Abstract
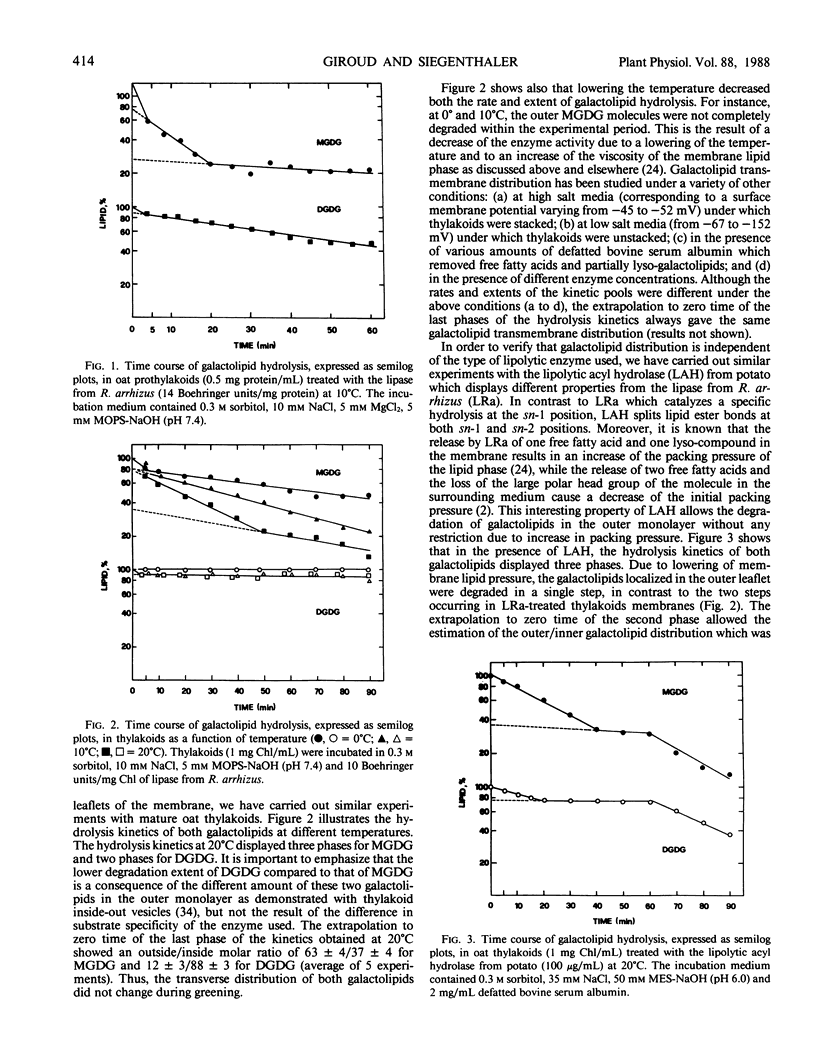
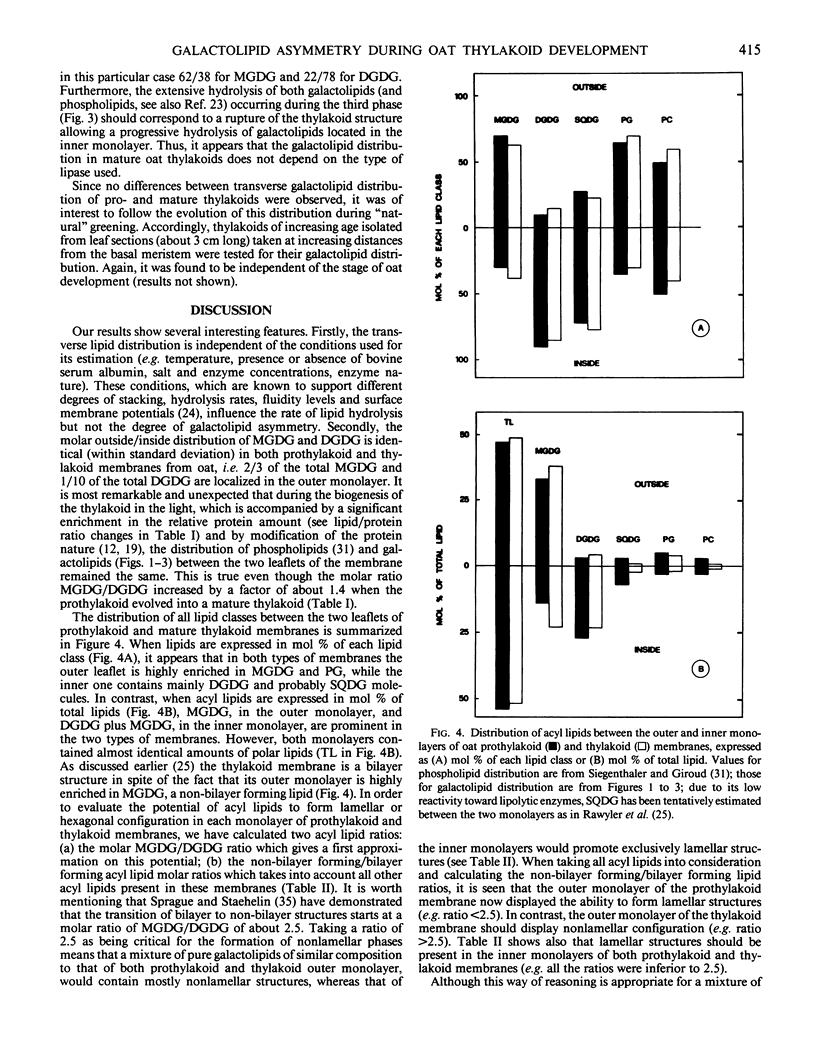
The lipase from Rhizopus arrhizus and the lipolytic acyl hydrolase from potato tubers have been used to determine the transmembrane distribution of monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG) in prothylakoids and thylakoids from oat (Avena sativa). Both galactolipids were found to be asymmetrically distributed. The molar outside/inside distribution was 70 ± 8/30 ± 8 for MGDG and 10 ± 4/90 ± 4 for DGDG in the prothylakoid membrane. Mature thylakoids presented a similar distribution, i.e. 63 ± 4/37 ± 4 for MGDG and 12 ± 3/88 ± 3 for DGDG. This distribution has been assessed under a variety of different conditions, namely (a) in media favoring thylakoid stacking or unstacking and inducing various membrane surface potentials, (b) in the presence of defatted bovine serum albumin which removed free fatty acids and partially lyso-galactolipids, (c) under various temperature conditions which resulted in different hydrolysis rates and degrees of fluidity of the membrane, and (d) in the presence of different enzyme concentrations which influenced the hydrolysis rate. The above distribution was found to be independent of the type of conditions used. Nonbilayer forming/bilayer forming lipid ratios suggest that both monolayers of the prothylakoid and the inner monolayer of oat thylakoid membranes should display lamellar structures (e.g. ratios <2.5). In contrast the outer monolayer of the thylakoid membrane should display non-lamellar configurations (e.g. ratio >2.5). Thus, it is concluded that the incorporation of chlorophyll-protein complexes into the nascent thylakoid membrane modifies neither the galactolipid nor the phospholipid transmembrane distribution. However, these complexes appear to be crucial to preserve a bilayer configuration to the greening membrane which, otherwise, would adopt nonlamellar structures. The possible origin of galactolipid transversal asymmetry which appears very early during the biogenesis of oat thylakoid membranes is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Galliard T. The enzymic deacylation of phospholipids and galactolipids in plants. Purification and properties of a lipolytic acyl-hydrolase from potato tubers. Biochem J. 1971 Feb;121(3):379–390. doi: 10.1042/bj1210379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounaris K., Barber J., Harwood J. L. The thylakoid membranes of higher plant chloroplasts. Biochem J. 1986 Jul 15;237(2):313–326. doi: 10.1042/bj2370313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths W. T. Reconstitution of chlorophyllide formation by isolated etioplast membranes. Biochem J. 1978 Sep 15;174(3):681–692. doi: 10.1042/bj1740681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBel D., Poirier G. G., Beaudoin A. R. A convenient method for the ATPase assay. Anal Biochem. 1978 Mar;85(1):86–89. doi: 10.1016/0003-2697(78)90277-4. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- Mathis J. N., Burkey K. O. Regulation of light-harvesting chlorophyll protein biosynthesis in greening seedlings : a species comparison. Plant Physiol. 1987 Dec;85(4):971–977. doi: 10.1104/pp.85.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawyler A., Siegenthaler P. A. Role of lipids in functions of photosynthetic membranes revealed by treatment with lipolytic acyl hydrolase. Eur J Biochem. 1980 Sep;110(1):179–187. doi: 10.1111/j.1432-1033.1980.tb04853.x. [DOI] [PubMed] [Google Scholar]
- Selstam E., Sandelius A. S. A Comparison between Prolamellar Bodies and Prothylakoid Membranes of Etioplasts of Dark-Grown Wheat Concerning Lipid and Polypeptide Composition. Plant Physiol. 1984 Dec;76(4):1036–1040. doi: 10.1104/pp.76.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraschi T. F., De Kruijff B., Verkleij A., Van Echteld C. J. Effect of glycophorin on lipid polymorphism. A 31P-NMR study. Biochim Biophys Acta. 1982 Feb 23;685(2):153–161. doi: 10.1016/0005-2736(82)90092-x. [DOI] [PubMed] [Google Scholar]
- Wellburn A. R., Hampp R. Appearance of photochemical function in prothylakoids during plastid development. Biochim Biophys Acta. 1979 Aug 14;547(2):380–397. doi: 10.1016/0005-2728(79)90019-7. [DOI] [PubMed] [Google Scholar]
- Wood P. M., Bendall D. S. The reduction of plastocyanin by plastoquinol-1 in the presence of chloroplasts. A dark electron transfer reaction involving components between the two photosystems. Eur J Biochem. 1976 Jan 15;61(2):337–344. doi: 10.1111/j.1432-1033.1976.tb10027.x. [DOI] [PubMed] [Google Scholar]
- Zachowski A., Fellmann P., Hervé P., Devaux P. F. Labeling of human erythrocyte membrane proteins by photoactivatable radioiodinated phosphatidylcholine and phosphatidylserine. A search for the aminophospholipid translocase. FEBS Lett. 1987 Nov 2;223(2):315–320. doi: 10.1016/0014-5793(87)80311-3. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Cullis P. R. The influence of poly(L-lysine) on phospholipid polymorphism. Evidence that electrostatic polypeptide-phospholipid interactions can modulate bilayer/non-bilayer transitions. Biochim Biophys Acta. 1980 Sep 2;601(1):235–240. doi: 10.1016/0005-2736(80)90528-3. [DOI] [PubMed] [Google Scholar]


