Abstract
Purpose
Survivors after acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19) are at high risk of developing respiratory sequelae and functional impairment. The healthcare crisis caused by the pandemic hit socially disadvantaged populations. We aimed to evaluate the influence of socio-economic status on respiratory sequelae after COVID-19 ARDS.
Methods
We carried out a prospective multicenter study in 30 French intensive care units (ICUs), where ARDS survivors were pre-enrolled if they fulfilled the Berlin ARDS criteria. For patients receiving high flow oxygen therapy, a flow ≥ 50 l/min and an FiO2 ≥ 50% were required for enrollment. Socio-economic deprivation was defined by an EPICES (Evaluation de la Précarité et des Inégalités de santé dans les Centres d’Examens de Santé - Evaluation of Deprivation and Inequalities in Health Examination Centres) score ≥ 30.17 and patients were included if they performed the 6-month evaluation. The primary outcome was respiratory sequelae 6 months after ICU discharge, defined by at least one of the following criteria: forced vital capacity < 80% of theoretical value, diffusing capacity of the lung for carbon monoxide < 80% of theoretical value, oxygen desaturation during a 6-min walk test and fibrotic-like findings on chest computed tomography.
Results
Among 401 analyzable patients, 160 (40%) were socio-economically deprived and 241 (60%) non-deprived; 319 (80%) patients had respiratory sequelae 6 months after ICU discharge (81% vs 78%, deprived vs non-deprived, respectively). No significant effect of socio-economic status was identified on lung sequelae (odds ratio (OR), 1.19 [95% confidence interval (CI), 0.72–1.97]), even after adjustment for age, sex, most invasive respiratory support, obesity, most severe P/F ratio (adjusted OR, 1.02 [95% CI 0.57–1.83]).
Conclusions
In COVID-19 ARDS survivors, socio-economic status had no significant influence on respiratory sequelae 6 months after ICU discharge.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-023-07180-y.
Keywords: ARDS, COVID-19, Socio-economic, Deprivation, Respiratory sequelae
Take-home message
| In this prospective multicenter cohort of 401 patients who survived acute respiratory distress syndrome due to coronavirus disease 2019, socio-economic status defined by EPICES score was not significantly associated with respiratory sequelae 6 months after intensive care unit (ICU) discharge in French healthcare settings. |
| The frequency of respiratory sequelae defined by the presence of at least one of the following elements: forced vital capacity < 80%, diffusing capacity of the lungs for carbon monoxide < 80%, oxygen desaturation during a 6-min walk test and fibrotic-like findings at chest computed tomography, was still high (80%) at 6 months after ICU discharge. |
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes severe cases of hypoxemic pneumonitis. During the first months of the coronavirus disease 2019 (COVID-19) crisis, around 5% of symptomatic patients required admission to the intensive care unit (ICU) and 50–70% of those needed mechanical ventilation, due to progression to acute respiratory distress syndrome (ARDS) [1–3]. COVID-19 ARDS survivors are at high risk of developing functional impairment and new disabilities, not only due to the severity of the pulmonary infection, but also because of potential multi-organ dysfunction and long ICU stay, as previously reported in non-COVID-19 ARDS [4]. Many COVID-19 cohort studies involved non-ARDS patients, and little is known about the respiratory sequelae late after ICU discharge in patients who survive ARDS [5–7]. A recent meta-analysis of 23 observational cohort studies showed in pooled analysis that lower socio-economic status was associated with higher mortality up to 30 days after admission to critical care, and may also be associated with functional status and discharge destination after ICU admission [8]. The healthcare crisis caused by the COVID-19 pandemic hit socially disadvantaged populations particularly hard [9–11], and may have exacerbated these social disparities in health [12]. In a study using surveillance data reported to the Swiss Federal Office of Public Health from March 1, 2020, to April 16, 2021, Riou et al. reported that during the COVID-19 pandemic in Switzerland, people living in neighbourhoods of low socio-economic position were less likely to be tested but more likely to test positive, be admitted to hospital, or die, compared with those living in areas of high socio-economic position [13]. A life-course trajectory of disadvantaged socio-economic status has been shown to be an important predictor of lower lung function during adulthood [14]. Thereby, socio-economic status may hamper recovery from long-term COVID-19 ARDS consequences. In this context, we conducted the prospective, multicentre RECOVIDS study, to assess the influence of socio-economic status on respiratory recovery in COVID-19 ARDS patients.
Methods
Study design
Details of the study design have previously been published [15]. Briefly, the RECOVIDS study is an observational, multicentre cohort study performed in 30 French centres during routine care for post-ICU functional recovery. The main objective was to evaluate respiratory recovery after COVID-19-related ARDS at 6 months after ICU discharge, according to patients’ socio-economic deprivation status. The study was approved by the ethics committee “Comité de Protection des Personnes Sud Méditerranée II” on 10/07/2020 under the number 2020-A02014-35. In line with French legislation, oral informed consent was obtained from all patients.
Patients
All adult patients admitted to an ICU for SARS-CoV-2 infection confirmed by polymerase chain reaction (PCR), in any of the 30 participating centres were eligible. To be included, patients had to have undergone chest computed tomography (CT) scan at the initial phase of management; have ARDS diagnosed according to the Berlin 2012 definition [16] or received high flow nasal oxygen with a flow of at least 50L/minute with FiO2 > 50% and a PaO2/FiO2 ratio ≤ 200. The main exclusion criteria included a walking perimeter < 50 m or World Health Organization (WHO) performance status 3 or 4; chronic respiratory insufficiency; patients not affiliated to the national health insurance and patients under legal protection (details in Supplemental eMethods 1). Moreover, patients who subsequently failed to attend the first evaluation at 6 months after ICU discharge or who did not have the required tests to enable evaluation of the primary endpoint were excluded from statistical analysis.
Procedures
As previously described [15], patients were screened at ICU discharge and during hospitalization in post-ICU units. If patients fulfilled the inclusion and exclusion criteria, they were pre-included. The scheduled follow-up was performed at 6 ± 1 months after ICU discharge and included a clinical exam with evaluation of dyspnea by the modified Medical Research Council dyspnea scale (mMRC) [17], a 6-min walk test (6MWT) performed according to the guidelines from the European Respiratory Society (ERS) and the American Thoracic Society (ATS) [18], pulmonary function tests (PFT) including spirometry, plethysmography and measurement of diffusing capacity of the lungs for carbon monoxide (DLCO) according to the ATS/ERS guidelines [19, 20], and chest CT without contrast. Dependency was evaluated using the activities of daily living (ADL) [21] and lower limb weakness using the Borg scale before and after the 6MWT. Respiratory quality of life was evaluated focusing on potential effects of respiratory status using the Visual Simplified Respiratory Questionnaire (VSRQ) [22] (Supplemental eFigure 1).
Social deprivation was assessed at the individual level using the EPICES (Evaluation of Deprivation and Inequalities in Health Examination Centers) score [23]. The EPICES score measures social and material deprivation using 11 items relating to social conditions, leisure activities and family/social support [24, 25] (Supplemental eFigure 2). Patients with an EPICES score ≥ 30.17 (corresponding to the lower boundary of the fourth quintile in the validation study of this score) were considered as socially deprived [25]. Ecological deprivation was also assessed at the level of the IRIS (aggregated units for statistical information) using the French European Deprivation Index (FEDI) based on a combination of 10 weighted census-derived variables [26].
Data related to socio-demographic characteristics and the ICU stay until hospital discharge are detailed in the Supplemental eMethods 2.
Outcomes
The primary outcome was the presence of lung sequelae at 6 months after ICU discharge, defined as the presence of any one or more of the following criteria:
- Alterations on PFTs:
- DLCO < 80% of the theoretical value
- And/or forced vital capacity (FVC) < 80% of the theoretical value
- Oxygen desaturation during a standardized 6MWT:
- Delta SpO2 (SpO2 pre walk-SpO2 post walk) ≥ 4% and SpO2 post walk < 90%
- Fibrotic-like pulmonary findings on thin-slice non-enhanced chest CT:
- traction bronchiectasis
- lung architectural distortion
- loss of lung volume with reticular and/or ground glass opacities
- honeycombing
Chest CT interpretation were centralized and performed independently by two experienced chest radiologists blinded to clinical information. In case of disagreement, a final decision was reached by consensus. The fibrotic-like findings were qualitatively measured and corresponded to the terms used to describe pulmonary fibrosis in the Glossary of Terms for Thoracic Imaging of the Fleischner Society [27].
The main secondary outcomes included the persistence of dyspnea, PFT results and muscular dependency, 6 months after ICU discharge (details in Supplemental eMethods 3).
Sample size calculation
Given the lack of specific COVID-19 data at the time the study was designed, the sample size was based on the expected number of admissions in the participating centers as estimated from the number of patients admitted to ICU during the first wave in Spring 2020. Accordingly, 500 patients were expected to be included, enabling detection of an odds ratio (OR) ranging from 1.664 to 2.924, at a two-sided significance level of 5%, power of 80%, with a social deprivation rate of 50% (based on the IVOIRE cohort study [23]), and assuming a respiratory sequelae rate at 6 months ranging from 50% to 90% in patients surviving ARDS [28].
Statistical analysis
Patient characteristics are described according to deprivation status as number and percentage for categorical variables, and mean ± standard deviation (SD) (or median and (Q1–Q3) depending on the distribution) for continuous variables. Data were compared using the Chi-2 or Fisher’s exact test for categorical variables, and the Student t or Wilcoxon tests for continuous variables, as appropriate.
The impact of social deprivation on the presence of lung sequelae at 6 months was analyzed using logistic regression. Analyses were adjusted for clinically relevant factors [age, gender, Simplified Acute Physiology Score II (SAPSII) and Sequential Organ Failure Assessment (SOFA) score at ICU admission, Charlson comorbidity score, body mass index (BMI) at ICU admission, most invasive respiratory support during ICU stay, most severe P/F ratio, hospital acquired pneumonia, length of ICU stay, corticosteroids, presence of rehabilitation between hospital discharge and 6-month evaluation, COVID-19 wave, and center characteristics (university vs non-university hospital)]. We also performed multilevel logistic regression using the FEDI: the first level was the FEDI, and the second was the patient, to take into account the deprivation effect at a contextual and individual level. The model was adjusted for the same factors as above. The main analysis was performed on complete cases. A sensitivity analysis was also performed on imputed data. Missing data were assumed to be missing at random. The chained equation method was used to impute missing data of any patients fulfilling the inclusion, non-inclusion and secondary exclusion criteria. Ten imputed data sets were generated and the modeling described above was applied to each. Finally, results of the 10 analyses were pooled to obtain OR and the associated 95% confidence interval (CI) for each parameter.
Collinearity between adjustment variables was verified by calculating the variance inflation factor (VIF); all values were < 5, indicating absence of multicollinearity.
All secondary outcomes are described in each group (deprived/non-deprived) and were compared between groups using the Chi-2, Student t or Wilcoxon test, as appropriate.
All analyses were performed using SAS Version 9.4 (SAS Institute Inc. Cary, NC, USA) with a bilateral significance threshold of p < 0.05.
Results
Study population
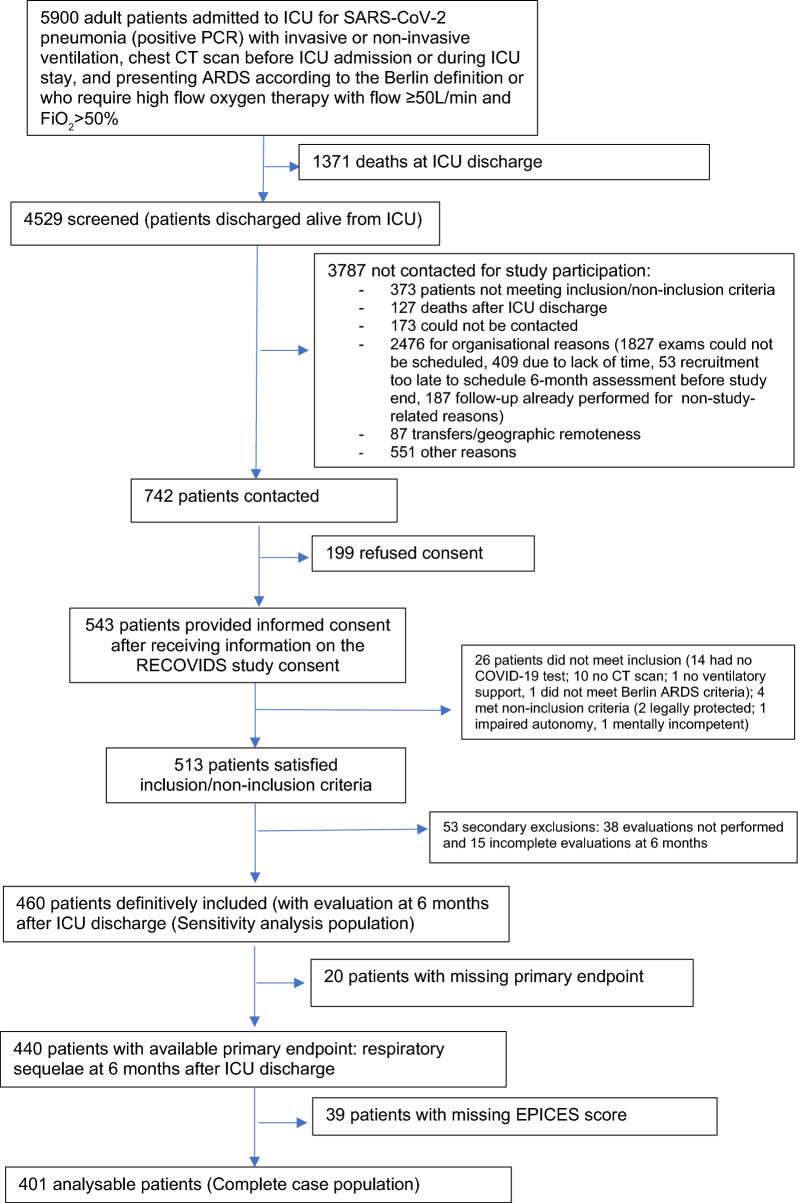
A total of 4529 adult patients with ARDS were discharged alive from 30 participating ICUs between September 2020 and June 2021. Among them, 742 (16%) were invited to participate, of whom 543 (73%) consented to participate. Finally, 401 patients were fully analyzable after verification of compliance with inclusion and non-inclusion criteria, and exclusion of patients who did not attend the 6-month evaluation, or for whom the primary endpoint or the EPICES score were not available (Fig. 1).
Fig. 1.
Flow chart of the study population
Imputed and analyzable patients were comparable regarding demographic and clinical characteristics at baseline (Supplemental eTable 1).
Among the 401 patients included in the final analysis, 160 (40%) were classed as deprived. Mean age (64 ± 11 years) did not differ significantly by deprivation status. Deprived patients were more frequently women (39% vs 28%; p = 0.02), obese (49% vs 35%; p = 0.007) and less often had a history of heart failure (1% vs 5%; p = 0.03) compared with non-deprived patients. Regarding social inequalities, deprived patients more frequently lived alone (31% vs 9%; p < 0.0001), were less often eligible to pay income tax (32% vs 62%; p < 0.001), had a lower rate of graduation from high school (69% vs 55%; p = 0.007) and were less often in employment before COVID (29% vs 37%; p = 0.001) than non-deprived patients (Table 1).
Table 1.
Comparison of patients at admission to the intensive care unit according to socio-economic status
| No./total (%) All patients, N = 401 |
No./total (%) Non-deprived, N = 241 |
No./total (%) Deprived, N = 160 |
p value | |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age, mean (SD), years | 64 (11) | 64 (11) | 62 (12) | 00.14 |
| Female | 129 (32) | 67 (28) | 62 (39) | 0.02 |
| EPICES score, mean (SD) | 27 (18) | 15 (9) | 44 (13) | |
| Quintiles of EPICES score | ||||
| 1 [0; 7,10[ | 40 (10) | 40 (17) | 0 (0) | |
| 2 [7,10; 16,56[ | 96 (24) | 96 (40) | 0 (0) | |
| 3 [16,56; 30,17[ | 105 (26) | 105 (44) | 0 (0) | |
| 4 [30,17; 48,52[ | 113 (28) | 0 (0) | 113 (71) | |
| 5 [48,52; 100] | 47 (12) | 0 (0) | 47 (29) | |
| Less than high school diploma | 238/392 (61) | 130/235 (55) | 108/157 (69) | 0.007 |
| Employment status | 0.001 | |||
| Employed | 134/396 (34) | 88/237(37) | 46/159 (29) | |
| Retired | 229/396 (58) | 139/237 (59) | 90/159 (57) | |
| Not working | 33/396 (8) | 10/237 (4) | 23/159 (14) | |
| Socioeconomic category | < .001 | |||
| Farmers | 11/358 (3) | 5/225 (2) | 6/133 (4) | |
| Self-employed/own business | 39/358 (11) | 31/225 (14) | 8/133 (6) | |
| Upper management and liberal professions | 70/358 (20) | 57/225 (25) | 13/133 (10) | |
| Intermediate professions | 46/358 (13) | 33/225 (15) | 13/133 (10) | |
| Employees | 105/358 (29) | 47/225 (21) | 58/133 (44) | |
| Labourers | 87/358 (24) | 52/225 (23) | 35/133 (26) | |
| Married or living maritally | 308/396 (78) | 211/238 (89) | 97/158 (61) | < .001 |
| Have children | 347/393 (88) | 210/235 (89) | 137/158 (87) | 0.43 |
| Living alone | 72/396 (18) | 22/237 (9) | 50/159 (31) | < .001 |
| Urban dwelling | 188/393 (48) | 106/236 (45) | 82/157 (52) | 0.16 |
| Eligible to pay income tax | 201/394 (51) | 150/235 (64) | 51/159 (32) | < .001 |
| Prefer not to say | 38 (9) | 25/235 (11) | 13/159 (8) | |
| Formal home help | 42/395 (11) | 21/236 (9) | 21/158 (13) | 0.17 |
| Katz ADL score, median (IQR) [no.] | 6 (6–6) [394] | 6 (6–6) [238] | 6 (6–6) [156] | |
| Pre‐existing conditions | ||||
| Alcohol | 0.009 | |||
| None (never or former) | 174/387 (45) | 90/229 (39) | 84/158 (53) | |
| At least one drink per day | 213/387 (55) | 139/229 (61) | 74/158 (47) | |
| Tobacco | 0.008 | |||
| Never | 191/400 (48) | 106/240 (44) | 85/160 (53) | |
| Current | 11/400 (3) | 3/240 (1) | 8/160 (5) | |
| Former | 198/400 (49) | 131/240 (55) | 67/160 (42) | |
| Charlson comorbidity index ≥ 1 | 183/400 (46) | 104/240 (43) | 79/160 (49) | 0.23 |
| Hypertension | 196 (49) | 114 (47) | 82 (51) | 0.44 |
| Diabetes | 110 (27) | 60 (25) | 50 (31) | 0.16 |
| Mild to severe chronic renal failure | 7/398 (2) | 2/239 (1) | 5/159 (3) | 0.12 |
| Congestive heart failure | 12 (3) | 11 (5) | 1 (1) | 0.03 |
| Chronic pulmonary disease | 28/400 (7) | 14/241 (6) | 14/159 (9) | 0.26 |
| Solid tumor with or without metastases (< 5 years) | 25 (6) | 13 (5) | 12 (8) | 0.4 |
| Hematological malignancy | 12 (3) | 8 (3) | 4/160 (3) | 0.77 |
| BMI | 0.02 | |||
| < 30 | 234/395 (59) | 155/240 (65) | 79/155 (51) | |
| [30; 40[ | 131/395 (33) | 67/240 (28) | 64/155 (41) | |
| ≥ 40 | 30/395 (8) | 18/240 (7) | 12/155 (8) | |
| COVID-19 epidemic wave | 0.75 | |||
| First wave | 141 (35) | 88 (36) | 53 (33) | |
| Second wave | 209 (52) | 122 (51) | 87 (54) | |
| Third wave | 51 (13) | 31 (13) | 20 (13) | |
| Severity of illness | ||||
| SOFA, median (IQR) | 4 (3–6) | 4 (3–6) | 4 (3–6) | 0.92 |
| SAPS II, median (IQR) [no.] | 34 (28–43) [399] | 34 (28–44) [240] | 35 (28–43) [159] | 0.59 |
ADL activity daily living, BMI body mass index, COVID-19 coronavirus disease 2019, EPICES evaluation of deprivation and inequalities in health examination centres (translated from French), IQR interquartile range, SD standard deviation, SOFA sequential organ failure assessment, SAPS II, simplified acute physiology score II
The characteristics of ICU and post ICU stay and management did not significantly differ between the 2 groups (Table 2), except for respiratory assistance at ICU discharge, which was more frequent in non-deprived vs deprived patients (82% vs 72%; p = 0.024) (Supplemental eTable 2). There were no significant differences between groups concerning ICU adverse events (Supplemental eTable 3).
Table 2.
Patient management in ICU and post ICU ward until hospital discharge according to socio-economic status
| No./total (%) All patients, N = 401 |
No./total (%) Non-deprived, N = 241 |
No./total (%) Deprived, N = 160 |
|
|---|---|---|---|
| Most invasive respiratory support | |||
| IMV | 205 (51) | 123 (51) | 82 (51) |
| NIV ≥ 24 h then IMV | 36 (9) | 21 (9) | 15 (9) |
| NIV (± HFOT) | 36 (9) | 20 (8) | 16 (10) |
| HFOT | 124 (31) | 77 (32) | 47 (29) |
| Most severe P/F ratio | |||
| [200; 300[ | 19/395 (5) | 15 (6) | 4 (3) |
| [100; 200[ | 170/395 (43) | 104 (43) | 66 (41) |
| < 100 | 206/395 (52) | 121 (50) | 85 (53) |
| Prone positioning | 219 (55) | 135 (56) | 84 (53) |
| Number of prone sessions, median (IQR) [no.] | 3 (2–5) [211] | 3 (2–5) [135] | 3 (1–5) [84] |
| Patients receiving IMV | 241/401 (60) | 144/240 (60) | 97/160 (61) |
| Duration, median (IQR), d [no.] | 17 (9–8) [227] | 16 (9–26) [135] | 17 [9–28] [92] |
| PEEP max, mean (SD), cm of water [no.] | 13 (2.9) [230] | 13.2 (3.1) [138] | 12.8 (2.7) [92] |
| Pplat max, mean (SD), cm of water [no.] | 29.4 (5.6) [200] | 29.1 (5.6) [124] | 29.8 (5.5) [76] |
| Cst min, mean (SD), ml per cm of water [no.] | 25.5 (12.7) [142] | 26.3 (11.7) [89] | 24 (14.2) [53] |
| Neuromuscular blockade | 220 (91) | 131 (91) | 89 (92) |
| Neuromuscular blockade duration, median (IQR), days [no.] | 5 (3–10) [215] | 5 (3–10) [130] | 4 (3–9) [85] |
| Inhaled nitric oxide | 33/239 (14) | 19/142 (13) | 14/97 (14) |
| VV ECMO | 14/240 (6) | 10/143 (7) | 4/97 (4) |
| VV ECMO duration, median (IQR), days [no.] | 10.5 (6–15) | 10.5 (7–12) | 9 (2–16) |
| IMV weaning | |||
| Tracheal re-intubation after weaning failure | 26/241 (11) | 13/144 (9) | 13/97 (13) |
| Tracheotomy | 52/241 (22) | 32 (22) | 20/97 (21) |
| IMV with tracheotomy duration, median (IQR), days [no.] | 18 (14–31) [33] | 16 (12–28) [19] | 22.5 (14–35) [14] |
| Patients receiving NIV during ICU stay | 133/401 (33) | 83/240 (35) | 50/160 (31) |
| Maximum Pressure support, median (IQR), cm of water [no.] | 9 (7–12) [109] | 10 (7–12) [70] | 8 (6–11) [39] |
| PEEP max, median (IQR), cm of water [no.] | 8 (6–10) [119] | 7 (6–8) [74] | 8 (6–10) [45] |
| FiO2 max, median (IQR), % [no.] | 60 (40–100) [129] | 60 (40–100] [79] | 60 (40–90] [50] |
| NIV duration, median (IQR), days [no.] | 3 (2–6) [121] | 3 (2–6) [77] | 4 (2–6.5) [44] |
| Patients receiving HFOT during ICU stay | 307/400 (77) | 184/241 (76) | 123/160 (77) |
| Flow max, median (IQR), L/min [no.] | 50 (50–60) [291] | 50 (50–60) [174] | 50 (50–60) [117] |
| FiO2 max, median (IQR), % [no.] | 80 [60–100) [294] | 80 (60–100) [175] | 80 (60–100) [119] |
| HFOT duration, median (IQR), days [no.] | 5 (3–8) [297] | 5 (3–8) [178] | 5 (3–8) [119] |
| Other organ support | |||
| Vasopressors | 191 (48) | 118 (49) | 73 (46) |
| Vasopressor duration, median (IQR), days [no.] | 6 (3–12) [183] | 6 (3–10) [116] | 5 (3–15) [67] |
| Inotropic agent | 10 (2) | 7 (3) | 3 (2) |
| Renal replacement therapy | 19 (5) | 9 (4) | 10 (6) |
| VA ECMO | 1 (0) | 0 (0) | 1 (1) |
| SARS-CoV-2 infection treatment | |||
| Antiviral therapies | 85/400 (21) | 51/241 (21) | 34/159 (21) |
| Corticosteroids therapies | 223/400 (56) | 133/241 (55) | 90/159 (56) |
| Other Immunomodulatory therapies | 23/400 (6) | 12/241 (5) | 11/159 (7) |
| Length of ICU stay, median (IQR), days | 13 (7–28) | 13 (7–27) | 13 (7–30) |
| Length of hospital stay, median (IQR), days | 23 (15–45) | 22 (15–45) | 25.5 (15–50) |
| Time from ICU discharge to hospital discharge, median (IQR), days | 7 (4–14) | 7 (4–14) | 7.5 (5–16) |
| Significant complications between ICU discharge and hospital discharge | |||
| ICU re-admission | 11/399 (3) | 5 (2) | 6 (4) |
| Tracheal intubation for IMV | 2/399 (1) | 1 (1) | 1 (1) |
ICU intensive care unit, IMV invasive mechanical ventilation, NIV non-invasive mechanical ventilation, HFOT high flow oxygen therapy, P/F ratio partial pressure of arterial oxygen divided by fraction of inspired oxygen, PEEP max maximum of positive end-expiratory pressure, Pplat max maximum of plateau pressure, Cst min minimum of static compliance of the respiratory system, VV ECMO veno-venous extracorporeal membrane oxygenation, FiO2 max maximum of fraction of inspired oxygen, AV ECMO arterio-venous extracorporeal membrane oxygenation, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2
The type of post-ICU rehabilitation was similar between deprived and non-deprived patients, as were the hospital discharge conditions (Supplemental eTable 4). In the overall cohort, 30% of patients were prescribed rehabilitation at hospital discharge, but half of the overall population (52%) actually performed rehabilitation once discharged, with no difference according to deprivation status.
Primary outcome
In all, 319 patients (80%) had lung sequelae at 6 months after ICU discharge (81% in deprived patients vs 78% in non-deprived patients) (Supplemental eTable 5), with no significant impact of deprivation (crude OR = 1.19 [0.72; 1.97]; p = 0.49) even after adjustment for clinically relevant factors (adjusted OR = 1.02 [0.57–1.83]; p = 0.95). Complementary analysis (multilevel modelling and analysis of the imputed population) yielded similar results (Table 3).
Table 3.
Results of primary analysis: effect of socio-economic status on lung sequelae 6 months after ICU discharge
| Complete case analysisa | Multilevel analysisb | Imputed data setsc | |||||
|---|---|---|---|---|---|---|---|
| OR [95% CI] | p value | Adjustedd OR [95% CI] | p value | Adjustedd OR [95% CI] | p value | Adjustedd OR [95% CI] | |
| EPICES | 0.49 | 0.95 | 0.99 | ||||
| Deprived | 1.19 [0.72; 1.97] | 1.02 [0.57; 1.83] | 1.00 [0.54; 1.87] | 1.08 [0.62; 1.87] | |||
| Non-deprived | Reference | Reference | Reference | Reference | |||
OR odds ratio, CI confidence interval, EPICES evaluation of deprivation and inequalities in health examination centres (translated from French), FEDI French European deprivation index, BMI body mass index, SOFA sequential organ failure assessment, SAPS II simplified acute physiology score II, P/F ratio partial pressure of arterial oxygen divided by fraction of inspired oxygen, ICU intensive care unit
aNumber of analyzed patients: 378
bAnalysis taking account of FEDI, number of analyzed patients: 366
cSensitivity analysis, number of analyzed patients: 460
dAdjusted for: center characteristics (university vs non-university Hospital), sex, age, COVID-19 wave, BMI at inclusion, corticosteroids, SAPS II and SOFA at inclusion, most invasive respiratory support during ICU stay, most severe P/F ratio, hospital acquired pneumonia, length of ICU stay, rehabilitation between hospital discharge and 6 month visit, Charlson comorbidity index
Secondary outcomes
Regarding respiratory evaluations (Table 4), deprived patients more often had dyspnea than non-deprived patients according to the mMRC scale (66% vs 50%; p = 0.004), with a lower walking distance on the 6MWT (411 m ± 131 vs 444 m ± 121]; p = 0.01), slightly lower level of SpO2 at the end of the 6MWT (95% [93–97] vs 94% [92–97]; p = 0.04) and a slightly more altered functional respiratory profile. Absolute values of volumes and flows collected on PFT were significantly lower in deprived patients, but when using predicted values, only forced expiratory volume in 1 s (FEV1) and FVC were significantly lower in deprived compared to non-deprived patients. Quality of life (QoL) assessed by the VSRQ score was lower in deprived patients.
Table 4.
Respiratory outcomes 6 months after ICU discharge according to socio-economic status
| No./total (%) All patients, N = 401 |
No./total (%) Non-deprived, N = 241 |
No./total (%) Deprived, N = 160 |
p value | |
|---|---|---|---|---|
| Dyspnea, mMRC scale ≥ 1 (n = 368) | 207/368 (56) | 112/223 (50) | 95/145 (66) | 0.004 |
| 6-min walk test | ||||
| Distance, mean (SD), m [no.] | 431 (126) [390] | 444 (121) [237] | 411 (131) [153] | 0.01 |
| Distance, mean (SD), % predicted | 86 (24) [329] | 87 (25) [205] | 83.1 (23) [124] | 0.11 |
| SpO2 before test, median (IQR), % [no.] | 97 (96–98) [387] | 97 (96–98) [235] | 97 (95–98) [152] | 0.43 |
| SpO2 at end of the test, median (QR), % [no.] | 95 (92–97) [385] | 95 (93–97) [236] | 94 (92–97) [149] | 0.04 |
| Delta SpO2, median (IQR), [no.] | − 2 (− 4–0) [383] | − 2 (− 4–0) [234] | − 2 (− 5–0) [149] | 0.13 |
| Dyspnea (Borg scale) before test, median (QR) [no.] | 0 (0–1) [376] | 0 (0–1) [232] | 0 (0–1) 144] | 0.73 |
| Dyspnea (Borg scale) after test, median (QR) [no.] | 2 (1–4) [377] | 2 (1–4) [233] | 3 (1.3–4.5) [144] | 0.05 |
| Delta dyspnea, median (IQR) [no.] | 2 (0.5–3) [376] | 2 (0–3) [232] | 2 (1–3) [144] | 0.07 |
| Pulmonary function tests | ||||
| FEV1, mean (SD), ml [no.] | 2769 (779) [394] | 2852 (768) [235] | 2647 (783) [159] | 0.01 |
| FEV1, mean (SD), % [no.] | 99 (21) [395] | 100 (20) [236] | 96 (21) [159] | 0.03 |
| FVC, mean (SD), ml [no.] | 3381 (955) [390] | 3492 (941) [232] | 3217 (956) [158] | 0.005 |
| FVC, mean (SD), % [no.] | 95 (20) [392] | 97 (20) [234] | 93 (20) [158] | 0.04 |
| FEV1/FVC, mean (SD), [no.] | 82 (8) [390] | 82 (8) [232] | 83 (9) [158] | 0.50 |
| FEV1/FVC < 70%, % [no.] | 26 (7) [390] | 14 (6) [232] | 12 (8) [158] | 0.55 |
| VC, mean (SD), ml [no.] | 3406 (1018) [344] | 3529 (1043) [208] | 3216 (952) [136] | 0.005 |
| VC, mean (SD), % [no.] | 94 (20 [343] | 96 (21) [207] | 92 (18) [136] | 0.12 |
| FRC, mean (SD), ml [no.] | 2901 (825) [377] | 3019 (818) [227] | 2723 (806) [150] | < 0.001 |
| FRC, mean (SD), % [no.] | 90 (21) [332] | 92 (22) [199] | 88 (21) [133] | 0.09 |
| TLC, mean (SD), ml [no.] | 5375 (1221) [383] | 5522 (1230) [231] | 5153 (1178) [152] | 0.004 |
| TLC, mean (SD), % [no.] | 88 (15) [387] | 89 (16) [234] | 86 (14) [153] | 0.05 |
| TLC < 80% predicted | 107/387 (28) | 61 (26) [234] | 46 (29) [153] | 0.40 |
| DLCO, mean (SD), % [no.] | 73 (19) [380] | 74 (17) [229] | 71 (22) [151] | 0.18 |
| KCO, mean (SD), % [no.] | 90 (18) [343] | 91 (16) [212] | 89 (21) [131] | 0.43 |
| VSRQ scale, median (IQR), [no.] | 56 (41–69) [391] | 59 (45–71) [235] | 50 (38–63.5) [156] | < 0.001 |
| Chest CT | ||||
| No lesions | 50/386 (13) | 35/233 (15) | 15/153 (10) | 0.13 |
| Fibrotic-like lesion(s)a | 148/389 (38) | 97/234 (41) | 51/155 (33) | 0.09 |
| Residual lesionsb | 336/386 (87) | 198/233 (85) | 138/153 (90) | 0.13 |
mMRC the modified Medical Research Council dyspnea scale, SpO2 peripheral capillary oxygen saturation, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, VC vital capacity, FRC functional residual capacity, TLC total lung capacity, DLCO diffusion capacity of the lung for carbon monoxide, KCO carbon monoxide transfer coefficient, VSRQ Visual Simplified Respiratory Questionnaire, CT computed tomography
aFibrotic-like lesions: traction bronchiectasis, lung architectural distortion, loss of lung volume with reticular and/or ground glass opacities, honeycombing
bResidual lesions: ground-glass opacities, reticulations or parenchymal bands
Fibrotic-like lesions on chest CT were found in 37%, with no significance difference between deprived and non-deprived patients (33% vs 41%; p = 0.09) (Table 4).
Concerning muscular dependency (Supplemental eTable 6), there was no difference between deprived and non-deprived patients.
Discussion
This study accrued one of the largest multicentre cohorts to date of COVID-related ARDS survivors, with extensive evaluation of respiratory and physical status at 6 months after discharge from the ICU. A high proportion (80%) of patients had persistent respiratory defects at 6 months, although we did not observe any difference in respiratory recovery according to socio-economic status. This corroborates a previous report, which found no difference across patient groups in self-perceived recovery after COVID-19, according to the Index of Multiple Deprivation [29].
In our study, the absence of any effect of social deprivation status on respiratory recovery could be explained by several factors. First, there was no difference in terms of rehabilitation after hospital discharge between deprived and non-deprived patients. The impact of socio-economic status on health has long been established [30], and this overall effect is largely mediated by lifestyle [31, 32]. However, in practical terms, lifestyle choices are often constrained by the socio-economic environment and the individual’s living conditions [33, 34], culminating in overall poorer health outcomes in individuals with higher levels of poverty or social deprivation [35, 36].
However, the majority of studies to date have mainly investigated the relation between socio-economic status and mortality, especially in the context of the COVID-19 pandemic [8, 13, 36–38]. There is no evidence that socio-economic status influences post ICU rehabilitation access or compliance, as has been described in patients with chronic obstructive pulmonary disease [39] or after surgery [40]. The long-term effects of rehabilitation after ICU in patients who received prolonged mechanical ventilation remain debated [41]. One study reported a positive effect of rehabilitation on physical function at 6 months after discharge [42]. It is thus possible that rehabilitation, including at the very least, regular physical activity, could help patients to limit the sequelae of COVID-19 and recommendations have been issued for the use of rehabilitation programs after COVID-19 [43–45]. Several studies have shown short-term benefits of early rehabilitation at ICU discharge, when performed in dedicated rehabilitation facilities, with effects observed in muscle strength, physical autonomy [46, 47] and respiratory function [46]. However, data are sparse regarding the value of pursuing rehabilitation after hospital discharge. One randomized, controlled trial showed that respiratory rehabilitation performed 6 months after COVID-19 in patients aged over 65 years yielded a significant improvement in lung function tests, and 6MWT distance [48]. In our cohort, 52% of the population were able to attend rehabilitation after hospital discharge, regardless of their level of social deprivation, even though only 30% of the population left the hospital with a prescription for rehabilitation. The dose of rehabilitation was similar in the 2 groups, although this finding should be taken with caution due to the few data available. The French health system provides access with full coverage for all citizens, likely indicating that there was equal access to rehabilitation services for all patients throughout the country. Together with the medical follow-up, this probably explains why there was no difference regarding rehabilitation.
A second potential explanation for our principal finding could be the choice of respiratory parameters to evaluate recovery. Indeed, the parameters chosen may not have been sensitive to the effects of social deprivation. We chose to evaluate residual pulmonary lesions [49], but not the clinical repercussions, apart from severe injury resulting in oxygen desaturation during exercise. The breathing difficulties that result from pulmonary lesions and/or from the deconditioning caused by a prolonged ICU stay are multifactorial in origin, and also depend on muscle strength, cardiac function, nutritional status and mental health [4].
Third, in our cohort, the proportion of socially deprived patients was lower (40%) than in the IVOIRE study (48%) [23]. Contrary to the IVOIRE study, we did not observe any difference at admission between deprived and non-deprived patients in terms of comorbidities (Charlson index) or autonomy (ADLs). These differences suggest that the socio-economic status was dissociated from the baseline health state in our cohort. The relative similarity between groups in terms of health status at admission, and progression through ICU up to discharge, suggest that all patients may have been equally affected by respiratory lesions in the ICU, regardless of their socio-economic status. The effect of deprivation might be expected to be most marked after hospital discharge.
In our study, deprived patients had more impaired respiratory function, as shown by lower values for clinical and functional parameters, oxygenation and quality of life associated with respiratory status. Yet, despite these differences in functional parameters, the mean values all remained above pathological levels. FVC was lower in deprived patients, but nevertheless remained above the threshold of 80% that defines a pathological state [50]. There was a significantly higher proportion of women and patients with obesity among the deprived, and this may also explain why the distance walked was shorter, with lower absolute values for volumes and flows. Indeed, obese patients generally harbour mild restrictive ventilatory disorders [51]. The theoretical values for volumes and flow that were used as reference are lower in women than in men [52]. The respiratory quality of life appears to be more altered in deprived patients, possibly meaning that the clinical and functional profile are really relevant.
The proportion of patients with respiratory sequelae at 6 months after COVID-19 was 80% in our study, which is higher than rates reported to date. This is likely related to the ICU stay and the severity of the initial pulmonary injury, with our patients classed among the most severe [53]. Of note, only 4% of patients still had respiratory support for prolonged respiratory insufficiency at 6 months. It has been shown that DLco was significantly associated with the severity of the initial pneumonia [6, 7]. Here, 64% of patients had DLco < 80% at 6 months after discharge from ICU, which is also higher than previously reported. Fibrotic-like pulmonary lesions have also been reported in the most severely ill COVID-19 patients [5, 54].
Our study has several limitations. First, a significant proportion of patients (84%) who were discharged alive from the ICU could not be included, although mainly related to organizational difficulties (65% of the mentioned reasons) due to the ongoing pandemic. Indeed, substantial resources were redirected to COVID-19 care, with a resultant shortage of personnel dedicated to other tasks, including research, and many complementary examinations were not available. Secondly, we cannot rule out the potential for selection bias, as around 30% of patients contacted at ICU discharge refused to participate in the study. These patients might be more frequently socially deprived than patients who consent to participate. Furthermore, patients who were not affiliated to any national health insurance system were excluded in accordance with French legislation. Third, certain patients could not be included in the final analysis, because either the EPICES score or 6-month outcome was missing. However, imputation of missing data analysis led to consistent results. Fourthly, the EPICES score has only been validated in France, and is more the reflection of overall vulnerability, rather than a purely social measure of deprivation [23]. Several studies performed outside of France have shown an association between socio-economic status and the severity of COVID-19, with an increased risk of hospitalization in ICU and death in socially disadvantaged patients [8, 13, 55, 56]. Therefore, it is possible that we evaluated a direct influence of social deprivation. In addition, the socio-economic deprivation measured at the time of recruitment into the study corresponds to the state prior to admission to ICU. We cannot exclude the possibility that it may have evolved over the course of the study period, up to the evaluation of the functional recovery at 6 months. Finally, the French health insurance system is quite specific in that it provides 100% coverage for the most disadvantaged citizens. Therefore, results cannot be extrapolated to other countries, where health systems likely differ. In particular, in this cohort, access to rehabilitation and physiotherapy during and after hospitalization was equitable thanks to public access to these services. Our results may not be generalizable to healthcare systems, where rehabilitation and physiotherapy services are not delivered through the public healthcare system.
Conclusion
In patients discharged alive after ARDS due to COVID-19, there was no significant difference in terms of respiratory sequelae at 6 months between patients who were socially deprived and those who were not.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank participating ICUs; the General Hospital Center of Dieppe, France and the Burgundy University Hospital of Dijon, France for their support; clinical research assistants for data entry; Emilie Galizzi, project leader (INSERM CIC1432 Epidémiologie Clinique, Dijon, France); Delphine Pecqueur, data‐manager (INSERM CIC1432 Epidémiologie Clinique, Dijon, France); and Fiona Ecarnot, PhD (EA3920, University Hospital Besancon, France) for translation and critical revision.
Members of the RECOVIDS study group: Mélody de Jesus, Sébastien Normant, Jean-Nicolas Dacher, Département de Radiologie, CHU de Rouen, Rouen, France; Thomas Stoup, Service de Pneumologie, CH de Béthune, France; Christophe Vinsonneau, Service de Médecine Intensive Réanimation, CH de Béthune, France; Anne Dewatine, Unité de Recherche Clinique, CH de Béthune, France; Pierre Cuchet, Service de Pneumologie, CHU de Caen, France; Delphine Rots, Unité de Recherche Clinique, CHU de Caen, France; Julien Calus, Service de Médecine Intensive Réanimation, CH Public du Cotentin, Cherbourg-en-Cotentin, France; Gabriel Le Moel, Service de Pneumologie, CH du Cotentin, Cherbourg-en-Cotentin, France; Pierre Kalfon, Gaëtan Badre, Jean-François Roy, Service de Médecine Intensive Réanimation, CH Chartres, France; Damien Roux, Jean-Damien Ricard, Service de Médecine Intensive Réanimation, Hôpital Louis Mourier, Colombes, France; Marie Marcq, Thomas Georges, Service de Pneumologie, CHD Vendée, France; Caroline Pouplet, Service de Médecine Intensive Réanimation, CH La Roche sur Yon, France; Laurie Lagache, Service de Réanimation Médico-Chirurgicale, Groupe Hospitalier du Havre, Le Havre, France; Nicolas Masson, Matthieu Devos, Pneumology unit, St Philibert hospital, Lille catholic university, Lille, France; Raphaël Favory, Sébastien Preau, Alexandre Gaudet, Service de Médecine Intensive Réanimation, CHRU Roger Salengro, Lille, France; Pierre Bouju, Service de Réanimation Polyvalente, Groupe Hospitalier Bretagne Sud, Lorient, France; Lidia Nichita, Pascal Maignan, Service de Pneumologie, Centre Hospitalier Mémorial de Saint Lô, France; Laurence Labourot, Service de Médecine Physique et Réadaptation, CH de Tourcoing, France; Francesco Molinari, Service de Radiologie, CH de Tourcoing, France; Laurence Thirard, Service de Pneumologie, CH de Tourcoing, France; Charlotte Larrat, Service de Médecine Intensive Réanimation, CHRU Bretonneau, Tours, France; Fernando Berdaguer, Service de Médecine Intensive Réanimation, Hôpital Nord-Franche Comté, Trevenans, France; Meltem Karakaya Akgun, Service de Pneumologie, Hopital Nord Franche-Comte, Trevenans, France; Yannick Fedun, Service de Réanimation Polyvalente, CHBA Vannes, Vannes, France; Thiphaine Guy, Service de Pneumologie, CHBA, Vannes, France; Marie Gousseff, Service de Médecine Interne et Maladies Infectieuses, CHBA Vannes, Vannes, France; Déborah Boyer, Medical intensive care unit, Rouen university hospital, France; Tristan Bonnevie, Normandie Univ, UNIROUEN, EA3830, CHU Rouen, ADIR Association; Elsa Demarest, Service de Médecine Intensive Réanimation, CHES Evreux, France; Sami Hraiech, Aude Sylvestre, Céline Sanz, Service de Médecine Intensive Réanimation, Hôpital Nord, Marseille, France; Anne Veinstein, Service de Médecine Intensive Réanimation, CHU de Poitiers, France.
Author contributions
PLD and JPQ had full access to all study data and take responsibility for the integrity of the data analysis. PLD, IF, EK, MD, JPQ, conceptualized and designed the study. All authors contributed to the analysis and/or interpretation of the data. PLD, IF, EK, JPQ drafted the original manuscript. All authors critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript for submission.
Funding
This study did not receive any external funding.
Data availability
The data that support the findings of this trial are available from the corresponding author (JPQ) upon reasonable request.
Declarations
Conflicts of interest
The authors declare no financial or non-financial interests directly or indirectly related to this work.
Ethics approval and consent to participate
The study was approved by the ethics committee “Comité de Protection des Personnes Sud Méditerranée II” on 10/07/2020 under the number 2020-A02014-35. In line with French legislation, oral informed consent was obtained from all patients.
Footnotes
The members of “RECOVIDS Study Group” are listed in the Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/27/2023
A Correction to this paper has been published: 10.1007/s00134-023-07217-2
Contributor Information
Pierre-Louis Declercq, Email: pdeclercq@ch-dieppe.fr.
Isabelle Fournel, Email: isabelle.fournel@u-bourgogne.fr.
Matthieu Demeyere, Email: matthieu.demeyere@chu-rouen.fr.
Anissa Berraies, Email: aberraies@ch-chartres.fr.
Eléa Ksiazek, Email: elea.ksiazek@u-bourgogne.fr.
Martine Nyunga, Email: martine.nyunga@ch-roubaix.fr.
Cédric Daubin, Email: daubin-c@chu-caen.fr.
Alexandre Ampere, Email: aampere@ch-bethune.fr.
Bertrand Sauneuf, Email: bertrandsauneuf@yahoo.fr.
Julio Badie, Email: julio.badie@hnfc.fr.
Agathe Delbove, Email: agathe.delbove@ch-bretagne-atlantique.fr.
Saad Nseir, Email: Saadalla.NSEIR@CHRU-LILLE.fr.
Elise Artaud-Macari, Email: elise.artaud-macari@chu-rouen.fr.
Vanessa Bironneau, Email: vanessa.bironneau@chu-poitiers.fr.
Michel Ramakers, Email: michel.ramakers@ch-stlo.fr.
Julien Maizel, Email: maizel.julien@chu-amiens.fr.
Arnaud-Felix Miailhe, Email: flexmiailhe@hotmail.com.
Béatrice Lacombe, Email: b.lacombe@ghbs.bzh.
Nicolas Delberghe, Email: nicolas.delberghe@ch-eureseine.fr.
Walid Oulehri, Email: walid.oulehri@chru-strasbourg.fr.
Hugues Georges, Email: hgeorges@ch-tourcoing.fr.
Xavier Tchenio, Email: xtchenio@ch-bourg01.fr.
Caroline Clarot, Email: clarot.caroline@ch-abbeville.fr.
Elise Redureau, Email: elise.redureau@chd-vendee.fr.
Gaël Bourdin, Email: gbourdin@chsjsl.fr.
Laura Federici, Email: laura.federici85@gmail.com.
Mélanie Adda, Email: melanie.adda@ap-hm.fr.
David Schnell, Email: david.schnell@ch-angouleme.fr.
Mehdi Bousta, Email: mehdi.bousta@ch-havre.fr.
Charlotte Salmon-Gandonnière, Email: charlotte.salmon.gandonniere@gmail.com.
Thierry Vanderlinden, Email: vanderlinden.thierry@ghicl.net.
Gaëtan Plantefeve, Email: gaetan.plantefeve@ch-argenteuil.fr.
David Delacour, Email: d.delacour@gmail.com.
Cyrille Delpierre, Email: cyrille.delpierre@inserm.fr.
Gurvan Le Bouar, Email: gurvan.le-bouar@chu-rouen.fr.
Nicholas Sedillot, Email: nsedillot@ch-bourg01.fr.
Gaëtan Beduneau, Email: gaetan.beduneau@chu-rouen.fr.
Antoine Rivière, Email: riviere.antoine@ch-abbeville.fr.
Nicolas Meunier-Beillard, Email: nicolas.meunier-beillard@u-bourgogne.fr.
Stéphanie Gélinotte, Email: sgelinotte@ch-dieppe.fr.
Jean-Philippe Rigaud, Email: jrigaud@ch-dieppe.fr.
Marie Labruyère, Email: marie.labruyere@chu-dijon.fr.
Marjolaine Georges, Email: marjolaine.georges@chu-dijon.fr.
Christine Binquet, Email: christine.binquet@u-bourgogne.fr.
Jean-Pierre Quenot, Email: jean-pierre.quenot@chu-dijon.fr.
the RECOVIDS trial investigators, the CRICS-TRIGGERSEP, BOREAL research networks:
Mélody De Jesus, Sébastien Normant, Jean-Nicolas Dacher, Thomas Stoup, Christophe Vinsonneau, Anne Dewatine, Pierre Cuchet, Delphine Rots, Julien Calus, Gabriel Le Moel, Pierre Kalfon, Gaëtan Badre, Jean-François Roy, Damien Roux, Jean-Damien Ricard, Marie Marcq, Thomas Georges, Caroline Pouplet, Laurie Lagache, Nicolas Masson, Matthieu Devos, Raphaël Favory, Sébastien Preau, Alexandre Gaudet, Pierre Bouju, Lidia Nichita, Pascal Maignan, Laurence Labourot, Francesco Molinari, Laurence Thirard, Charlotte Larrat, Fernando Berdaguer, Meltem Karakaya Akgun, Yannick Fedun, Thiphaine Guy, Marie Gousseff, Déborah Boyer, Tristan Bonnevie, Elsa Demarest, Sami Hraiech, Aude Sylvestre, Céline Sanz, and Anne Veinstein
References
- 1.Covid-Icu Group on behalf of the REVA Network, C-I-C-U Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiumello D, Coppola S, Froio S, Gotti M. What's next after ARDS: long-term outcomes. Respir Care. 2016;61:689–699. doi: 10.4187/respcare.04644. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez J, Benitez ID, Carmona P, et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160:187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guler SA, Ebner L, Aubry-Beigelman C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021 doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHenry RD, Moultrie CEJ, Quasim T, Mackay DF, Pell JP. Association between socioeconomic status and outcomes in critical care: a systematic review and meta-analysis. Crit Care Med. 2023;51:347–356. doi: 10.1097/CCM.0000000000005765. [DOI] [PubMed] [Google Scholar]
- 9.Baggett TP, Keyes H, Sporn N, Gaeta JM. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323:2191–2192. doi: 10.1001/jama.2020.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapostolle F, Goix L, Vianu I, Chanzy E, De Stefano C, Gorlicki J, Petrovic T, Adnet F. COVID-19 epidemic in the Seine-Saint-Denis Department of Greater Paris: one month and three waves for a tsunami. Eur J Emerg Med. 2020;27:274–278. doi: 10.1097/MEJ.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 11.Wadhera RK, Wadhera P, Gaba P, Figueroa JF, Joynt Maddox KE, Yeh RW, Shen C. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flor LS, Friedman J, Spencer CN, et al. Quantifying the effects of the COVID-19 pandemic on gender equality on health, social, and economic indicators: a comprehensive review of data from March, 2020, to September, 2021. Lancet. 2022;399:2381–2397. doi: 10.1016/S0140-6736(22)00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riou J, Panczak R, Althaus CL, Junker C, Perisa D, Schneider K, Criscuolo NG, Low N, Egger M. Socioeconomic position and the COVID-19 care cascade from testing to mortality in Switzerland: a population-based analysis. Lancet Public Health. 2021;6:e683–e691. doi: 10.1016/S2468-2667(21)00160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha V, Stringhini S, Henriques A, Falcao H, Barros H, Fraga S. Life-course socioeconomic status and lung function in adulthood: a study in the EPIPorto cohort. J Epidemiol Community Health. 2020;74:290–297. doi: 10.1136/jech-2019-212871. [DOI] [PubMed] [Google Scholar]
- 15.Declercq PL, Fournel I, Demeyere M, et al. Influence of socioeconomic status on functional recovery after ARDS caused by SARS-CoV-2: a multicentre, observational study. BMJ Open. 2022;12:e057368. doi: 10.1136/bmjopen-2021-057368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Williams N. The MRC breathlessness scale. Occup Med (Lond) 2017;67:496–497. doi: 10.1093/occmed/kqx086. [DOI] [PubMed] [Google Scholar]
- 18.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 19.Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, MacIntyre NR, Thompson BR, Wanger J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017 doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 20.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 22.Perez T, Arnould B, Grosbois JM, Bosch V, Guillemin I, Bravo ML, Brun M, Tonnel AB, Group TS Validity, reliability, and responsiveness of a new short Visual Simplified Respiratory Questionnaire (VSRQ) for health-related quality of life assessment in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2009;4:9–18. [PMC free article] [PubMed] [Google Scholar]
- 23.Quenot JP, Helms J, Labro G, et al. Influence of deprivation on initial severity and prognosis of patients admitted to the ICU: the prospective, multicentre, observational IVOIRE cohort study. Ann Intensive Care. 2020;10:20. doi: 10.1186/s13613-020-0637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bihan H, Ramentol M, Fysekidis M, et al. Screening for deprivation using the EPICES score: a tool for detecting patients at high risk of diabetic complications and poor quality of life. Diabetes Metab. 2012;38:82–85. doi: 10.1016/j.diabet.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Labbe E, Blanquet M, Gerbaud L, Poirier G, Sass C, Vendittelli F, Moulin JJ. A new reliable index to measure individual deprivation: the EPICES score. Eur J Public Health. 2015;25:604–609. doi: 10.1093/eurpub/cku231. [DOI] [PubMed] [Google Scholar]
- 26.Pornet C, Delpierre C, Dejardin O, Grosclaude P, Launay L, Guittet L, Lang T, Launoy G. Construction of an adaptable European transnational ecological deprivation index: the French version. J Epidemiol Community Health. 2012;66:982–989. doi: 10.1136/jech-2011-200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 28.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 29.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health. 1992;82:816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Contoyannis P, Jones AM. Socio-economic status, health and lifestyle. J Health Econ. 2004;23:965–995. doi: 10.1016/j.jhealeco.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Geng L. Effects of socioeconomic status on physical and psychological health: lifestyle as a mediator. Int J Environ Res Public Health. 2019 doi: 10.3390/ijerph16020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cockerham WC, Abel T, Lüschen G. Max Weber, formal rationality, and health lifestyles. Sociol Quart. 1993;34:413–425. doi: 10.1111/j.1533-8525.1993.tb00119.x. [DOI] [Google Scholar]
- 34.Link BG, Phelan J (1995) Social conditions as fundamental causes of disease. J Health Soc Behav; Spec No: 80–94 [PubMed]
- 35.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood) 2002;21:60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 36.Little C, Alsen M, Barlow J, Naymagon L, Tremblay D, Genden E, Trosman S, Iavicoli L, van Gerwen M. The impact of socioeconomic status on the clinical outcomes of COVID-19; a retrospective cohort study. J Community Health. 2021;46:794–802. doi: 10.1007/s10900-020-00944-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faramarzi A, Javan-Noughabi J, Mousavi SA, Bahrami Asl F, Shabanikiya H. Socioeconomic status and COVID-19-related cases and fatalities in the world: a cross-sectional ecological study. Health Sci Rep. 2022;5:e628. doi: 10.1002/hsr2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bastian K, Hollinger A, Mebazaa A, et al. Association of social deprivation with 1-year outcome of ICU survivors: results from the FROG-ICU study. Intensive Care Med. 2018;44:2025–2037. doi: 10.1007/s00134-018-5412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone PW, Hickman K, Steiner MC, Roberts CM, Quint JK, Singh SJ. Predictors of pulmonary rehabilitation completion in the UK. ERJ Open Res. 2021 doi: 10.1183/23120541.00509-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stonner MM, Keane G, Berlet L, Goldfarb CA, Pet MA. The impact of social deprivation and hand therapy attendance on range of motion after flexor tendon repair. J Hand Surg Am. 2022;47:655–661. doi: 10.1016/j.jhsa.2022.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Fuke R, Hifumi T, Kondo Y, Hatakeyama J, Takei T, Yamakawa K, Inoue S, Nishida O. Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: a systematic review and meta-analysis. BMJ Open. 2018;8:e019998. doi: 10.1136/bmjopen-2017-019998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris PE, Berry MJ, Files DC, et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. JAMA. 2016;315:2694–2702. doi: 10.1001/jama.2016.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailly M, Pelissier L, Coudeyre E, et al. Systematic review of COVID-19-related physical activity-based rehabilitations: benefits to be confirmed by more robust methodological approaches. Int J Environ Res Public Health. 2022 doi: 10.3390/ijerph19159025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imamura M, Mirisola AR, de Quadros RF, Ramos De Pretto L, Marcon Alfieri F, Ramos Delgado V, Rizzo Battistella L. Rehabilitation of patients after COVID-19 recovery: an experience at the Physical and Rehabilitation Medicine Institute and Lucy Montoro Rehabilitation Institute. Clinics. 2021;76:e2804. doi: 10.6061/clinics/2021/e2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang F, Liu N, Hu JY, Wu LL, Su GS, Zhong NS, Zheng ZG. Pulmonary rehabilitation guidelines in the principle of 4S for patients infected with 2019 novel coronavirus (2019-nCoV) Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:180–182. doi: 10.3760/cma.j.issn.1001-0939.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Puchner B, Sahanic S, Kirchmair R, et al. Beneficial effects of multi-disciplinary rehabilitation in postacute COVID-19: an observational cohort study. Eur J Phys Rehabil Med. 2021;57:189–198. doi: 10.23736/S1973-9087.21.06549-7. [DOI] [PubMed] [Google Scholar]
- 47.Woo H, Lee S, Lee HS, et al. Comprehensive rehabilitation in severely ill inpatients with COVID-19: a cohort study in a tertiary hospital. J Korean Med Sci. 2022;37:e262. doi: 10.3346/jkms.2022.37.e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020;39:101166. doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta P, Rosas IO, Singer M. Understanding post-COVID-19 interstitial lung disease (ILD): a new fibroinflammatory disease entity. Intensive Care Med. 2022;48:1803–1806. doi: 10.1007/s00134-022-06877-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godfrey MS, Jankowich MD. The vital capacity is vital: epidemiology and clinical significance of the restrictive spirometry pattern. Chest. 2016;149:238–251. doi: 10.1378/chest.15-1045. [DOI] [PubMed] [Google Scholar]
- 51.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol (1985) 2010;108:206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 52.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Characterisation WWGotC, Management of Covid-infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Writing Committee for the Comebac Study Group. Morin L, Savale L, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lone NI, McPeake J, Stewart NI, et al. Influence of socioeconomic deprivation on interventions and outcomes for patients admitted with COVID-19 to critical care units in Scotland: a national cohort study. Lancet Reg Health Eur. 2021;1:100005. doi: 10.1016/j.lanepe.2020.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nordberg P, Jonsson M, Hollenberg J, Ringh M, Kiiski Berggren R, Hofmann R, Svensson P. Immigrant background and socioeconomic status are associated with severe COVID-19 requiring intensive care. Sci Rep. 2022;12:12133. doi: 10.1038/s41598-022-15884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this trial are available from the corresponding author (JPQ) upon reasonable request.