Graphical abstract

Keywords: Celery dietary fiber, Celery flavonoids, Non-group feeding cages, Colitis, Gut microbiota
Highlights
-
•
Kale and red chicory significantly relieved DSS-induced colitis of mice.
-
•
A slight remission of colitis by celery was associated with the antagonistic effects of CSDF and CF.
-
•
CSDF and CF significantly relieved DSS-induced colitis of mice.
-
•
CSDF can inhibit CF-induced Akkermansia to weaken the colitis relieving effect of CF.
-
•
Non-group feeding cages for mice were designed in this study.
Abstract
Introduction
Dietary fiber and flavonoids are promising drugs reported in the treatment of inflammatory bowel disease (IBD). However, it is unclear the interaction between dietary fiber and flavonoids in gut health.
Objective
The therapeutic effect of celery, kale, and red chicory powders on colitis mice using non-group feeding cages was investigated. Further, the efficacy of whole celery, celery soluble dietary fiber (CSDF), celery insoluble dietary fiber (CIDF), celery flavonoids (CF), CSDF + CF and CIDF + CF in IBD mice model was assessed to dissect protective effect to attribute to which component(s) in such complex matrix.
Methods
3% Dextran sulfate sodium salt (DSS) was used to induce mice colitis model. Multiple molecular biological methods were employed to evaluate the severity of mice colitis and the gut microbial composition of mice.
Results
Administration of kale and red chicory significantly restored body weight, DAI score, and colon length in colonic mice, and celery showed the weakest effects. Administration of either CSDF or CF markedly improved the histological damage, increased colonic mucus expression, and reduced colonic MPO/iNOS activities, and IL-6/IL-1β levels. However, CSDF + CF showed weaker improvement than CF or SDF in most physical and biochemical signs. Furthermore, CSDF and CF decreased intestinal g_Escherichia-Shihella and g_Clostridium_sensu_stricto_1 induced by DSS administration. Interestingly, celery flavonoid promoted g_Akkermansia proliferation both in vivo and in vitro, and which can be inhibited by CSDF.
Conclusions
This study revealed for the first time that CSDF can suppress the protective effect of CF on intestinal health by inhibiting g_Akkermansia, and clarified that the decreased efficacy of celery whole food on colitis was mediated by an antagonism between CSDF and CF. Moreover, this study presents for the first time that interaction between soluble dietary fiber and flavonoids in vivo can ameliorate the efficacy of dietary fiber or flavonoids when administered alone suggestive for an antagonistic effect.
Introduction
Inflammatory bowel disease (IBD), mainly divided into ulcerative colitis (UC) and Crohn's disease (CD), is a chronic and recurrent intestinal inflammatory disease with high risks of developing colon cancer [1]. According to statistics, millions of people are suffering from IBD worldwide, especially in America and northern Europe [2]. Although the etiology of IBD is not completely eliminated, studies have found that heredity, immune and environment are key factors in the predisposal to colitis [3]. Moreover, diet is a crucial environmental factor for patients with colitis. Diets with high fat, high sugar, high protein, and low fiber have been proposed to increase the risk of IBD [4]. Therefore, changing dietary habits is regarded as an effective measure to manage colitis symptoms [5].
IBD is associated with the impairment of colonic intestinal barrier function, and the production of inflammatory cytokines by colonic infiltrating inflammatory cells [6]. Weight loss and malnutrition may be developed due to inflammation, impaired nutrient absorption, and nutrient loss from damaged tissues of IBD patients [7]. Moreover, recent studies have shown that gut microbiota dysbiosis is an important pathogenic factor in IBD [8]. Clinical and experimental data have demonstrated changes in gut microbiota, with observed expansion of inflammation associated bacteria, such as Enterobacteriaceae [9]. Recently, dietary fiber and flavonoids has been shown to reduce inflammation and improve gut tight junction protein integrity [10], [11]. Meanwhile, they can also improve the composition of intestinal flora, inhibit harmful bacteria proliferation and promote the growth of probiotics, such as Bifidobacterium and Akkermansia, which contribute to the treatment of IBD.
Vegetables contain many functional nutrients beneficial to intestinal health, especially dietary fiber and flavonoids, which are considered to be beneficial to reduce the risk of colitis [12]. Although some reports showed the non chemical adsorption between dietary fiber and flavonoids, few studies reported the interaction of dietary fiber and flavonoids on intestinal health in vivo. Therefore, we aimed to explore the effects of three vegetables (celery, kale, and red chicory) rich in dietary fiber and flavonoids on IBD [13], and further compare the effects of celery soluble dietary fiber (CSDF), celery insoluble dietary fiber (CIDF), celery flavonoids (CF) and dietary fiber-flavonoids (CSDF + CF or CIDF + CF) mixture for colitis prevention. The results of this study provided a new paradigm of the potential adverse effects of dietary fiber-flavonoids interaction in the context of colitis for the first time, and support the future dietary choices and regimens design.
Materials and methods
Materials and reagents
Celery (Apium graveolens Linn.) was produced in Weifang City, Shandong Province. Red chicory aerial part (Cichorium intybus L.) was produced in Chengde, Hebei province. Kale (Brassica oleraceae L. var. acephala) was cultivated in Qingdao, Shandong province. Reference standards of apigenin 7-O-apiosylglucoside (Apiin, purity > 98 %), chrysoeriol 7-O-apiosylglucoside (CA, purity > 98 %), and luteolin 7-O-apiosylglucoside (LA, purity > 98 %), were purchased from Yongjian Pharmaceutial (Taizhou, China). Dextran sulfate sodium salt (DSS) (MW 40–50 kD) was purchased from MP Biomedicals (Santa Ana, California). Amyloglucosidase from Aspergillus niger and heat-stable α-amylase were purchased from Aladdin reagent (Shanghai, China). Protease was purchased from Pangbo Bioen-gineering (Nanning, China). AIN93G diet were customized from FBSH Biotechnology Co.,ltd (Shanghai, China). anti-ZO-1 and anti-Occludin antibodies were obtained from Abcam (Shanghai, China).
Animals
Male C57BL/6J mice were used as experimental animals in this study. Mice were purchased from Vital River Laboratories (Beijing, China). Animal feeding conditions: room temperature was 25 ± 0.5 °C, relative humidity was 50 ± 5 %, and light/dark cycle was 12 h/12 h. Mice were adapted for one week before experiments.
Ethics statement
All procedures involving animals were performed according to the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institute of Health (NIH, Publication No. 85–23, 1996) and approved by the Animal Care Review Committee (Animal application approval number 0064257) at Nanchang University, China.
Non-group feeding cages
Mice have the habit of eating feces, and the intestinal flora of mice will be affected by other individuals in the same cage [14]. In this study, all animals were raised in non-group feeding cages to prevent mice from being affected by the same cage effect (Fg. S1A). The cage was modified based on M1 type mouse cage (290 × 178 × 160 mm). A partition board (Fg. S1B) was added to divide the cage into two independent spaces. Each mouse was raised and given independent feed and water.Fig. 2
Fig. 2.
The flowchart of celery dietary fiber and flavonoids purification.
Experimental design of the three vegetables effects on DSS-induced colitis mice
Mice were randomly assigned into 5 groups (n = 8), including control group, DSS group, celery group (2 %), kale group (4.5 %), and red chicory group (3 %). The normal control group and DSS group were given normal AIN93G diet throughout the experiment, and other groups were given customized diets containing vegetable powder adjusted based on AIN93G diet (Tab. S1). Fresh diets were changed every-two days. From day 7th to day 14th, the DSS group and vegetable treatment groups were given 3 % DSS sterile water. Control group was given sterile water during the 16 days experiment.
The equation (1) was used for calculating vegetables powder content in customized mice diets.
| (1) |
PV represent vegetables powder content in customized mice diets (%). PM represents moisture content of fresh vegetables (%). The PM of celery, kale, and red chicory are 95.47 ± 0.05 %, 89.6 ± 0.01 %, and 93.6 ± 0.04 %, respectively. With reference to the nutritional components of vegetables in the database (celery [15], kale [15], and chicory [16]), the contents of protein, dietary fiber, fat, vitamins and minerals in the feed were adjusted appropriately. It is estimated that 300 g, 60 kg and 12.3 are the fresh weight of daily intake of vegetables for human (g), body weight of human (kg) and equivalent surface area dosage conversion factors between human and mouse [17]. 20 g represent body weight of mice, and 3 g represent feed intake of mice every day. In summary, the vegetables’ dose for mice is approximately equivalent to 300 g/day fresh vegetables for 60 kg adult humans [17].
Evaluation of the disease activity index (DAI)
After giving DSS water, each mouse was evaluated for several traits [18]: the scores of stool consistency (0, normal; 2, soft; and 4, diarrhea), the scores of hematochezia (0, none; 2, blood; and 4, abundant bleeding) and the scores of body weight loss (0, < 1 %; 1, = 1–5 %; 2, = 5–10 %; 3, = 10–20 %; 4, > 20 %). DAI score for each animal is the average of these three indicators scores.
Extraction and purification of CF
The extraction and purification of CF were performed following the protocol described [19], with some modifications. Briefly, chopped fresh celery was oven dried at 50 °C, and pulverized. Celery total flavonoids were extracted using 80 % ethanol (w/v = 1/20) at 50 °C for 1 h, centrifuged, and supernatant was obtained. The extraction was performed four times. The marc left after flavonoid extraction was further used for the extraction of dietary fiber. After concentration and centrifugation, the supernatant was collected for further flavonoids purification. 100 mL Celery flavonoid extract was loaded in a 100 mL polyamide resin (80–100 mesh) column at 2 BV/ h, washed by 3 BV distilled water, and eluted with 3 BV 40 % ethanol at 2 BV/h. After concentration of eluted ethanol and lyophilization, purified CF was obtained.
Quantitative detection of flavonoids in CF and its content in celery powder
The method of celery flavonoid detection was performed following the protocol described [20], with some modifications. The purified CF is dissolved in 80 % methanol and filtered by 0.22 μm organic filter membrane for detection. The flavonoid in celery powder was extracted using 80 % methanol (25 mL/ 50 mg celery powder) by ultrasonic for 1 h, centrifuged, and supernatant was was filtered by 0.22 μm organic filter membrane for further analysis using UPLC-MS.
Samples were detected using a UPLC-QQQ-MS/MS system (Agilent Technologies, American) equipped with a pump with a vacuum degasser (1290 Bin Pump VL), thermostated column compartment (1290 TCC), auto-sampler (1290 Sampler), a 6460 QQQ-MS/MS with ESI source, and a ZORBAX Eclipse Plus C18 column (4.6 mm × 100 mm, 3.5 µm). The mobile phase used consisted of solvent A (99.9 % water and 0.1 % formic acid) and solvent B (100 % methanol). The gradient elution of solvent B was performed as follows: 5–30 % (0–5 min), 30–40 % (5–11 min), 40–45 % (11–18 min), 45–50 % (18–23 min), 50–100 % (23–24 min), 100–100 % (24–26 min), 100–5 % (26–27 min) and 5–5 % (27–30 min), and column temperature was set at 35 °C. The seperation was performed using 2 µL initial injected volume and 0.3 mL/min flow rate. The identification and quantification of flavonoid were operated using electrospray ion source (ESI) positive ion and multiple reaction monitoring (MRM) detection method. Standards including apiin, CA and LA were used to build calibration curve, and the MRM parameters were list in Tab. S1.
Dietary fiber preparation
Dietary fiber was extracted by the methods in the references and modified appropriately [21]. In short, the celery residue is enzymatically hydrolyzed in 95 °C hot water containing 0.5 % (w/v) heat-stable α-amylase for 1 h, in 60 °C hot water containing 0.5 % (w/v) protease for 1 h, and in 60 °C hot water containing 0.5 % (w/v) starch glucosidase (pH 4.5) for 1 h, respectively. All enzymes were inactivated at 100 °C for 15 min. After centrifuged, to obtain CIDF, hot water and 95 % ethanol were used to wash the precipitate. 80 % alcohol was used to precipitate the supernatant, and 8000–10000 Da dialysis membranes was used to dialyzed in distilled water for 2 days. After concentration and lyophilization, CSDF was obtained.
Chemical composition
The content of neutral sugar was detected by phenol sulfuric acid method [22]. The content of uronic acid was determined by sulfate carbazole method [23]. The official methods were used to detected the contents of protein and ash [24]. A total starch detection kit (Megazyme, Ireland) was used to determine the starch content.
Molecular weight distribution
The molecular weight (Mw) of CSDF was determined by a liquid chromatography system [25]. It includes Waters e2695 system collocated with 2414 RI refractive index detector and Waters UltrahydrogelTM Linear (7.8 mm × 300 mm) column (Milford, USA). The mobile phase was 0.02 % NaN3 and flow rate was set to 0.6 mL/min. The standard curve were established with standards of T-10, T-40, T-70, T-500 and glucose.
Fourier transform infrared spectrum
Dried potassium bromide was mixed with dried dietary fiber and prepared into a transparent sheet. Then, using a Nicolet 5700 fourier transform infrared spectrophotometer (FT-IR) (Thermo Electron, USA) acquire the signal with wavenumbers from 4000 to 400 cm.
Monosaccharides composition
The methods of acid hydrolysis and monosaccharides determination were performed following the protocol previously described [26]. In brief, samples were dissolved in 12 M H2SO4 (5 mg/0.5 mL) in the condition of ice-bath for 0.5 h, respectively. 2.5 mL distilled water was added to solution, and samples were treated with 100 °C oil-bath for 2 h. The samples were diluted, filtered and tested with a Dionex ICS-5000 ion chromatography (Dionex, USA). Dionex CarboPac™ PA20 column (3 mm × 150 mm) and guard column (3 mm × 30 mm) were used for sample separation. 1 M CH3COONa, 250 mM NaOH and H2O were used as the mobile phase. The standard curve were established with standards of rhamnose (Rha), fucose (Fuc), galactose (Gal), arabinose (Ara), xylose (Xyl), glucose (Glc), fructose (Fru), mannose (Man), galacturonic acid (GalA) and glucuronic acid (GlcA).
13C NMR analysis
CP/MAS 13C NMR spectra were measured on a Bruker Avance 400 M spectrometer (Bruker, Germany) at 100.61 MHz. Samples were pulverized, loaded into clean rotors, and placed in a catheter on the upper part of the magnet. A CP/MAS mode was used for sampling. The sampling temperature was set at room temperature and the grinding angle rotation frequency was at 12 MHz. The contact time was 2.0 ms. Acquisition time was 0.0127 s. The cycling delay was set at 4 s. The number of scans was 1500.
Experimental design of celery dietary fiber and flavonoids effects on DSS-induced colitis mice
Mice were randomly assigned into 8 groups (n = 10), including Control group, DSS group, Whole celery group (2 %), CSDF group (0.052 %), CF group (0.004 %), CIDF group (0.64 %), CSDF + CF group (0.052 % CSDF and 0.004 % CF), and CIDF + CF group (0.64 % CIDF and 0.004 % CF). In this experiment, dietary fiber and flavonoid were added to diet, which is obviously not applicable to common drugs. For this reason, drug control group was not set.
Control group and DSS group mice were given normal AIN93G diet, whereas other treatment groups were administered adjusted AIN93G diet (Tab. S3). From the 7th day till the 13th day, 3 % DSS water was given to mice except for Control group. Normal water was given to Control group mice(Fig. 3A). Based on equivalent surface area dosage conversion factors, the vegetable intake of a mouse is equivalent to a daily intake of 300 g of fresh vegetables for a 60 kg adult [17]. Celery dietary fiber or flavonoid content in adjusted AIN93G diets was calculated using the equation (2).
| (2) |
Fig. 3.
The effects of celery dietary fiber and flavonoids on basic physiological indicators of DSS-induced colitis. (A) animal experiment design; (B) Body weight change after DSS on the last day; (C) DAI score on the last day; (D-E) Colon image and colon length. CSDF, celery soluble dietary fiber; CF, celery flavonoids; CIDF, celery insoluble dietary fiber; CSDF + CF, celery soluble dietary fiber plus celery flavonoids; CIDF + CF, celery insoluble dietary fiber plus celery flavonoids. All values expressed as mean ± SEM (n = 10). *, p < 0.05; **, p < 0.01; ***, p < 0.001. Data with no identical letter denotes a significant difference.
PD is celery dietary fiber or flavonoid content in adjusted AIN93G diets. 2 % represents celery powder content in adjusted AIN93G diets. PC is the detected content of celery dietary fiber or flavonoid in celery powder.
Analysis of intestinal permeability
Mice received intragastric administration of FITC-dextran (MW 4kd, Sigma-Aldrich) to trace intestinal permeability at 500 mg/kg body weight. Serum was collected after 4 h gavage, and FITC-dextran (4 kDa) was detected by a multifunctional spectrophotometer (Varioskan, Thermo Fisher Scientific Co., American).
H&E staining and histological damage score
Colon sections were stained with hematoxylin and eosin (H&E). The H&E staining results pictures were obtained by a camera (Nikon Co., Japan) and a Zeiss Primo Vert microscope (Carl Zeiss Suzhou Co., ltd). Three scoring criteria of Wlodarska et al. were referred to score the histological damage [27]: crypt damage (0 = normal, 3 = severe crypt damage), inflammation (0 = normal, 3 = severe inflammatory infiltrate), muscle thickening (0 = no muscle thickening present, 3 = significant muscle thickening present). Histological damage score for each animal is the sum of these three scores.
Measurement of mucus expression area
Alcian blue/periodic acid-Schiff (AB-PAS) method was used to stain the mucus expression area of colon tissues [28]. The AB-PAS staining results pictures were obtained by a camera (Nikon Co., Japan) and a Zeiss Primo Vert microscope (Carl Zeiss Suzhou Co., ltd).
Image pro plus software 6.0 was used to measure the mucus expression area. The mucus expression area was calculated using the equation (3).
| (3) |
Immunofluorescence
Paraffin-embedded tissues Carnoys-fixed were dewaxed and rehydrated[29]. The tissue antigen was repaired by microwave. Immunostaining was carried out with anti-ZO-1 and anti-Occludin primary antibodies followed by the incubation with a Alexa Fluor 568-conjugated or FITC-conjugated secondary antibody, and 4′,6-diamidino-2-phenylindole (DAPI) were used for DNA counter-stain. Images were acquired using an inverted fluorescence microscope (Nikon Eclipse Ti-SR, Nikon Co., Japan).
ELISA analysis
100 mg Colon tissue was mixed with 1 mL PBS and then homogenized using a KZ-II high speed tissue grinder (Servicebio Co., Wuhan, China). The tissue homogenate was centrifuged at 3,000 rpm/min for 20 min, and the supernatant was obtained for ELISA detection. The concentrations of IL-6 and IL-1β and the activity levels of MPO and iNOS inside the colon tissue were measured using commercial ELISA kits (Nanjing SenBeiJia Biological Technology Co., Nanjing, China). The data were normalized to protein content measured by a BCA protein assay kit (Beyotime Biotechnology Co., Shanghai, China).
Fecal microbiome composition analysis
A DNA extraction kit (TianGen DNA Stool Kit, TianGen Biotech Co., ltd., China) was used to extracted feces microbial DNA. The V4 hypervariable region of bacterial 16S rDNA gene was amplified using primers 515F 5′-GTGCCAGCMGCCGCGGTAA-3′ and 806R 5′-GGACTACHVGGGTWTCTAAT-3′. The amplified DNA was recovered using agarose gel electrophoresis. DNA libraries of the V4 hypervariable region were constructed. A MiSeq platform (Illumina Co., American) was used to sequence paired-end (2 × 250 bp) amplicon.
In vitro faecal fermentation experiments
C57BL/6J mice feces were collected, and added to the sterilized BHI medium (without glucose) according to 5 mL/g. After homogenization, it was filtered with a 40 µm filter screen. 1 % bacterial solution was added into BHI medium (without glucose). Then add different doses of apiin (10 μM and 100 μM) and CSDF (3 mg/mL). After incubation at 37 °C in an anaerobic incubator for 48 h, the bacterial fluid was collected. Akkermansia muciniphila (A. muciniphila) and all bacteria (All) were quantified by quantitative real-time PCR using specific primers, A. muciniphila: F: 5′-CCTTGCGGTTGGCTTCAGAT-3′, R: 5′-CAGCACGTGAAGGTGGGGAC-3′[30], and All: F: 5′-ACTCCTACGGGAGGCAGCAG-3′, R: 5′-ATTACCGCGGCTGCTGG-3′[31].
Statistical analysis
Statistics were obtained using IBM SPSS statistical software 23 (SPSS Inc., Chicago, IL, USA). T test and Duncan's multiple range test were performed. Results were expressed as the mean ± SEM. Data with *p < 0.05,**p < 0.01, ***p < 0.001 or no identical letter between samples were considered statistical significant.
16S rDNA sequencing data analysis was performed in QIIME2 (https://qiime2.org) platform. The DADA2 software package was used to splice data and remove primer and chimeric sequences. Species annotation was performed basing Silva_132 database. LEfSe analysis and PICRUSt analysis were performed via galaxy platform (http://huttenhower.sph.harvard.edu/galaxy). PCA analysis and heatmap were performed via a Personalbio gene cloud platform (https://www.genescloud.cn) and R software (3.6.3), respectively.
Results
Celery slightly alleviated DSS-induced colitis
In this study, vegetable powder was added to mice diet, and 3 % DSS water was given to induce mice colitis model (Fig. 1A). Results showed that DSS group showed significant weight loss, severe loose stools and hematochezia after given 3 % DSS water (Fig. 1B and C). The administration of celery, kale and red chicory significantly decreased DAI score, but only kale and red chicory that led to increased body weight compared with DSS group. Representative photos of colon are shown in Fig. 1D. Kale and red chicory, but not celery that significantly alleviated DSS induced colon shortening in this experiment (Fig. 1E).
Fig. 1.
The effects of celery, kale, and red chicory intakes on basic physiological indicators of DSS-induced colitis mice. (A) animal experimental design; (B) body weight change; (C) disease activity index (DAI) score. (D) representative photos and (E) length of colon. All values are expressed as mean ± SEM (n = 8); *, p < 0.05; **, p < 0.01; ***, p < 0.001.
CF chemical composition
Subsequently, celery was selected to study the interaction between dietary fiber and flavonoids, and tried to investigate further why celery was the least effective among the 3 vegetables to alleviate colitis. Prior to exploring the biological functions of dietary fiber and flavonoids, it is important to know the chemical composition and structure characteristics.
In this study, CF was obtained using ethanol extraction and polyamide purification. The main composition of CF is listed in Table 1. CF encompassed 67.85, 9.76, and 10.2 mg/100 mg of apiin, CA, and LA, respectively. Moreover, celery powder contained 0.13, 0.02, and 0.02 mg/100 mg of apiin, CA, and LA (Table 1). These results showed a high purity of CF fraction obtained by purification in this study with ca. 88 % flavonoids content. The chemical structure and extracted ion chromatograms of major flavonoids in CF are shown in Figure S2.
Table 1.
The content of the three major flavonoids in CF and celery powder (dry weight).
| Compound name | CF (mg/100 mg) | Celery powder (mg/100 mg) |
|---|---|---|
| Apigenin 7-O-apiosylglucoside (Apiin) | 67.85 ± 0.89 | 0.13 ± 0.01 |
| Chrysoeriol 7-O-apiosylglucoside (CA) | 9.76 ± 0.23 | 0.02 ± 0.00 |
| Luteolin 7-O-apiosylglucoside (LA) | 10.22 ± 0.05 | 0.02 ± 0.00 |
CSDF and CIDF chemical composition
Dietary fiber (CSDF and CIDF) were obtained using an enzymatic method [21]. The yields of CSDF and CIDF in celery powder were at 2.28 % and 27.16 % (Table 2). CSDF possessed 49.27 % neutral sugar and 39.95 % uronic acid, and CIDF consisted of 49.45 % neutral sugar and 17.58 % uronic acid. These data indicated that carbohydrate accounted for ca. 90 % in CSDF and 67 % in CIDF. There was a small amount of starch detected in CSDF and CIDF, while a relative high content of protein was present in CIDF (6.04 %).
Table 2.
The chemical composition and monosaccharides composition of CSDF and CIDF.
| CSDF (%) | CIDF (%) | |
|---|---|---|
| Yielda | 2.28 ± 0.00 | 27.16 ± 0.81 |
| Neutral sugar | 49.27 ± 1.19 | 49.45 ± 1.92 |
| Uronic acid | 39.95 ± 0.79 | 17.58 ± 1.19 |
| Protein | 2.39 ± 0.27 | 6.04 ± 0.62 |
| Ash content | 7.72 ± 0.47 | 8.89 ± 0.45 |
| Starch | 0.24 ± 0.00 | 0.26 ± 0.00 |
| Rhamnose | 6.53 ± 0.08 | 8.88 ± 0.84 |
| Arabinose | 21.85 ± 0.34 | 5.43 ± 0.35 |
| Galactose | 30.78 ± 1.30 | 4.15 ± 0.17 |
| Glucose | 2.32 ± 0.49 | 55.18 ± 1.11 |
| Xylose | – | 3.51 ± 0.57 |
| Mannose | – | 4.47 ± 0.5 |
| Galacturonic acid | 36.56 ± 1.46 | 18.37 ± 0.91 |
| Glucuronic acid | 1.96 ± 0.16 | – |
- means not detected.
Yield of dietary fiber is presented by mass percentage based on the dry celery powder.
Structure characterization of CSDF and CIDF
The monosaccharides composition of CSDF and CIDF is listed in Table 2. CSDF mainly consisted of 6.53 % Rha, 21.85 % Ara, 30.78 % Gal, 2.32 % Glc, 36.56 % GalA, 1.96 % GlcA, while CIDF contained 8.88 % Rha, 5.43 % Ara, 4.15 % Gal, 55.18 % Glc, 3.51 % Xyl, 4.47 % Man, 18.37 % GalA.
The HPGPC chromatogram of CSDF showed that there were three distinct peaks with peak Mw of 482 kmajor peak, Peak 1), 29 kDa (Peak 2), and 2.1 kDa (Fg. S3A).
The structural features of CSDF and CIDF were measured using FT-IR spectroscopy. As shown in Fg. S3B, in FTIR spectra of CSDF and CIDF, there were absorption bands at 3400 cm−1, 1621 cm−1, 1421 cm−1, 3000–2800 cm−1 and 1760–1700 cm−1. In addition, In the FTIR spectra of the CSDF, the characteristic absorption bands existed at 1099.2 cm−1 and 1020.2 cm−1. In the FTIR spectra of the CIDF, the characteristic absorption bands existed at 1062.6 cm−1.
To further compare structural differences of CSDF from that of CIDF, a solid-state NMR was employed to better characterize CSDF and CIDF. Firstly, the 13C solid-state NMR spectra results of CSDF are shown in Fg. S4A. The signal peak at 68.61 ppm corresponded to chemical shifts of C-2, C-3, C-4 and C-5. The signal peak at 101.25 ppm corresponded to the chemical shift of anomeric C1, whereas downfield chemical shifts at 171.12 ppm and 175.17 ppm were assigned to C6 (–COOH) of GalA and GlcA, respectively and confirming the presence of uronic acids as typical in SDF. Methoxylation and acetylation in CSDF was evident from signal peak at 53.57 ppm attributed to the chemical shift of OMe and that at 21.08 ppm corresponding to the chemical shift of OAc, respectively. The 13C solid-state NMR spectra of CIDF is presented in Fg. S4B. The signal peak at 72.23 ppm corresponded to the chemical shifts of C-2, C-3, C-4 and C-5, whereas anomeric sugar peak was detected at 104.97 ppm assigned to the chemical shift of C1. Likewise, signal peak at 173.75 ppm corresponded to the chemical shift of C6 (–COOH) of GalA, whereas methylation was evident from signal peak at 53.62 ppm as observed in CSDF. The signal peak at 21.26 ppm inferred for OAc in CIDF. Compared to NMR signals of CSDF, there were several characteristic signal peaks in the NMR signals of CIDF including that at 62.63 and 83.55 ppm assigned to the chemical shift of C-6 [32] and C-4 in amorphous cellulose, respectively [33]. The presence of lignin moiety in CIDF was evident from signal peak at 128.98 ppm in the aromatic region [34], and absent from CSDF.
CSDF or CF alleviated physiological parameters indicative of DSS‑induced colitis
The effects of celery dietary fiber (CSDF and CIDF) combined CF on DSS induced colitis mice were assessed. Treatment with CSDF or CF alone significantly increased body weight change of colitis mice compared to DSS group (from 88 % to 94 % and 93 %), while body weight change of CSDF + CF group was significantly lower than that in CSDF or CF group, only 90 % (Fig. 3B). All treatment groups significantly alleviated the symptoms compared to DSS group, though with DAI score of CSDF + CF group (about 2.5 points) found markedly higher than that of the CSDF group (about 1.7 points) (Fig. 3C). In addition, colonic shortening by DSS was significantly improved in all treatment groups, with no significant difference among the treatment groups (Fig. 3D & E).
Combined use of CSDF and CF antagonized the effect of improved barrier function
To visually characterize the lesions, colon tissues were subjected to pathological examination using H&E staining. Severe inflammatory cell infiltration, crypt damage, and intestinal wall thickening were observed in the colonic sections of the DSS group (Fig. 4A). Our study showed that all treatment groups showed a significant improvement in colonic pathological damage (from 9 points down to<5 points), except for CSDF + CF group (Fig. 4B).
Fig. 4.
The effects of celery dietary fiber and flavonoids on microstructure levels of DSS-induced colitis. (A-B) H&E staining of the colon tissues and histological scores (n = 10); (C-D) AB-PAS staining of the colon tissues and area of mucus expression (n = 10); (E) Immunofluorescence sections of colon were stained for ZO-1 (red) and occludin (green), and nuclei were stained with DAPI (blue); (F) FITC-dextran (4 kDa) level in serum (n = 6). Values expressed as mean ± SEM; *, p < 0.05; **, p < 0.01; ***, p < 0.001. Data with no identical letter denotes a significant difference.
Mucus is secreted by goblet cells to maintain intestinal barrier function and limit the translocation of intestinal flora [35]. Therefore, mucus was examined using AB-PAS staining. Results showed that CSDF and CF significantly increased area of mucus expression in colon with comparison of the DSS group (from 2.2 % up to 4.5 % and 7.2 %), while the promoting effect was not observed in CSDF + CF group (only 3.7 %) (Fig. 4C-D).
Intestinal barrier function damage typically leads to an increase of FITC-dextran (4 kDa) in serum after gavage of FITC-dextran (4 kDa) [36]. Our study showed likewise that serum FITC-dextran (4 kDa) level was remarkably elevated in the DSS group compared to normal group, while CF markedly reduced FITC-dextran (4 kDa) level in serum (from 6.9 μg/mL down to 1.6 μg/mL) (Fig. 4F). However, CSDF weakened the decreasing effect of CF on serum FITC-dextran (4 kDa) level (up to 6.2 μg/mL).
It has been shown that tight junction proteins play a vital role in preserving epithelial barrier functions [37]. Hence, expression of tight junction protein, including ZO-1 and Occludin, was determined using double-immunofluorescent stain. Colitis resulted in reduction in ZO-1 and Occludin expression levels, failing to form a continuous linear distribution above the nucleus (Fig. 4E). Compared with DSS group, expression of Occludin and ZO-1 was distinctly increased in CSDF and CF groups, albeit not in CSDF + CF group.
CSDF ameliorates the suppression of inflammation related proteins by CF
To further explore the molecular mechanism of CSDF + CF attenuation of the ameliorative effect of CSDF or CF in DSS-induced colitis mice, inflammation related proteins in mice colon were assessed. Compared with the DSS group, administration of CSDF or CF markedly reduced MPO and iNOS activities and the levels of IL-6 and IL-1β inside the colon, while administration of CIDF and whole celery only inhibited iNOS activity and IL-1β levels, respectively (Fig. 5A-D). More importantly, significant differences in colonic MPO activity, IL-6 and IL-1β levels were observed between CSDF + CF group and CF group (Fig. 5A-D) indicating that CSDF attenuated the suppressive effect of CF on inflammation related proteins in colon (Fig. 5A-D).
Fig. 5.
The effects of celery dietary fiber and flavonoids on inflammatory-related protein levels of DSS-induced colitis. Activities of (A) MPO and (B) iNOS, and levels of (C) IL-6 and (D) IL-1β in colon tissue. All values expressed as mean ± SEM (n = 6); *, p < 0.05; **, p < 0.01; ***, p < 0.001. Data with no identical letter denotes a significant difference.
Combined use of CSDF and CF antagonized regulating gut microbiota composition
To further investigate the mechanisms underlying the weakened ameliorative effect of CSDF or CF in mice with DSS induced colitis, we performed 16 s rDNA microbiota analysis of mice feces. The shannon index was used to analyze the α diversity of the gut microbiota. Results showed that the shannon index was markedly decreased after giving DSS water (from 5.5 down to 3.9). The shannon index of CSDF + CF group (3.5) was significantly lower than that of CSDF or CF group (4.6 and 4.5) (Fig. 6 A). Moreover, PCA analysis was used to analyze the similarities in microbial community composition. Results showed that gut microbiota composition in DSS group was significantly different from that in control mice. Whereas, CF treatment not CSDF + CF that markedly regulated the changes in the gut microbiota of colitis mice (Fig. 6 B). Moreover, CIDF + CF group samples in PCA analysis were far away from samples of the control group and DSS group (Fig. 6 B). Results of gut microbial compositions at phyla levels and top 35 gut bacteria at genus levels are depicted in Fig. 6 C and D, respectively.
Fig. 6.
The effects of celery dietary fiber and flavonoids on gut microbiota structure of colitis mice. (A) The shannon index; (B) PCA analysis results; (C) Gut microbial compositions at phyla levels; (D) Heatmap of top 35 gut bacteria at genus levels. All values are expressed as mean ± SEM (n = 6); *, p < 0.05; **, p < 0.01; ***, p < 0.001. Data with no identical letter denotes a significant difference.
To screen out the key bacteria in mice after dietary fiber combined CF administration, LEfSe analysis was performed for two parts, CSDF part and CIDF part. Results showed that the characteristic bacteria in the CSDF + CF group were g_Escherichia-Shigella, g_Paraclostridium, g_Clostridium_sensu_stricto_1, g_Paeniclostridium and g_Enterococcus (LDA > 2), whereas characteristic bacteria in the CIDF + CF group were g_Bacteroides and g_Muribaculum (LDA > 2) (Fig. 7A-B). Moreover, g_Akkermansia (LDA > 2) was found as a key bacterium in CF group in both CSDF and CIDF analysis parts as revealed from LEfSe analysis.
Fig. 7.
Analysis of the significantly differential intestinal bacteria of colitis mice. Taxonomic cladogram generated from LEfSe analysis of CSDF with CF part (A) and CIDF with CF part (B).
To further validate the relationship between the characterized bacteria at the genus level and disease status, spearman correlation analysis was performed. Our results showed that the abundance of g_Escherichia_Shigella, g_Clostridium_sensu_stricto_1, g_Terrisporobacter, g_Paraclostridium and g_Enterococcus were positively associated with FITC-dextran (4 kDa) level in serum, DAI score and H&E score, and negatively with body weight change (Fig. 8A). The relative abundance of g_Escherichia-Shigella and g_Clostridium_sensu_stricto_1 was correlated positively with IL-6 and iNOS levels. Whereas, the abundance of g_Akkermansia was negatively associated with IL-1β, MPO and iNOS levels, but positively associated with mucus expression area.
Fig. 8.
(A) Correlation analysis between the significantly differential intestinal bacteria and biochemical indexes; (B-F) Relative abundances of g_Escherichia-Shigella, g_Clostridium_sensu_stricto_1, g_Paraclostridium, and g_Enterococcus, and g_Akkermansia. All values expressed as mean ± SEM (n = 6); *, p < 0.05; **, p < 0.01; ***, p < 0.001. Data with no identical letter denotes a significant difference.
Moreover, our results showed that the relative abundance of g_Escherichia-Shigella, g_Clostridium_sensu_stricto_1, g_Paraclostridium and g_Enterococcus in DSS group significantly increased from<2.5 % to 24 %, 4.9 %, 9.3 %, and 0.12 %, respectively. After giving CSDF or CF separately, g_Escherichia-Shigella (down to 14.5 % and 9.5 %), g_Clostridium_sensu_stricto_1 (down to 0.4 % and 0.9 %), and g_Paraclostridium (down to 3.5 % and 5.6 %) were significantly inhibited. The relative abundance of g_Escherichia-Shigella, g_Clostridium_sensu_stricto_1, g_Paraclostridium and g_Enterococcus in the CSDF + CF group was significantly higher than that in CSDF and CF groups, up to 26 %, 3.8 %, 12.8 %, and 0.32 % (Fig. 8B-G). CF can greatly promote the relative abundance of g_Akkermansia (from 3.2 % to 20 %) (Fig. 8F). CSDF had no significant effect on the relative abundance of g_Akkermansia (5.6 %). Nevertheless, it was interesting that combined use of CSDF and CF (only 3.3 %) suppressed the response observed of g_Akkermansia for CF. There was no significant difference in the relative abundance of g_Akkermansia between CIDF + CF group (12 %) and CF group (20 %).
Functional prediction of changes in gut microbiota composition
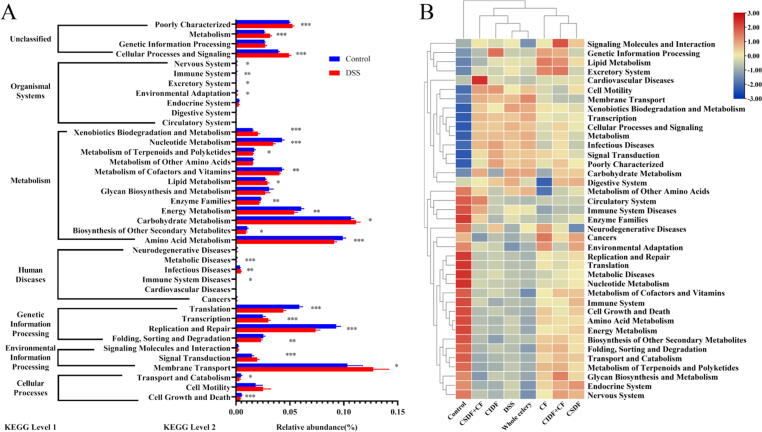
To further predict functional changes of intestinal flora, a PICRUSt analysis was performed. Alterations in multiple KEGG functional pathways were detected in the DSS group compared with the normal group, such as amino acid metabolism, carbohydrate metabolism, energy metabolism, immune system (Fig. 9A). In cluster analysis of the function prediction result across all groups, treatment groups were divided into two clusters (Fig. 9B). The CF, CSDF and CIDF + CF group were distributed in the same cluster showing generally improvement in most functional pathways, such as immune system, cell growth and death, amino acid metabolism, and energy metabolism.
Fig. 9.
Functional prediction of gut microbiota in colitis mice. (A) Differential metabolic pathways (Control vs DSS); (B) Heatmap of functional prediction results. All values are expressed as mean ± SEM (n = 6); *, p < 0.05; **, p < 0.01; ***, p < 0.001.
CSDF inhibited the pro-proliferative effect of apiin on A. Muciniphila in vitro
In order to further explore the mechanism of CSDF inhibiting CF activity in vivo, in vitro mixed fermentation was used to explore the effect of the combination of CSDF and apiin (the main component of CF) on A. muciniphila. The results are as shown in the Fig. 10. In the mixed fermentation experiment, 10 μ M and 100 μM of apiin significantly promoted the abundance of A. muciniphila (4-fold and 5.5-fold of control group). Treatment with CSDF alone also significantly promoted A. muciniphila abundance (1.8-fold of control group), but it was much lower than that of apiin group. However, when 10 μM apiin and 100 μM apiin were mixed with CSDF, the abundance of A. muciniphila decreased significantly (only 1.2-fold and 0.9-fold of control group). In addition, the proportion of A. muciniphila in total bacteria was consistent with the trend of relative quantitative results (Fig. 10B).
Fig. 10.
CSDF inhibited the pro-proliferative effect of apiin on Akkermansia muciniphila (A. muciniphila) in vitro faecal fermentation. (A) Abundance changes of A. muciniphila; (B) Changes in the proportion of A. muciniphila to all bacteria (All). All values are expressed as mean ± SEM (n = 3); *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
Colitis is a serious threat to human health worldwide, and it is a typical intestinal inflammatory disease. Numerous studies have shown that treatment with dietary fibers or flavonoids alone that can effectively regulate intestinal barrier function and inhibit intestinal dysfunction. Our data herein indicated that the combined use of CSDF and CF can weaken the effect of CF on alleviating colitis via inhibiting g_Akkermansia.
Flavonoids and dietary fiber in vegetables are considered beneficial for intestinal health. Dietary control is one of the important intervention to ameliorate colitis. A lot of reports about dietary fiber [38] and flavonoids [39] improving colitis present strong evidence for such management. Previous studies confirmed the enrichment of flavonols i.e., kaempferol (46.8 mg/100 g), quercetin (22.6 mg/100 g), and isorhamnetin (23.6 mg/100 g) in raw kale [13], whereas high levels of procyanidin A2 (24.0 mg/100 g) and rutin (5.4 mg/100 g) predominated in red chicory [40]. Meanwhile, raw celery is rich in flavones i.e., apigenin and its derivatives (1.7–24.0 mg/100 g) [13]. Moreover, according to the USDA database [15] and China Food Composition [16], the content of dietary fiber in celery, kale, and chicory was estimated at 1.6 %, 4.1 %, and 0.9 % wet weight, respectively. In the database, the content of flavonoids in specific categories of these three vegetables is the highest. Consequently, celery, kale, and red chicory were selected as treatments in this study according to phytochemical screening to compare the effects of vegetables on colitis. Our results showed that administration of kale and red chicory significantly increased body weight and colon length, concurrent with a decreased DAI index (Fig. 1B-E) suggestive for their potential for prevention of colitis, which may be explained by the abundant flavonoids in these vegetables. Interestingly, celery only slightly alleviated colitis and in accordance with reports in parsley (Petroselinum crispum) for relieving colitis basal physiological indexes [41]. Moreover, it has been reported that apigenin (5 mg/kg) was beneficial for the control of IBD in mice [42]. However, in our results, the efficacy of celery whole food with a comparable content of apigenin for colitis was not prominent.
We speculated that ome joint effects between the different components in celery exist to interfere with the function exerted by celery flavonoids. Considering that dietary fiber and flavonoids are the most common functional nutrients in vegetables [43], celery dietary fiber (CSDF and CIDF) and flavonoids were obtained for investigating the interaction between dietary fiber and flavonoids. In this study, flavonoids in CF is enriched to reach 88 %, with apiin as main component (68 %) (Table 1). Moreover, results showed that the main components in CSDF and CIDF were sugars (Table 2). CSDF contained a higher content of uronic acid (39.95 %) than that in CIDF (17.58 %) (Table 2), which was also confirmed in the monosaccharides composition analysis results (36.56 % GalA and 1.96 % GlcA in CSDF; 18.37 % GalA in CIDF) (Table 2). The CSDF contained 30.78 % Gal, 21.85 % Ara and a small amount of Rha and Glu (Table 2). These results indicated that CSDF was mainly a pectin-based dietary fiber [44]. Furthermore, 55.18 % of CIDF in the monosaccharides composition results was Glc (Table 2).
In the result of FTIR spectra (Figure S3 B), there was a major absorption band at 3400 cm−1 corresponding to the absorption of an O—H stretching vibration [45]. Absorption band 3000–2800 cm−1 could be attributed to C—H stretching vibration [46]. Absorption band at 1760–1700 cm−1 corresponded to COOH stretching vibration associated with the uronic acid residues [47]. Moreover, in FTIR spectra of CIDF, the absorption band at 1062.6 cm−1 was characteristic for cellulose [48]. In FTIR spectra of CSDF, the specific bands in the 1099.2 and 1020.2 cm−1 region corresponded to ring vibrations overlapping with stretching vibrations of (C–OH) side groups and the (C—O—C) glycosidic band vibration [49]. Bands around 1621 cm−1 and 1421 cm−1 might be linked to –OH flexural vibrations [50]. Combined FTIR spectral results (1062.6 cm−1 of cellulose characteristic peak) (Figure S3 B), 13C NMR characteristic signals of amorphous cellulose (62.63 ppm chemical shift of C-6 and 83.55 ppm chemical shift of C-4) (Figure S4 B), along with CIDF insolublity in water indicated that the main component in CIDF was amorphous cellulose [33].
Treatment alone with celery dietary fiber (CSDF and CIDF) or flavonoids was compared with the combined use of celery dietary fiber and flavonoids in alleviating DSS-induced colitis (Fig. 3A). Our results revealed that the administration of CSDF, or CF alone and CIDF + CF were effective in protecting against DSS-induced acute intestinal injury by ameliorating body weight loss, DAI and colon shortening (Fig. 3B-E). However, whole celery, CIDF and CSDF + CF did not alleviate body weight loss. On the other hand, enterocyte can express mucus and tight junction proteins, including Occludin, and ZO-1, which shape a natural gut barrier, preventing microbial antigens and toxins through the lamina propria [51]. It was proved that a decrease of mucus or tight junction protein expression contributed to the development of experimental colitis [52]. Our data showed that CSDF and CF ameliorated crypt damage, reduced infiltration of inflammatory cells and enhanced the expression of mucus (Fig. 4A-D). Furthermore, CF recovered intestinal tight junction proteins expression and distribution in colon of colitis mice and significantly reinforced intestinal barrier function (Fig. 4E-F). Unexpectedly, the combined use of CSDF and CF showed significantly lower ameliorated effects than CSDF group or CF group alone, while similar results were not observed in the CIDF + CF group (Fig. 4F). Additionally, whole celery did not increase the expression of mucus and intestinal barrier function suggestive that there are indeed some interactions between celery CSDF and CF in colitis mice to weaken celery effect in alleviating colitis (Fig. 4D).
Pro-inflammatory cytokines (such as IL-1β and IL-6) have been proved to be involved in the pathogenesis of IBD [53]. An increase in iNOS activity can produce large amounts of NO known to be involved in inflammatory response. Furthermore, MPO is an enzyme mainly present in neutrophils [54]. Its activity is a reliable indicator to evaluate the degree of neutrophils infiltration in tissues. Our results showed that MPO activities and IL-6 and IL-1β levels in CSDF + CF group were markedly higher than CF group, although colonic MPO and iNOS activities and IL-6 and IL-1β levels in DSS-induced mice were significantly reduced by giving CSDF or CF (Fig. 5A-D). Meanwhile, whole celery only weakly suppressed effects related to colon inflammatory levels, such as IL-1β (Fig. 5D). Several studies have reported the inhibition of intestinal inflammatory factors by dietary fiber i.e., pectin or flavonoids alone i.e., galangin, which can inhibit colon IL-6 and IL-1β in colitis mice[55],[56]. However, there are few reports on the interaction between dietary fiber and flavonoids. The results presented herein further suggested that CSDF exerted an antagonism effect against alleviating colitis effects of CF based on monitoring of inflammation-related proteins expression levels.
There is a symbiotic relationship between gut microbiota and host, and intestinal flora disorder is one of the whole marks of colitis pathogenesis [57]. Based on the results of 16 s rDNA high-throughput sequencing, it was found that intestinal flora balance of colitis mice was disrupted. The α diversity (the shannon index) and the β diversity were used to characterize the diversity of intestinal flora and analyze the overall difference between groups. Results showed that CSDF and CF improved the diversity or composition of intestinal flora induced by DSS administration, but CSDF + CF did not play a role (Fig. 6A-B). Further, in LEfSe and correlation analysis, some bacterial genera found to be affected by the interaction between CSDF and CF were screened out (Fig. 7A-B). Most Proteobacteria are thought to be opportunistic bacteria in ulcerative colitis [58], with Escherichia-Shigella as the typical genus of Proteobacteria. g_Clostridium_sensu_stricto_1 was associated with intestinal inflammation and found to increase in patients with colitis [59]. In addition, present studies have shown that the strains of g_Paraclostridium (Paraclostridium bifermentans)[60] and g_Enterococcus (Enterococcus faecalis)[61] exacerbated experimental colitis in mice. After treatment with CSDF combined with CF, the inhibitory effect of CSDF or CF alone on g_Escherichia-Shigella, g_Clostridium_sensu_stricto_1, g_Paraclostridium, and g_Enterococcus disappeared (Fig. 8B-G). Moreover, the correlation analysis suggested that the relative abundance of Akkermansia was positively correlated with the mucus expression (Fig. 8A), but negatively correlated with pro-inflammatory cytokines suggestive for its protective role in colitis [62]. CF significantly increased the relative abundance of g_Akkermansia. Similarly, epigallocatechin gallate (EGCG) has been reported to effectively relieve colitis, and enhance the Akkermansia colonization [63]. However, CSDF inhibited the promotion of CF on the g_Akkermansia abundance (Fig. 8F), which may be the potential mechanism by which CSDF weakened the improvement of CF on DSS-induced colitis in mice. Such effect was interestingly not observed in case of combined CF and CIDF combination suggestive that soluble dietary but not insoluble fibers that show such antagonistic effect. Subsequent PICRUSt analysis further demonstrated that CSDF interfered with the intestinal flora function, especially immune system, to decrease colitis alleviating effect of CF (Fig. 9B).
Finally, in order to verify the promoting effect of CF on A. muciniphila in vivo and the weakening effect of CSDF in this process. The effect of apiin (the main component of CF) and CSDF on A. muciniphila abundance was studied by fecal bacteria fermentation in vitro. Our results show that celery flavonoids can dramatically promote the abundance of A. muciniphila in vivo and in vitro (Fig. 8, Fig. 10). This again showed that celery flavonoids can alleviate colitis by promoting the proliferation of A. muciniphila. However, it is interesting that CSDF also interfered with A. muciniphila proliferation in this process, both in vivo and in vitro. This demonstrated that, in gut microbiota, CSDF directly inhibited the response of A. muciniphila, thereby weakening the relieving effect of celery flavonoids on colitis.
Conclusion
The present study revealed for the first time that CSDF and CF can significantly relieve DSS-induced colitis, but CSDF weakened the ameliorative effect of CF on colitis. Specifically, CSDF and CF significantly decreased the concentration and activity of colonic inflammation related proteins and intestinal permeability, increased mucus expression, and regulated intestinal flora disorders of colitis mice. However, after combined use of CSDF and CF, the effects of suppressing intestinal inflammation and abundance of harmful bacteria and enhancing intestinal barrier function and the abundance of beneficial bacteria (especially g_Akkermansia) by CF were weakened. In addition, CIDF is mainly composed of cellulose, while CSDF is pectin-type dietary fiber. Compared with CSDF, CIDF had a weaker effect on the remission of colitis and the interference of CF in colitis mice, which was related to their structural differences. More importantly, we verified the promoting effect of celery flavonoid on g_Akkermansia, as well as the inhibition of this effect by CSDF, both in vivo and in vitro. This study illustrated for the first time that the interaction between CSDF and celery flavonoids in vivo can weaken the efficacy of flavonoids when administered alone by inhibiting the proliferation of g_Akkermansia. Therefore, the activities in vivo of purified functional nutrients may be influenced by other components of the whole food. This study provides a reference for elucidating the interaction between different dietary components on intestinal health and further in other diseases. For the population with intestinal inflammation, increased vigilance may be necessary when they have a diet with high amounts of both dietary fiber and flavonoids. In the future work, deeper action mechanisms of the combined CSDF and CF antagonism in alleviating colitis will be further explored and in other diseases to be more conclusive. Examination of such antagonism in other dietary sources rich dietary fibers and flavonoids should also be considered to provide improved formulation or extract targeting a certain effect to be considered as nutraceuticals.
CRediT authorship contribution statement
Hui Wang: Writing – original draft, Methodology, Data curation, Investigation, Formal analysis, Visualization. Xiaojun Huang: Conceptualization, Methodology, Data curation. Shengkun Xia: Methodology, Investigation. Chunhua Chen: Methodology, Investigation. Xiaomin Chen: Methodology, Investigation. Yanli Zhang: Data curation, Investigation. Mohamed A. Farag: Conceptualization, Writing – review & editing. Jianbo Xiao: Conceptualization, Supervision, Writing – review & editing. Shaoping Nie: Conceptualization, Funding acquisition, Supervision, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The financial support from the National Natural Science Foundation of China for Distinguished Young Scholars (31825020), Key Technology Project in Jiangxi Province (20212AAF01005), Technological Innovation Guidance Plan of Jiangxi Province (20203AEI91007), Key Laboratory of Bioactive Polysaccharides of Jiangxi Province (20212BCD42016), Ramón y Cajal Programme (RYC2020-030365-I) and Axudas para a consolidación de unidades de investigación competitivas (Excelencia-ED431F2022/01) were gratefully acknowledged.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.01.013.
Contributor Information
Jianbo Xiao, Email: jianboxiao@yahoo.com.
Shaoping Nie, Email: spnie@ncu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Matsuoka K., Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(M'Koma, A. E., Inflammatory bowel disease: an expanding global health problem, Clinical Medicine Insights. Gastroenterology. 6 (2013) 33-47. 10.4137/CGast.S12731. [DOI] [PMC free article] [PubMed]
- 3.Orel R., Trop T.K. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World Journal of Gastroenterology. 2014;20:11505–11524. doi: 10.3748/wjg.v20.i33.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsiountsioura M., et al. Detailed assessment of nutritional status and eating patterns in children with gastrointestinal diseases attending an outpatients clinic and contemporary healthy controls. European Journal of Clinical Nutrition. 2014;68:700–706. doi: 10.1038/ejcn.2013.286. [DOI] [PubMed] [Google Scholar]
- 5.Lewis J.D., Abreu M.T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology. 2017;152:398–414. doi: 10.1053/j.gastro.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto K., et al. Oxidative stress induces gastric epithelial permeability through claudin-3. Biochemical And Biophysical Research Communications. 2008;376:154–157. doi: 10.1016/j.bbrc.2008.08.140. [DOI] [PubMed] [Google Scholar]
- 7.Altomare R., et al. Enteral nutrition support to treat malnutrition in inflammatory bowel disease. Nutrients. 2015;7:2125–2133. doi: 10.3390/nu7042125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida A., et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clinical journal of gastroenterology. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 9.Yang H., et al. Ulcerative Colitis-associated E. coli pathobionts potentiate colitis in susceptible hosts. Gut Microbes. 2020;12 doi: 10.1080/19490976.2020.1847976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon K.H., Murakami A., Tanaka T., Ohigashi H. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: attenuation of pro-inflammatory gene expression. Biochemical Pharmacology. 2005;69:395–406. doi: 10.1016/j.bcp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Xie Q., You L., Cheung P.C.-K., Zhao Z. Behavior of Non-Digestible Polysaccharides in Gastrointestinal Tract: A Mechanistic Review of its Anti-Obesity Effect. eFood. 2021;2:59–72. doi: 10.2991/efood.k.210310.001. [DOI] [Google Scholar]
- 12.Jaeger C., Tischkau S.A. Role of aryl hydrocarbon receptor in circadian clock disruption and metabolic dysfunction. Environmental health insights. 2016;10:133–141. doi: 10.4137/ehi.s38343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David B. Haytowitz, X. W., and Seema Bhagwat, USDA database for the flavonoid content of selected foods, Release 3.3. U.S. Department of Agriculture: Washington, D., USA, 2018., Ed. 2018.
- 14.Caruso R., Ono M., Bunker M.E., Nunez G., Inohara N. Dynamic and asymmetric changes of the microbial communities after cohousing in laboratory mice. Cell Reports. 2019;27:3401–3412.e3. doi: 10.1016/j.celrep.2019.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agriculture, U. S. D. o., Agricultural Research Service. 2020, USDA Food and Nutrient Database for Dietary Studies 2017-2018. Food Surveys Research Group Home Page, http://www.ars.usda.gov/nea/bhnrc/fsrg.
- 16.Yuexing-Yang,Guangya-Wang,Xingchang-Pan, China Food Composition. Beijing: Peking University Medical Press: 2012; p 384.
- 17.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. Journal of basic and clinical pharmacy. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McHenga S.S.S., Wang D., Li C., Shan F., Lu C. Inhibitory effect of recombinant IL-25 on the development of dextran sulfate sodium-induced experimental colitis in mice. Cellular & Molecular Immunology. 2008;5:425–431. doi: 10.1038/cmi.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, K.; Yan, Z.; Luo, J.; Chen, L., Study on separating and purification technology of flavonoid from Apium graveolens L. with polyamide resin, Food & Machinery. 25 (2009) 46-50.
- 20.Lin L.-Z., Lu S., Harnly J.M. Detection and quantification of glycosylated flavonoid malonates in celery, Chinese celery, and celery seed by LC-DAD-ESI/MS, Journal Of Agricultural And. Food Chemistry. 2007;55:1321–1326. doi: 10.1021/jf0624796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S., et al. Removal of bound polyphenols and its effect on antioxidant and prebiotics properties of carrot dietary fiber. Food Hydrocolloids. 2019;93:284–292. doi: 10.1016/j.foodhyd.2019.02.047. [DOI] [Google Scholar]
- 22.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.T., Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28:350–356. doi: 10.1038/168167a0. [DOI] [Google Scholar]
- 23.Bitter T. A modified uronic acid carbazole reaction. Analytical Biochemistry. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 24.AOAC, Official methods of analysis, Association of Official Analytical. (2006).
- 25.Tang W., et al. Purification of polysaccharide from Lentinus edodes water extract by membrane separation and its chemical composition and structure characterization. Food Hydrocolloids. 2020;105 doi: 10.1016/j.foodhyd.2020.105851. [DOI] [Google Scholar]
- 26.Feng, L.; Yin, J.; Nie, S.; Wan, Y.; Xie, M., Fractionation, physicochemical property and immunological activity of polysaccharides from Cassia obtusifolia, International Journal Of Biological Macromolecules. 91 (2016) 946-953. doi: 10.1016/j.ijbiomac.2016.05.030. [DOI] [PubMed]
- 27.Wlodarska M., et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156:1045–1059. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou, X., et al., Exopolysaccharides from Lactobacillus plantarum NCU116 enhances colonic mucosal homeostasis by controlling epithelial cell differentiation and c-Jun/Muc2 signaling, Journal Of Agricultural And Food Chemistry. 67 (2019) 9831-9839. doi: 10.1021/acs.jafc.9b03939. [DOI] [PubMed]
- 29.Poritz L.S., et al. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. Journal Of Surgical Research. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 30.Schneeberger M., et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Scientific Reports. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fierer, N.; Jackson, J. A.; Vilgalys, R.; Jackson, R. B., Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays, Applied And Environmental Microbiology. 71 (2005) 4117-4120. 10.1128/aem.71.7.4117-4120.2005. [DOI] [PMC free article] [PubMed]
- 32.Haslinger S., Hietala S., Hummel M., Maunu S.L., Sixta H. Solid-state NMR method for the quantification of cellulose and polyester in textile blends. Carbohydrate Polymers. 2019;207:11–16. doi: 10.1016/j.carbpol.2018.11.052. [DOI] [PubMed] [Google Scholar]
- 33.Dalvi, L. C., et al., Study of xylan and cellulose interactions monitored with solid-state NMR and QCM-D, Holzforschung. 74 (2020) 643-653. 10.1515/hf-2019-0221.
- 34.Mao, J.; Holtman, K. M.; Scott, J. T.; Kadla, J. F.; Schmidt-Rohr, K., Differences between lignin in unprocessed wood, milled wood, mutant wood, and extracted lignin detected by C-13 solid-state NMR, Journal Of Agricultural And Food Chemistry. 54 (2006) 9677-9686. 10.1021/jf062199q. [DOI] [PubMed]
- 35.Katayama, M.; Xu, D. Z.; Specian, R. D.; Deitch, E. A., Role of bacterial adherence and the mucus barrier on bacterial translocation - Effects of protein malnutrition and endotoxin in rats, Annals Of Surgery. 225 (1997) 317-326. 10.1097/00000658-199703000-00012. [DOI] [PMC free article] [PubMed]
- 36.Zhou, Z., et al., Inhibition of epithelial TNF-alpha receptors by purified fruit bromelain ameliorates intestinal inflammation and barrier dysfunction in colitis, Frontiers In Immunology. 8 (2017) 1468. doi: 10.3389/fimmu.2017.01769. [DOI] [PMC free article] [PubMed]
- 37.Gonzalez-Mariscal L., Betanzos A., Nava P., Jaramillo B.E. Tight junction proteins. Progress In Biophysics & Molecular Biology. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong, H., Mander, I., Zhang, Z., Armstrong, D., Wine, E., Not all fibers are born equal; Variable response to dietary fiber subtypes in IBD, Frontiers In Pediatrics. 8 2021 10.3389/fped.2020.620189. [DOI] [PMC free article] [PubMed]
- 39.Li, M., Weigmann, B., A novel pathway of flavonoids protecting against inflammatory bowel disease: modulating enteroendocrine system Metabolites. 12 2022 10.3390/metabo12010031. [DOI] [PMC free article] [PubMed]
- 40.Migliorini A.A., et al. Red chicory (Cichorium intybus) extract rich in anthocyanins: chemical stability, antioxidant activity, and antiproliferative activity in vitro. Journal Of Food Science. 2019;84:990–1001. doi: 10.1111/1750-3841.14506. [DOI] [PubMed] [Google Scholar]
- 41.Jia, H., et al., Multi-faceted integrated omics analysis revealed parsley (Petroselinum crispum) as a novel dietary intervention in dextran sodium sulphate induced colitic mice, Journal of Functional Foods. 11 (2014) 438-448. 10.1016/j.jff.2014.09.018.
- 42.Ganjare A.B., Nirmal S.A., Patil A.N. Use of apigenin from Cordia dichotoma in the treatment of colitis. Fitoterapia. 2011;82:1052–1056. doi: 10.1016/j.fitote.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Ganesan K., Quiles J.L., Daglia M., Xiao J., Xu B. Dietary phytochemicals modulate intestinal epithelial barrier dysfunction and autoimmune diseases, Food. Frontiers. 2021;2:357–382. doi: 10.1002/fft2.102. [DOI] [Google Scholar]
- 44.Buss Marenda, F. R., et al., Advances in studies using vegetable wastes to obtain pectic substances: A Review, Journal Of Polymers And the Environment. 27 (2019) 549-560. doi: 10.1007/s10924-018-1355-8.
- 45.Mahloko L.M., Silungwe H., Mashau M.E., Kgatla T.E. Bioactive compounds, antioxidant activity and physical characteristics of wheat-prickly pear and banana biscuits. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su, Y.; Li, L., Structural characterization and antioxidant activity of polysaccharide from four auriculariales, Carbohydrate Polymers. 229 (2020) 115407. doi: 10.1016/j.carbpol.2019.115407. [DOI] [PubMed]
- 47.Xia, L.; Li, B.; Lu, Y.; Chen, D., Structural characterization and anticomplement activity of an acidic polysaccharide containing 3-O-methyl galactose from Juniperus tibetica, International Journal Of Biological Macromolecules. 132 (2019) 1244-1251. doi: 10.1016/j.ijbiomac.2019.04.029. [DOI] [PubMed]
- 48.Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y., Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective, Food Chemistry: X. 12 (2021) 100168. 10.1016/j.fochx.2021.100168. [DOI] [PMC free article] [PubMed]
- 49.Molaei, H.; Jahanbin, K., Structural features of a new water-soluble polysaccharide from the gum exudates of Amygdalus scoparia Spach (Zedo gum), Carbohydrate Polymers. 182 (2018) 98-105. doi: 10.1016/j.carbpol.2017.10.099. [DOI] [PubMed]
- 50.Wang, L.; Chen, L.; Li, J.; Di, L.; Wu, H., Structural elucidation and immune-enhancing activity of peculiar polysaccharides fractioned from marine clam Meretrix meretrix (Linnaeus), Carbohydrate Polymers. 201 (2018) 500-513. doi: 10.1016/j.carbpol.2018.08.106. [DOI] [PubMed]
- 51.Vivinus-Nebot M., et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;(63):744–752. doi: 10.1136/gutjnl-2012-304066. [DOI] [PubMed] [Google Scholar]
- 52.Kim, J. J.; Shajib, M. S.; Manocha, M. M.; Khan, W. I., Investigating intestinal inflammation in DSS-induced model of IBD, Jove-Journal Of Visualized Experiments. (2012) 3678. 10.3791/3678. [DOI] [PMC free article] [PubMed]
- 53.Alex, P., et al., Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis, Inflammatory Bowel Diseases. 15 (2009) 341-352. 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed]
- 54.Hakansson, A., et al., Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice, Clinical and Experimental Medicine. 15 (2015) 107-120. 10.1007/s10238-013-0270-5. [DOI] [PMC free article] [PubMed]
- 55.Wu, D., et al., Protective effects of six different pectic polysaccharides on DSS-induced IBD in mice, Food Hydrocolloids. 127 (2022) 10.1016/j.foodhyd.2021.107209.
- 56.Xuan, H.; Ou, A.; Hao, S.; Shi, J.; Jin, X., Galangin Protects against Symptoms of Dextran Sodium Sulfate-Induced Acute Colitis by Activating Autophagy and Modulating the Gut Microbiota, Nutrients. 12 (2020) 10.3390/nu12020347. [DOI] [PMC free article] [PubMed]
- 57.Ferreira, C. M., et al., The central role of the gut microbiota in chronic inflammatory diseases, Journal of Immunology Research. 2014 (2014) 10.1155/2014/689492. [DOI] [PMC free article] [PubMed]
- 58.Ohkusa, T.; Koido, S., Intestinal microbiota and ulcerative colitis, Journal Of Infection And Chemotherapy. 21 (2015) 761-768. 10.1016/j.jiac.2015.07.010. [DOI] [PubMed]
- 59.Yu, J.; Li, H.; Hu, Q., Research on Gut Microbiota Diversity in Patients with Ulcerative Colitis by High-throughput Sequencing, Acta Medicinae Universitatis Scientiae et Technologiae Huazhong. 47 (2018) 460-465.
- 60.Kutsuna, R.; Tomida, J.; Morita, Y.; Kawamura, Y., Paraclostridium bifermentans exacerbates pathosis in a mouse model of ulcerative colitis, Plos One. 13 (2018) 10.1371/journal.pone.0197668. [DOI] [PMC free article] [PubMed]
- 61.Fan, T.-J.; Goeser, L.; Lu, K.; Faith, J. J.; Hansen, J. J., Enterococcus faecalis Glucosamine Metabolism Exacerbates Experimental Colitis, Cellular And Molecular Gastroenterology And Hepatology. 12 (2021) 1373-1389. 10.1016/j.jcmgh.2021.06.017. [DOI] [PMC free article] [PubMed]
- 62.Bian X., et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Frontiers In Microbiology. 2019;10 doi: 10.3389/fmicb.2019.02259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu, Z., et al., Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis, Microbiome. 9 (2021) 184. doi: 10.1186/s40168-021-01115-9. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.