128/aac.38.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]