Abstract
The safety and pharmacokinetics of once-daily oral levofloxacin in 16 healthy male volunteers were investigated in a randomized, double-blind, placebo-controlled study. Subjects were randomly assigned to the treatment (n = 10) or placebo group (n = 6). In study period 1, 750 mg of levofloxacin or a placebo was administered orally as a single dose on day 1, followed by a washout period on days 2 and 3; dosing resumed for days 4 to 10. Following a 3-day washout period, 1 g of levofloxacin or a placebo was administered in a similar fashion in period 2. Plasma and urine levofloxacin concentrations were measured by high-pressure liquid chromatography. Pharmacokinetic parameters were estimated by model-independent methods. Levofloxacin was rapidly absorbed after single and multiple once-daily 750-mg and 1-g doses with an apparently large volume of distribution. Peak plasma levofloxacin concentration (Cmax) values were generally attained within 2 h postdose. The mean values of Cmax and area under the concentration-time curve from 0 to 24 h (AUC0–24) following a single 750-mg dose were 7.1 μg/ml and 71.3 μg · h/ml, respectively, compared to 8.6 μg/ml and 90.7 μg · h/ml, respectively, at steady state. Following the single 1-g dose, mean Cmax and AUC0–24 values were 8.9 μg/ml and 95.4 μg · h/ml, respectively; corresponding values at steady state were 11.8 μg/ml and 118 μg · h/ml. These Cmax and AUC0–24 values indicate modest and similar degrees of accumulation upon multiple dosing at the two dose levels. Values of apparent total body clearance (CL/F), apparent volume of distribution (Vss/F), half-life (t1/2), and renal clearance (CLR) were similar for the two dose levels and did not vary from single to multiple dosing. Mean steady-state values for CL/F, Vss/F, t1/2, and CLR following 750 mg of levofloxacin were 143 ml/min, 100 liters, 8.8 h, and 116 ml/min, respectively; corresponding values for the 1-g dose were 146 ml/min, 105 liters, 8.9 h, and 105 ml/min. In general, the pharmacokinetics of levofloxacin in healthy subjects following 750-mg and 1-g single and multiple once-daily oral doses appear to be consistent with those found in previous studies of healthy volunteers given 500-mg doses. Levofloxacin was well tolerated at either high dose level. The most frequently reported drug-related adverse events were nausea and headache.
Levofloxacin is a fluoroquinolone antibiotic which is the levorotatory isomer of the racemate ofloxacin. The antibacterial activity of ofloxacin is known to reside almost entirely within the l isomer, while the d and l isomers contribute equally to the toxicological profile (25). Levofloxacin has broad spectrum of in vitro activity against both gram-positive and gram-negative organisms, including Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes, Haemophilus influenzae, Moraxella catarrhalis, Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, and Pseudomonas aeruginosa (10, 14, 25). It also has in vitro activity against some anaerobes and other pathogens including Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydia pneumoniae (2, 17, 19).
The pharmacokinetics of levofloxacin following 500-mg oral and intravenous doses have been studied (4). Levofloxacin pharmacokinetics were linear and predictable for single and multiple 500-mg once-daily oral and intravenous dosing regimens, and the pharmacokinetic parameter values obtained following oral and intravenous administrations were similar. The oral bioavailability was virtually complete, with a mean absolute bioavailability of ≥99%. Levofloxacin was excreted renally, with a renal clearance of approximately 99 ml/min and a long elimination half-life of 7 h.
Data from a variety of in vitro animal and human studies have suggested that the bactericidal activity of the fluoroquinolones is rapid and concentration dependent (3, 8, 9). Preliminary findings for humans have supported the in vitro data (12, 22). Fluoroquinolones also exhibit significant postantibiotic effect against the susceptible organisms (13, 18). Thus, for the fluoroquinolones, high doses given infrequently (e.g., once daily) may be more efficacious than smaller doses given more frequently and may be less likely to result in the emergence of resistant organisms.
Levofloxacin at 500 mg once daily has been shown to be safe and efficacious for the treatment of several commonly encountered infections in adults, including community-acquired pneumonia, sinusitis, acute bacterial exacerbations of chronic bronchitis, uncomplicated skin and skin structure infections, and urinary tract infections (1, 7, 11, 20, 23). However, difficult-to-treat infections, for example, those due to more resistant organisms or those in poorly perfused tissues, may require higher doses. Before higher doses of levofloxacin can be tested in clinical trials, it is imperative that the safety and linear pharmacokinetics be confirmed. Therefore, the primary objective of this investigation was to evaluate the safety and pharmacokinetics of oral levofloxacin following single and multiple once-daily 750-mg and 1-g doses given to healthy male volunteers. These doses are 50 and 100% higher, respectively, than the usual dose (500 mg once daily) currently approved for antimicrobial treatment in the United States.
(This study was presented at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 18 September, 1996.)
MATERIALS AND METHODS
Subjects.
Healthy male subjects between the ages of 18 and 55 years were eligible for entry into the study. Subjects were qualified for the study if they had normal findings following a prestudy medical history and physical examination performed within 2 weeks of study entry. Subject eligibility was restricted to those with no evidence of significant major organ dysfunction; abnormal electrocardiogram; or clinically significant abnormal hematologic, serum chemistry, or urinalysis laboratory values. Additionally, all participants had body weights within 30% of the ideal. Key exclusion criteria were a previous history of allergy to a fluoroquinolone, alcohol or controlled-substance abuse, or use of an investigational agent within 30 days of study entry. Potential subjects were also excluded if they used any medication within 3 days prior to administration of the first study dose. All subjects signed an informed consent form approved by the institutional review board.
Study design and drug administration.
A randomized, double-blind, placebo-controlled, parallel-design study was conducted. Subjects were randomly assigned to either the levofloxacin (n = 10) or placebo (n = 6) treatment group. The levofloxacin group received 750 mg (one 500-mg tablet and two 125-mg tablets) of levofloxacin and the placebo group received identically appearing placebo tablets. All tablets were provided by The R. W. Johnson Pharmaceutical Research Institute, Raritan, N.J. A single dose of the study drug was administered to each subject on day 1; there was a washout period on days 2 and 3, followed by once-daily dosing from days 4 to 10. Following a 3-day washout period (days 11 to 13), subjects received 1 g of levofloxacin (two 500-mg tablets) or identically appearing placebo tablets according to an identical dosing scheme (days 14 to 26). Each dose was administered in the morning with 8 oz of water. Dosing on days 1, 4, 7, 10, 14, 17, 20, and 23 was completed in a fasting state that lasted from 8 h prior to dosing to 2 h postdosing. On these days, clinical laboratory tests were performed and/or blood was drawn for pharmacokinetic evaluation. Fasting on other drug administration days lasted from 2 h before dosing to 2 h after dosing. Ingestion of alcohol, caffeine, substances containing methylxanthine (e.g., chocolate), or antacids was not permitted during the study period. Subjects were confined from 12 h prior to administration of the first dose until after all final plasma and urine samples had been collected on day 24.
Sample collection.
Samples (5 ml) of venous blood for the determination of plasma levofloxacin concentrations were collected from an indwelling catheter on days 1 and 14 immediately prior to the first dose and then at the following times postdosing: 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 8.0, 12.0, 24.0, 36.0, 48.0, and 60.0 h. On days 4 to 9 and 17 to 22 of therapy, a single blood sample was obtained just prior to the morning dose. Following the last dose on days 10 and 23, blood samples were obtained immediately prior to dosing and at the following times postdosing: 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 8.0, 12.0, 24.0, 36.0, 48.0, 60.0, and 72.0 h. Blood samples were collected in heparinized tubes and centrifuged; the plasma was separated and stored at −20°C until it was assayed.
Urine samples for assessment of levofloxacin concentrations were collected beginning 8 h prior to the first dose on days 1 and 14 and at the following intervals postdosing: 0 to 2, 2 to 4, 4 to 8, 8 to 12, 12 to 24, 24 to 48, and 48 to 72 h. The volume and pH of each urine sample were recorded, and a 20-ml aliquot from each collection was frozen at −20°C until it was assayed.
Safety analysis.
Adverse events were monitored on a daily basis for the duration of the study. Each adverse event was assessed by the investigator as to severity (mild, moderate, or severe) and relationship to the study drug (definite, probable, possible, remote, unlikely). Fasting clinical laboratory tests (hematology, serum chemistry, and urinalysis) were performed in the morning on study days 1, 2, 4, 7, 11, 14, 15, 17, 20, and 24. Any abnormal laboratory result that resulted in additional testing or therapy was considered to be clinically significant and was to be reported as an adverse event. Laboratory values that were substantially outside the range of normal values (usually 10 to 20% depending on the analyte) were considered to be markedly abnormal. A complete physical examination was conducted on day 24, and results were compared to those of the predosing physical examination. Ophthalmologic examinations, including funduscopy and slit lamp, visual acuity, and color perception (Farnesworth-Hue color test) tests, were performed on study days 0, 11, 13, and 24.
Analytical procedures.
The concentration of total levofloxacin in plasma and urine was determined by a high-pressure liquid chromatography method (26). Briefly, the procedure utilized single-step liquid-liquid extraction with methyl-tert-butyl ether. A reversed-phase C18 column was used to separate levofloxacin and the internal standard (ciprofloxacin). Elution was accomplished isocratically by using a mobile phase consisting of 0.005 M copper(II) sulfate pentahydrate in 0.01 M l-isoleucine–methanol (87.5:12.5 [vol/vol]) at a flow rate of 1.0 ml/min. UV detection (330 nm) was used to measure peak area. For plasma, the range of detection was linear from 0.08 to 5.12 μg/ml; the inter- and intra-assay precision values (expressed as percent coefficient of variation) for levofloxacin were consistently below 10%, and the accuracy values were consistently within ±10% of the target. For urine, the range of detection was linear from 25 to 2,000 μg/ml; the inter- and intra-assay precision values for levofloxacin were consistently below 10%, and the accuracy values were consistently within ±10% of the target. Samples were diluted in the appropriate matrix and reassayed when concentrations were outside the range of detection.
Pharmacokinetic analysis.
Levofloxacin plasma and urine concentration-time data were analyzed by standard noncompartmental methods (15). The levofloxacin absorption rate following oral administration was assumed to be zero order and complete at the time (Tmax) to reach the peak concentration (Cmax) of the drug in plasma. Elimination was assumed to be linear and first order. This model has been successfully applied to characterize the pharmacokinetics of levofloxacin following oral administration to healthy and human immunodeficiency virus-seropositive subjects (5, 16). Estimated pharmacokinetic parameters of levofloxacin included the area under the plasma concentration-time curve from 0 to t h (AUC0–t) as measured by the trapezoidal-summation method; the AUC for the 24-h dosing interval (AUC0–24); AUC0–∞ calculated as AUC0–last + Cplast/kel, where Cplast is the last measurable plasma concentration; kel is the terminal elimination rate constant, i.e., the slope of the plasma concentration-versus-time profile at the terminal log-linear phase, as determined by ordinary least-squares regression; and AUC0–last is the AUC from 0 h to the time corresponding to Cplast; the terminal plasma elimination half-life (t1/2), calculated as 0.693/kel; the apparent total body clearance (CL/F) calculated as dose/AUC0–∞ for single dosing and as dose/AUC0–24 at steady state; apparent volume of distribution (Vss/F) calculated as CL/F · (MRT − Tmax/2), where MRT refers to the mean residence time following drug administration determined in accordance with the method of Smith and Schentag (24). The Cmax and Tmax values were estimated by visual inspection of the plasma drug concentration-versus-time data. Renal clearance (CLR) of the drug was estimated as Aet/AUC0–t, where Aet refers to the cumulative amount of levofloxacin recovered in the urine at time t. The degree of accumulation was determined from the ratios of Cmax and AUC0–24 at steady state to the single-dose values.
Statistical analysis.
Page’s test (21) was used to test for the attainment of steady state based on the trough concentrations measured during multiple dosing. Page’s test is a nonparametric test for increasing (or decreasing) trends. The null hypothesis in testing for the attainment of steady state is that the mean trough concentration values on the different days under consideration are equal, and the alternative hypothesis is that there is an increasing trend in the mean values over the days under consideration. Steady state is concluded if the null hypothesis is not rejected at a 5% level of significance.
RESULTS
Patient population.
The characteristics of the treatment groups receiving levofloxacin or the placebo were comparable. Sixteen healthy male subjects ranging in age from 19 to 51 years were enrolled (mean ages ± standard deviations [SD], 26.3 ± 6.0 and 35.2 ± 10.5 years for levofloxacin and placebo groups, respectively). Weights ranged from 62 to 105 kg (mean weights ± SD of 78 ± 14 kg and 80 ± 14 kg for levofloxacin and placebo groups, respectively). Fifteen of the 16 subjects completed the study. One subject in the placebo group was prematurely discontinued from the study because of deterioration of color perception and photopsia. All 16 subjects were included in the safety analysis; pharmacokinetic analysis was limited to the 10 levofloxacin group participants. No subject required a dose reduction or concomitant therapy during the study period.
Pharmacokinetics.
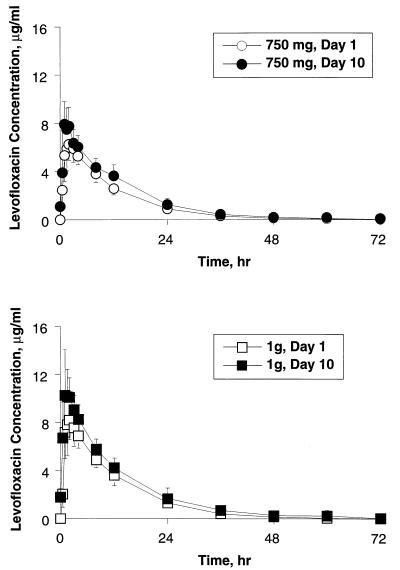
The mean levofloxacin plasma concentration-versus-time profiles for the single and multiple once-daily doses are illustrated in Fig. 1. The test of Page (21) suggested that steady state was attained 24 h from the start of multiple once-daily dosing for both the 750-mg and the 1-g doses. Levofloxacin was rapidly absorbed after single and multiple once-daily 750-mg and 1-g doses, with an apparently large volume of distribution. Mean Cmax and AUC0–24 values ± SD following a single 750-mg dose were 7.13 ± 1.44 μg/ml and 71.3 ± 10.3 μg · h/ml, respectively, compared to 8.60 ± 1.86 μg/ml and 90.7 ± 17.6 μg · h/ml at steady state (Table 1). Following the single 1-g dose, mean Cmax and AUC0–24 values ± SD were 8.85 ± 1.86 μg/ml and 95.4 ± 16.0 μg · h/ml, respectively; corresponding values at steady state were 11.8 ± 2.52 μg/ml and 118 ± 18.9 μg · h/ml. The mean ratios of the values of Cmax and AUC0–24 at steady state to the corresponding single-dose values ± SD were 1.22 ± 0.25 and 1.27 ± 0.11, respectively, for the 750-mg dose; and 1.34 ± 0.16 and 1.24 ± 0.06, respectively, for the 1-g dose. These indicate modest and similar degrees of accumulation of levofloxacin for the two dose levels upon multiple dosing.
FIG. 1.
Mean plasma levofloxacin concentration-versus-time profiles following single and multiple once-daily 750-mg and 1-g oral doses of levofloxacin for 10 healthy subjects. Error bars indicate SD.
TABLE 1.
Summary of levofloxacin pharmacokinetic parameter estimates for 10 healthy subjectsa
| Levofloxacin dose and day | Cmax (μg/ml) | Tmax (h) | AUCb (μg · h/ml) | CL/F
|
Vss/F
|
t1/2 (h) | Cumaxc (μg/ml) | Aed (% dose) | CLR
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ml/min | ml/min/kg | Liters | Liters/kg | ml/min | ml/min/kg | |||||||
| 750 mg | ||||||||||||
| Day 1 (single dose) | 7.13 ± 1.44 | 1.9 ± 0.7 | 82 ± 14 | 157 ± 28 | 2.06 ± 0.42 | 90 ± 14 | 1.17 ± 0.10 | 7.7 ± 1.3 | 403 ± 249 | 75 ± 6 | 118 ± 28 | 1.55 ± 0.36 |
| Day 10 (steady state) | 8.60 ± 1.86 | 1.4 ± 0.5 | 91 ± 18 | 143 ± 29 | 1.88 ± 0.43 | 100 ± 16 | 1.29 ± 0.13 | 8.8 ± 1.5 | 822 ± 437 | 79 ± 5 | 116 ± 28 | 1.52 ± 0.39 |
| 1 g | ||||||||||||
| Day 14 (single dose) | 8.85 ± 1.86 | 1.7 ± 0.4 | 111 ± 21 | 156 ± 34 | 2.05 ± 0.49 | 96 ± 22 | 1.24 ± 0.15 | 7.9 ± 1.5 | 667 ± 286 | 73 ± 8 | 113 ± 2.6 | 1.48 ± 0.36 |
| Day 23 (steady state) | 11.8 ± 2.52 | 1.7 ± 0.6 | 118 ± 19 | 146 ± 29 | 1.91 ± 0.40 | 105 ± 27 | 1.36 ± 0.17 | 8.9 ± 2.5 | 992 ± 377 | 71 ± 5 | 106 ± 23 | 1.38 ± 0.31 |
Values are means ± SD.
The AUC is the AUC0–∞ for single doses (days 1 and 14) and the AUC0–24 at steady state (days 10 and 23).
Cumax, peak urinary levofloxacin concentration.
Ae, cumulative amount of levofloxacin recovered from urine from 0 to 72 h for single doses (days 1 and 14) and from 0 to 24 h at steady state (days 10 and 23).
Apparent CL/F, Vss/F, and t1/2 values for the two doses were similar and did not vary between single and multiple dosing (Table 1). In addition, the pharmacokinetics of levofloxacin were consistent at the two dose levels based on the mean ratio of the 1-g to 750-mg disposition parameters (Table 2).
TABLE 2.
Ratios of the levofloxacin pharmacokinetic parameter estimates (1-g dose versus 750-mg dose)
| Dose condition | Ratio of indicated parameter value for 1-g dose to value for 750-mg dosea
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cmax | Tmax | AUC | CL/F | Vss/F | t1/2 | Cumax | Ae | CLR | |
| Single dose | 1.25 ± 0.21 | 0.95 ± 0.40 | 1.35 ± 0.14 | 0.99 ± 0.10 | 1.06 ± 0.11 | 1.02 ± 0.06 | 2.02 ± 1.12 | 0.98 ± 0.12 | 0.97 ± 0.15 |
| Steady state | 1.40 ± 0.27 | 1.28 ± 0.47 | 1.31 ± 0.16 | 1.03 ± 0.12 | 1.05 ± 0.19 | 1.00 ± 0.16 | 1.5 ± 0.96 | 0.91 ± 0.06 | 0.92 ± 0.09 |
For definitions of AUC, Cumax, and Ae, see the footnotes to Table 1. Values are means ± SD.
Elimination of levofloxacin by renal routes is summarized in Table 1. Mean renal clearance values were found to be not significantly different when day 1 and steady-state values at either dose level were compared. Renal clearance accounted for approximately 70 to 80% of apparent total plasma clearance. Mean ratios of the 1-g to 750-mg renal elimination parameters were nearly identical following both single and multiple dosing (Table 2).
Safety.
Of the 10 subjects receiving levofloxacin, 3 subjects reported adverse events considered to be related to levofloxacin after receiving the 750-mg dose and 4 subjects reported drug-related adverse events following the 1-g dose. Most of the adverse events were considered mild and were resolved spontaneously without therapeutic intervention or drug discontinuation. Of the six subjects in the placebo group, three reported adverse events considered to be drug related. The most frequently reported adverse events (regardless of relationship to the study drug) were nausea (one event, 750-mg levofloxacin dose; one event, placebo group; three events, 1-g levofloxacin dose) and headache (two events, 750-mg levofloxacin dose; two events, placebo group; one event, 1-g levofloxacin dose). The overall distribution and frequencies of these and other adverse events among the placebo-treated subjects and the levofloxacin-treated subjects were comparable at both dose levels. No clinically meaningful alterations in laboratory parameters, vital signs, and repeat physical examination (including ophthalmologic evaluation) results from baseline measurements were recognized for either treatment group over the course of the study. No markedly abnormal laboratory values were noted. In general, single- and multiple-dose administration of 750 mg and 1 g of levofloxacin to healthy subjects was found to be safe and well tolerated.
One placebo-treated subject, a 45-year-old Caucasian male without significant medical history, was prematurely discontinued from the study due to an adverse event.
DISCUSSION
This is the first study to report the safety and pharmacokinetic disposition of levofloxacin following multiple once-daily 750-mg and 1-g doses given to healthy male subjects. The pharmacokinetics of oral levofloxacin at both dose levels, following single and multiple once-daily doses, were found to be similar based on comparable values of apparent clearance and volume of distribution, estimates of elimination half-life, and levels of urinary excretion of unchanged drug. Expected dose-related differences in mean steady-state Cmax and AUC values between the two dose levels were observed. The pharmacokinetics of levofloxacin following the 750-mg and 1-g doses were also similar to those obtained in a previous study with 500-mg levofloxacin doses (4), showing that levofloxacin exhibits linear pharmacokinetics over this dose range (500 mg to 1 g).
This study also demonstrated that high doses of levofloxacin are safe. The incidence of adverse events following multiple 750-mg and 1-g levofloxacin doses was nearly identical to that reported during placebo administration. In addition, the adverse events reported in this study, nausea and headache, have been reported in clinical studies utilizing the 500-mg daily dose of levofloxacin. Thus, there were no apparent dose-related adverse effects seen in this study.
Although not noted in clinical trials with levofloxacin (500 mg once daily), visual disturbances, including blurred vision, dimmed vision, disturbed vision, diplopia, change in color perception, overbrightness of lights, flashing lights, decreased visual activity, photophobia, eye pain, visual loss, constriction of visual fields, and cataracts, have resulted from trials of other fluoroquinolones (6). Therefore, a thorough ophthalmologic examination was performed for subjects treated with high doses of levofloxacin. No evidence of treatment-emergent visual disturbances were found.
In conclusion, since fluoroquinolones are concentration dependent in their kill rates, it is expected that administration of levofloxacin once daily would result in better efficacy than administering the same total daily dose in a divided fashion. The safety and pharmacokinetic findings of this study support further clinical evaluation of these high once-daily doses of levofloxacin for difficult-to-treat infections.
REFERENCES
- 1.Adelglass J, DeAbate C A, McElvaine P, Fowler C L. A comparison of levofloxacin and amoxicillin clavulanate for the treatment of acute bacterial sinusitis. Presented at the Infectious Diseases Society of America 34th Annual Meeting, New Orleans, La., 18 to 20 September 1996. 1996. [Google Scholar]
- 2.Baltch A L, Smith R P, Ritz W. Inhibitory and bactericidal activities of levofloxacin, ofloxacin, erythromycin, and rifampin used singly and in combination against Legionella pneumophila. Antimicrob Agents Chemother. 1995;39:1661–1666. doi: 10.1128/aac.39.8.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser J, Stone B B, Groner M C, Zinner S H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987;31:1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien S-C, Rogge M C, Gisclon L G, Curtin C, Wong F, Natarajan J, Williams R R, Fowler C L, Cheung W K, Chow A T. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob Agents Chemother. 1997;41:2256–2260. doi: 10.1128/aac.41.10.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Child J, Mortiboy D, Andrews J M, Chow A T, Wise R. Open-label crossover study to determine pharmacokinetics and penetration of two dose regimens of levofloxacin into inflammatory fluid. Antimicrob Agents Chemother. 1995;39:2749–2751. doi: 10.1128/aac.39.12.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christ, W., and B. Esch. 1994. Adverse reactions to fluoroquinolones in adults and children. Infect. Dis. Clin. Pract. 3(Suppl. 3):S168–S176.
- 7.DeAbate C A, Russell M, McElvaine P, Faris H, Upchurch J, Fowler C L, Polak E M, Morgan N S. Safety and efficacy of oral levofloxacin versus cefuroxime axetil in acute bacterial exacerbation of chronic bronchitis. Respir Care. 1997;42:206–213. [Google Scholar]
- 8.Drusano G L, Johnson D E, Rosen M, Standiford H C. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob Agents Chemother. 1993;37:483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley, M. N., H. D. Mandler, D. Gilbert, J. Ericson, K. H. Mayer, and S. H. Zinner. 1987. Pharmacokinetics and pharmacodynamics of intravenous ciprofloxacin. Am. J. Med. 82(Suppl. 4A):363–368. [PubMed]
- 10.Eliopoulos G M, Wennersten C B, Moellering R C., Jr Comparative in vitro activity of levofloxacin and ofloxacin against gram-positive bacteria. Diagn Microbiol Infect Dis. 1996;25:35–41. doi: 10.1016/0732-8893(96)00069-7. [DOI] [PubMed] [Google Scholar]
- 11.File T M, Jr, Segreti J, Dunbar L, Player R, Williams R R, Kojak C, Rubin A. A multicenter, randomized study comparing the efficacy and safety of intravenous and/or oral levofloxacin versus ceftriaxone and/or cefuroxime axetil in treatment of adults with community-acquired pneumonia. Antimicrob Agents Chemother. 1997;41:1965–1972. doi: 10.1128/aac.41.9.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu K P, Foleno B, Rosenthale M E. The postantibiotic suppressive effect of l-ofloxacin, an optically active isomer of ofloxacin. Diagn Microbiol Infect Dis. 1992;15:375–378. doi: 10.1016/0732-8893(92)90028-r. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto T, Mitsuhashi S. In vitro antibacterial activity of DR-3355, the S-(−) isomer of ofloxacin. Chemotherapy. 1990;36:268–276. doi: 10.1159/000238777. [DOI] [PubMed] [Google Scholar]
- 15.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 16.Goodwin D S, Gallis H A, Chow A T, Wong F A, Flor S C, Bartlett J A. Pharmacokinetics and safety of levofloxacin in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1994;38:799–804. doi: 10.1128/aac.38.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammerschlag M R, Wumei K K, Roblin P M. In vitro activities of azithromycin, clarithromycin, levofloxacin, and other antibiotics against Chlamydia pneumoniae. Antimicrob Agents Chemother. 1992;36:1573–1574. doi: 10.1128/aac.36.7.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald P J, Pruul H. Quinolones and the immune system. In: Siporin C, et al., editors. The new generation of quinolones. New York, N.Y: Marcel Dekker; 1990. pp. 107–122. [Google Scholar]
- 19.Molitoris E, Wexler H M, Finegold S M. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro activity of levofloxacin against bacteria from skin and soft tissue infections, abstr. E88; p. 97. [Google Scholar]
- 20.Nicols R L, Smith J W, Gentry L O, Gezon J, Campbell T, Sokol P, Williams R R. Multicenter, randomized study comparing levofloxacin and ciprofloxacin for uncomplicated skin and skin structure infections. South Med J. 1997;90:1193–1200. doi: 10.1097/00007611-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Page E B. Ordered hypotheses for multiple treatments: a significance test for linear ranks. J Am Stat Assoc. 1963;58:216–230. [Google Scholar]
- 22.Peloquin C A, Cumbo T J, Nix D E, Sands M F, Schentag J J. Intravenous ciprofloxacin in patients with nosocomial lower respiratory tract infections: impact of plasma concentration, organism MIC, and clinical condition on bacterial eradication. Arch Intern Med. 1989;149:2269–2273. [PubMed] [Google Scholar]
- 23.Richard G A, Klimberg I N, Fowler C, Callery-D’Amico S. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. A combined analysis of two studies comparing levofloxacin (LVFX) with two other fluoroquinolones for the treatment of acute pyelonephritis (pyelo), abstr. LM3; p. 281. [Google Scholar]
- 24.Smith I L, Schentag J J. Noncompartmental determination of the steady-state volume of distribution during multiple dosing. J Pharm Sci. 1984;73:281–282. doi: 10.1002/jps.2600730239. [DOI] [PubMed] [Google Scholar]
- 25.Une T, Fujimoto T, Sato K, Osada Y. In vitro activity of DR-3355, an optically active ofloxacin. Antimicrob Agents Chemother. 1988;32:1336–1340. doi: 10.1128/aac.32.9.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong F A, Juzwin S J, Flor S C. Rapid stereospecific high-performance liquid chromatographic determination of levofloxacin in human plasma and urine. J Pharm Biomed Anal. 1997;15:765–771. doi: 10.1016/s0731-7085(96)01890-0. [DOI] [PubMed] [Google Scholar]