Abstract
Purpose of Review
Extramammary Paget disease (EMPD) is a rare entity which is more frequently localized at the vulva, though it only accounts for 1–2% of vulvar neoplasms. It is a primary cutaneous adenocarcinoma whose cell of origin is still a matter of controversy: it can either arise from apocrine/eccrine glands or from stem cells. The diagnosis demands a biopsy and entails a histopathological analysis by which cells show similar characteristics as breast Paget disease.
Recent Findings
Treatment approach can entail surgery, radiotherapy, photodynamic therapy, systemic chemotherapy, and topical chemotherapy. For metastatic disease, many different chemotherapy regimens have been explored and even targeted therapy can play an important role in this disease. Since almost 30–40% of patients overexpress HER-2, trastuzumab and anti-HER-2 therapies can be employed in this setting.
Summary
Due to its low incidence, there is almost no specific evidence on therapeutic interventions for this disease. Thus, there is a neat unmet need for molecular characterization of EMPD and diagnostic tools that allow clinicians to guide treatment both in the early and in the advanced disease settings. In this review, we aim to summarize available evidence about diagnosis and treatment of EMPD, both localized and metastatic, and to provide a comprehensive analysis that may help clinicians for therapeutic decisions.
Keywords: Extramammary Paget disease, Imiquimod, Radiotherapy, Chemotherapy, HER-2
Introduction
Vulvar cancer is the 4th gynecological neoplasm by frequency in women in the USA after uterus, ovary, and cervix. However, it is a very rare pathology, with about 6000 cases per year and a mortality of about 1000 cases in the USA [1]. Squamous cell is the most frequent histology, representing up to 75% [2]. Other histologies include melanoma, basal cell carcinoma, adenocarcinoma of the Bartholin glands, sarcomas, and Paget’s disease.
Paget’s disease was described by Sir James Paget in 1874 [3]. The first case of extramammary Paget’s disease (EMPD) was described by Crocker in 1889 [4] being the vulva the most affected region (54–65%) [5, 6]. In contrast, EMPD accounts for only 1–2% of vulvar neoplasms [7, 8]. Although it may be asymptomatic, the majority present with pruritus, burning, or decreased sensitivity [9••], being white postmenopausal women the most frequently population affected [10]. In general, it is well delimited, with slightly raised edges and a red background, often with small pale islands. It is usually multifocal and can appear anywhere on the vulva, perineum, or inner thighs.
The diagnosis is histological, and there may be foci of invasive adenocarcinoma in up to 4–17%, inside and/or below the lesion [11, 12]. In addition, it can be associated with other malignancies such as breast, rectum, bladder, urethra, cervix, or ovary in up to 10–42% of cases [13, 14].
Histopathology and Immunohistochemistry
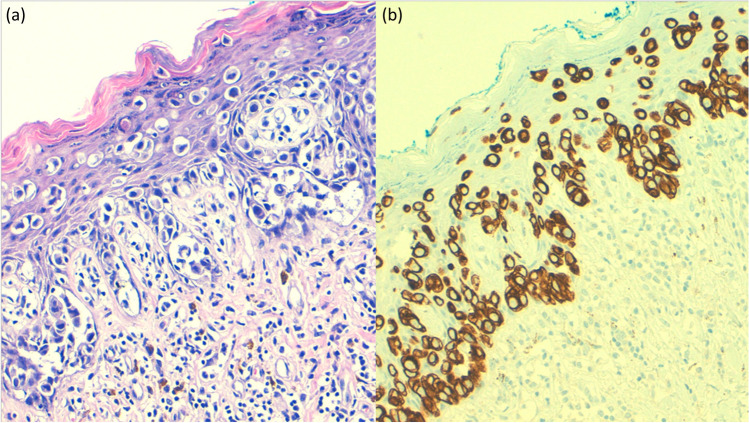
The diagnosis is histopathological. The tumor cells of EMPD, as well called Paget’s cells, have abundant pale cytoplasm and large nuclei with a prominent, vesicular nucleus, with unusual mitotic figures [15] (Fig. 1a). The tumor usually locates in the epidermis, with occasional dermal invasion. This entity can be confused with other neoplasms, such as melanoma, Bowen’s disease, or sebaceous carcinoma, which can show similar morphological findings. In these cases, periodic acid-Schiff (PAS) and Alcian blue stains can help to make an accurate diagnosis, due to the abundant mucin production of this entity. Immunohistochemistry may provide helpful information to make a proper diagnosis. On the one hand, EMPD is usually positive for carcinoembryonic antigen (CEA), cytokeratin (CK) 7 (Fig. 1b), CAM5.2, and gross cystic disease fluid protein (GCDFP15). On the other hand, it is negative for S100 protein and other melanocytic markers (melan-A, MITF, HMB45, etc.) [16].
Fig. 1.
a Paget disease (hematoxylin and eosin): nests of pale-staining tumor cells are arranged singly or in small cluster along the vulvar epidermis. b CK7 immunohistochemistry in a Paget disease
Notwithstanding, primary and secondary EMPD can show different immunophenotypes [17–19]. Usually, primary EMPD is positive for CK7 and GCDFP15 (with CK20 negative). Nevertheless, some locations may present different immunohistochemistry profiles. For instance, there are cases of primary perianal EMPD with GCDFP15-positive and CK20-negative [17], whereas other cases of primary vulvar EMPD show both markers positive [19].
A recent study has described a high presence of EpCAM (epithelial cell adhesion molecule, CD326) in EMPD and a negative correlation between its expression levels and the presence of distant metastasis [20].
It has also been reported a frequent overexpression of cyclin-dependent kinase 4 and cyclin D1 in EMPD [21], which could play a role in the disease treatment [22].
Prognostic Factors
Several factors of poor prognosis have been identified in EMPD. Vaginal localization had higher mortality than those localized in vulva or lips, as well as those with metastatic involvement (HR 3.26, both). Male sex (HR 1.42), being older (HR 1.09), and different races from white or black (HR 0.81 Vs. 0.65) also have worse prognosis [23].
In another analysis, receiving radiotherapy was related with poorer prognosis (HR 1.6 vs. 1.09). Therefore, they re-analyzed the risk factors in a separate multivariate analysis according to whether they had received radiotherapy or not. The location in the vagina and the presence of metastases continued to be a factor of poor prognosis, while having undergone surgery was a factor of good prognosis in all patients. Moreover, the race ceased to be a prognostic factor and sex only continued to be so in patients who did not receive radiotherapy [6].
Other authors had previously described tumor depth, lymphovascular invasion, and the presence of metastases as factors of poor prognosis [24, 25], likewise the number of affected lymph nodes [24, 26•], or positive sentinel lymph node biopsy (SLNB) [26•].
Positron Emission Tomography (PET)–Computed Tomography (CT)
There is limited evidence about the role of PET-CT in EMPD to assess the presence of disease at a distance [27,28,29,30,31,32, 33•].
A retrospective study reviewed 10 cases with newly diagnosed disease or suspected recurrence [32], with two cases of recurrence confirmed and one false positive. Nodal involvement was detected in 6 cases, with 20.4% of them measuring less than 1 cm. Other metastatic locations included bone, liver, lung, and adrenal glands. Compared to the conventional study, 4 out of 10 patients experienced changes in staging and the treatment was modified based on the findings in 3 of them [32].
Inversely, other study analyzed the relationship of SUVmax with the presence of lymph node metastases. They evaluated 15 cases of patients with localized EMPD in which pre-surgical PET and SLNB had been performed. The SLNB was positive in 7 patients (of 37 nodes analyzed). These positive nodes showed a SUVmax between 3.7 and 11.7, while it was ≤1.74 in the negative nodes. Three nodes were suspected in CT scan, resulting in a negative histological study, while 2 nodes with no suspicion of malignancy in the PET scan were affected. The authors proposed a SUVmax cut-off point of 2.5 [33•].
According to the groups established in Table 1, there were no cases of false positives or false negatives. Based on their results, the authors concluded that PET is a valid tool for the detection of regional lymph node involvement, suggesting the need for a SLNB in cases with a SUVmax ≤2.5.
Table 1.
Groups established by Fujiwara et al.
| Histology | |||
|---|---|---|---|
| + | - | ||
| PET-CT | + | 7 | 0 |
| - | 0 | 19 | |
PET-CT, positron emission tomography-computerized tomography
Although there is no robust evidence to recommend standard use of PET-CT for the evaluation of both localized and metastatic EMPD (mEMPD), it seems to provide useful information for disease staging.
Sentinel Lymph Node Biopsy
The need to carry out SLNB is controversial and the available evidence shows dissimilar results. Hatta et al. published a case series of 13 patients who underwent SLNB (unilaterally or bilaterally), being positive 4 of them. Three of the four patients with a positive SLNB had invasive disease, while all patients with in situ disease had a negative SLNB. Those 3 patients underwent lymphadenectomy, being positive all of them. Subsequently, two of them presented distant metastases [34].
Ogata presented similar results in a case series of 59 patients in whom surgery and SLNB were performed. The SLNB was positive in 10 patients, performing a subsequent lymphadenectomy in 9 of them (the other patient refused it). There was a total of 27 nodes out of 139 with a median of affected nodes per patient of 2.4. The probability of a positive SLNB was correlated with the level of invasion of the primary tumor. The 5-year overall survival (OS) in patients with a positive SLNB was 24%, while in the negative SLNB group was 100%. Similarly, the number of affected nodes was also found as a prognostic factor, with a 5-year survival rate of 100% for those who had 2 or less and 0% for those who had 3 or more [26•].
However, Fujisawa et al. presented divergent results. They analyzed a total of 151 patients who underwent SLNB, performing lymphadenectomy in 113 of them. Of the 107 patients without clinical lymphadenopathy, 16 had lymph node involvement. Although the level of invasion was again shown as a poor prognosis factor, there were no differences between positive or negative SLNB. The relapse rate was also similar among patients with positive and negative SLNB [35].
Despite discordant results from the Fujisawa study, it seems that the level of invasion and having affected nodes (either after lymphadenectomy or SLNB) worsen the prognosis. Nevertheless, performing a lymphadenectomy after a positive SLNB has not clearly been demonstrated to improve these patients’ prognosis. Given the comorbidities associated with lymphadenectomies, we believe that each case must be individualized when indicating a SLNB.
Other authors have suggested the use of ultrasound imaging to identify suspicious lymph nodes, based on morphology and perfusion criteria (balloon shape, loss of central echo, and presence of peripheral perfusion) which are as useful as the longitudinal/transversal ratio of 2 or less. They also propose to perform a lymph node biopsy instead of immediate lymphadenectomy [36].
Surgery
Lesion excision is the standard treatment for localized disease, avoiding radical vulvectomy, but ensuring margins of 2 cm. The degree of depth of the lesion and lymphovascular involvement are poor prognosis factors [15], so some authors have proposed lymphadenectomy if there are foci of adenocarcinoma [37].
Sarmiento et al. evaluated the need for lymphadenectomy in a study where 34 patients were classified into 4 groups: carcinoma in situ (CIS), microinvasion to the papillary dermis (MIPD), invasion of the reticular dermis (IRD), and invasion of subcutaneous tissue (IST) [38]. Patients with CIS or MIPD did not undergo lymphadenectomy and no disease-related deaths were reported. Among the 6 patients with IRD, 4 underwent lymphadenectomy, with 3 of them presenting node metastases, who would later die. All the 6 patients with IST underwent lymphadenectomy, and all of them had node involvement, dying due to the disease. Based on this, the authors recommended lymphadenectomy if the lymph node biopsy is positive and in case of IRD or IST, being unnecessary in cases of CIS or MIPD. Notwithstanding, no survival improvement was demonstrated with lymphadenectomy [38]; thus, its execution should be individualized.
Despite performing surgery with free margins, up to 12–60% of cases can present local relapse [12, 39–45]. In these cases, a new resection can be attempted. Since it is possible to need 5-cm margins of “apparently normal skin” to achieve free margins, some authors have proposed resection by Mohs surgery to achieve it [46] with good results in retrospective series [47]. A recent study reported 11% of recurrences in 19 patients treated with Mohs surgery compared with 36% of recurrences in 45 patients treated with conventional surgery [48••].
Non-surgical Treatments
In those cases, in which surgical resection is not possible, due to patient comorbidities or unresectable disease, there are alternatives such as radiotherapy, photodynamic therapy, systemic chemotherapy, and topical chemotherapy, especially imiquimod.
Imiquimod binds to the Toll-like receptor 7, inducing an innate and cell-mediated immune response [49]. There are several articles reporting benefits in the use of 5% topical imiquimod. Overall response rates (ORR) between 56 and 100% have been described, with a recurrence rate up to 62% at the end of treatment, although most of the lesions responded again after reintroducing imiquimod [49–57]. Three times a week application was the most recurrent schedule in those studies. The most frequent toxicities are erythema, erosions, and local pain. Some patients may require the use of concomitant topical corticosteroids or anesthetics due to these adverse events.
There is a recently published study which explored the application of 5% imiquimod in patients with non-invasive recurrent vulvar EPMD (vEMPD). In this multicenter, prospective, observational, and open-label study, 24 patients were included, receiving imiquimod 3 times a week for 16 weeks. The reported results showed an ORR of 82.6%, with 10 histological complete responses (CR) and 7 partial responses [58••].
Photodynamic therapy is a non-invasive treatment that uses photosensitizing drugs such as 5-aminolevulinic acid or 16% methyl-aminolevulinic acid. The area where it is applied is afterwards exposed to light with the appropriate wavelength that destroy tumor cells. The ORR rages between 53 and 68% but there is a high recurrence rate, between 56 and 100% [59–61]. The predominant side effects include burns and pain.
Radiotherapy
Tolia et al. published in 2016 a deep review about the use of radiotherapy in vEMPD [62]. They reviewed 10 articles between 1992 and 2015 with a total of 57 patients with invasive vEMPD treated with radiotherapy. The authors concluded that surgical resection continues to be the standard of treatment in vEMPD, although recurrence rates after it varies from 12 to 60% [12, 31–37].
Hence, they recommend the use of radiotherapy with adjuvant intention in case of affected margins, locoregional lymph node involvement, multifocal disease, or presence of adenocarcinoma. Likewise, they also consider the use of radiotherapy as an alternative in cases where surgery is not possible. Given the heterogeneity of the reported cases, they do not establish an optimal dose of radiotherapy, but for definitive treatment, it ranges between 44 and 81.6 Gy in the primary tumor and between 44 and 70.2 Gy in the locoregional lymph nodes, and in the adjuvant setting, it ranges between 44 and 64.8 Gy and 32 and 50.4 Gy, respectively [62].
There is a more recent systematic review, in which 195 patients were analyzed [63••]. For definitive treatment, doses of radiotherapy between 30 and 80.2 Gy (3–43 fractions) were observed, with CR rates of 50–100% and relapse/persistence rates between 0 and 80%. The authors recommend, at least, doses of 60 Gy. In the adjuvant setting, the doses used were between 32 and 64.8 Gy (20–30 fractions) with relapse rates of 0–62.5%. The authors recommend doses between 45 and 60 Gy in cases of dermal invasion by the tumor of proximal involvement to the margins, and at least 60 Gy in cases of positive postoperative margins or nodal involvement. This study has the same limitations as that of Tolia et al. with respect to the great heterogeneity it presents regarding tumor staging and the different techniques used, as well as the volumes and doses of radiotherapy used.
In a large retrospective series that collected the data from Surveillance, Epidemiology, and End Results (SEER) program of 1439 patients with EMPD, the vulva was the most commonly affected region (781 cases, 81.3%) [6]. In this series, 1230 patients (86.4%) underwent surgery. The majority of patients (1335, 93.6%) did not receive radiotherapy. Of those who underwent radiotherapy (92, 6.4%), 51 received it as adjuvant treatment after surgery and 40 as sole treatment. Patients who received radiotherapy had a disease-specific survival (DSS) of 134.7 months (95% confidence interval (CI) 113.7–155.6), being lower than those who did not receive radiotherapy (342.1 months 95% 330.5–353.7, p<0.001). Patients who underwent surgery exclusively showed better prognosis with a DSS of 346.8 months (95% CI 335–358.6) compared to patients who did not undergo surgery or radiotherapy (median DSS 255.1 months, 95% CI 221.1–289.2, p=0.002), to patients who only received radiotherapy (median DSS 143.3 months, 95% CI 119.2–167.5, p=0.004), and to patients who underwent surgery and radiotherapy (median DSS 120.6 months, 95% CI 93.6–147.6, p<0.001).
This detriment in DSS in those patients who received radiotherapy (including those who also underwent surgery) could not be explained by the authors, in spite of adjusting for sex, age, location, and stage of the disease. The authors suggested radiotherapy may induce biological alterations in the tumor that render it greater aggressiveness, although they could not prove it. They also concluded that the heterogeneity of the data could affect the analysis and its interpretation.
By their hand, Tolia et al. explained that these results could be due to the fact that the use of radiotherapy was related to advanced stages or recurrent disease. They also believed that the interpretation of the data may be affected by the heterogeneity of radiotherapy schemes, dose, and fields of application [62].
By our hand, we agree with the conclusions of Tolia et al., reserving RT for those situations in which surgery is not possible, or as an adjuvant treatment in case of margins affected, locoregional lymph node involvement, multifocal disease, or presence of adenocarcinoma.
Systemic Treatment for Advanced Disease
There is no standard treatment for mEMPD and no randomized clinical trials have been performed in order to settle the optimal treatment for this disease. Given its low frequency, the vast majority of the available evidence proceeds from multiple isolated cases and small case series.
The first report cases date from the late 1980s and early 1990s. Piedbois published a case of a woman who presented a CR after been treated with mitomycin-C, vincristine, 5-fluorouracil, doxorubicin, cisplatin, and bleomycin [64]. Balducci would only use mitomycin-C and 5-fluorouracil in a male patient, who obtained a CR as well [65]. In the early 1990s, the case of a woman treated with mitomycin-C, cisplatin, and vincristine would also be published, reaching a partial response (PR) for 5 months [66]. In the frame of this thinking, the FECOM scheme was established, consisting in a combination of mitomycin-C, epirubicin, vincristine, carboplatin, and 5-fluorouracil. It was firstly used in a case with a PR and a 15-month survival [67]. Additionally, in a series of 7 patients, an ORR of 100% and a 1-year survival rate of 43% were reported with the use of FECOM schedule [68]. However, there are also reported cases of poor response [69, 70].
Combinations with fluoropyrimidines and platins have been widely explored in the last decade. Holger presented a case of a patient treated with 5-fluorouracil in combination with carboplatin with a CR for 12 months [71]. Subsequently, carboplatin was substituted in favor of cisplatin. Kariya et al. achieved a PR maintained for 16 months [72]. In a retrospective analysis of 22 cases, an ORR of 59% and a 1-year survival rate of 50% were obtained [73]. Kato et al. would also perform a retrospective analysis in 8 patients treated with this scheme, with an ORR of 50% and an OS of 19 months [74].
In 2019, a case of mEMPD treated with a non-previously described combination of topic 5-fluorouracil and systemic pemetrexed was reported. The disease showed a PR for more than 6 months [75].
Some cases treated with anthracyclines either in monotherapy or in combination have likewise been reported. A patient with cerebral involvement that did not respond to polychemotherapy and other therapies (tamoxifen, thalidomide) obtained a PR with liposomal doxorubicin and progression free survival (PFS) of 1 year [76]. Anecdotally, a case of complete remission of localized vEMPD in a patient who was simultaneously treated with anthracyclines and taxanes for a breast cancer has been reported [77].
However, at the beginning of the twenty-first century, taxanes became the most used drugs in mEMPD, frequently combined with 5-fluorouracil and carboplatin.
Docetaxel monotherapy has been broadly used as first-line treatment. Fujisawa would use a 2:1 week scheme with a PR maintained for 13 months [78], while other authors would use a 3:1 week scheme, obtaining in this case a CR after 9 cycles and PFS of 2 years [79]. Yoshino et al. would present a series of 13 patients using monthly docetaxel with an ORR of 58% (all of them were PR), a PFS of 7.1 months, and OS of 16.6 months, with a 1-year survival of 75% [80]. Docetaxel has been used likewise as second line and beyond. Oashi et al. published a series of 3 patients who received such therapy after progression to the FECOM scheme [68]. Other authors used it as a third-line treatment after progression to FECOM scheme and 5-fluorouracil plus cisplatin, achieving a PR maintained for 12 months [70].
Taxanes have also been combined with 5-fluorouracil, S-1 (consisting of tegafur, 5-chloro-2,4-dihydroxypyridine, and potassium oxalate), and platins.
Zhu et al. presented a series of 10 patients, of which 2 were treated with docetaxel alone, 2 in combination with cisplatin, and 3 in combination with 5-fluorouracil, achieving an ORR of 57% [29]. Because the data is not separately by schemes, it is difficult to draw conclusions from this study. A case of a patient treated with docetaxel and carboplatin reached a PFS of 13 months [81]. There is also larger series, with one patient treated with paclitaxel alone (PR) and 2 patients in combination with carboplatin (one progression and one stable disease) [82].
Some authors have used S-1 in combination with docetaxel. A series of 4 patients reported a PR for 30 weeks (after progression to FECOM) [69], a CR maintained for 1 year [83], and a CR and PR for more than 10 months [84]. The use of S-1 has been also used to reverse the resistance to docetaxel, achieving response in a total of 3 cases [85, 86]. Fukuda et al. also reported a series of 8 cases where patients were treated with docetaxel and S-1 after progression to docetaxel. They obtained 2 PR and a disease control rate (DCR) of 88%. Median PFS and OS were 8 and 10 months respectively [87].
Finally, Hirai et al. explored an unusual combination of cisplatin, epirubicin, and paclitaxel (PET regimen) in a series of 5 patients. Three patients received it as first-line regimen, one as second line and the other as third line. There were 4 PR and 1 PD, with a PFS of 8 months and an OS of 20.1 months. The dosing schedules were diverse: biweekly for one patient, 2 weeks on and 2 weeks off for two patients, and triweekly for another patient [88].
Targeted Therapy
Overexpression of HER2 has been described in up to 30–40% of mEMPD. The first case was described in a breast Paget in 1989 [89], and 2 years later in EMPD [90]. It has been postulated that the alteration of this pathway could contribute to the progression of the disease, as all PI3K and ERK pathways [91].
We summarized in Table 2 the current evidence of HER2 overexpression prevalence in EMPD [88, 91,92,93,94,95,96,97,98,99,100,101,102,103••]. Among 486 cases of localized disease and 99 of metastatic disease, there is an overall expression of HER2 of 28.2% and 40% respectively.
Table 2.
Prevalence of HER2 amplification in metastatic and localized extramammary Paget’s disease
| First author | Year | No. of patients | Stage | HER2 positive | Detection methods |
|---|---|---|---|---|---|
| Takata [92] | 1999 | 27 | Localized | 6 (22%) | IHC, FISH |
| 4 | LNM | 2 (50%) | |||
| Tanskanen [93] | 2003 | 21 | Localized | 11 (52%) | ICH, FISH |
| 2 | LNM | 1 (50%) | |||
| Brummer [94] | 2004 | 10 | Localized | 8 (80%) | IHC |
| Reich [95] | 2005 | 6 | Localized | 4 (66.7%) | FISH |
| Ogawa [91] | 2005 | 34 | Localized | 3 (8.8%) | IHC |
| Bianco [96] | 2006 | 15 | Localized | 1 (6.67%%) | IHC, CISH |
| Plaza [97] | 2009 | 47 | Localized | 15 (32%) | IHC |
| Richter [98] | 2010 | 33 | Localized | 19 (57.6%) | IHC, FISH |
| Miyamoto [99] | 2010 | 23 | Localized | 13 (69.5%) | IHC, FISH |
| 9 | Metastatic | 7 (77.7%) | |||
| Hikita [100] | 2012 | 17 | Localized | 4 (23.5%) | IHC, FISH |
| Tanaka [101] | 2013 | 78 | Localized | 8 (10%) | IHC, FISH |
| 26 | Metastatic | 4 (15%) | |||
| Kang [102] | 2015 | 227 | Localized | 45 (18.3%) | IHC |
| 19 | LNM | ||||
| Hirai [88] | 2019 | 47 | Metastatic | 23 (49%) | IHC, FISH |
| Lu [103••] | 2019 | 11 | Metastatic | 3 (27.2%) | FISH |
| Total | 486 | Localized | 137 (28.2%) | ||
| 99 | Metastatic and LNM | 40 (40%) | |||
LNM, lymph node metastases; IHC, immunohistochemistry; FISH, fluorescent in situ hybridization; CISH, chromogenic in situ hybridization
In the same way, we have summarized the efficacy of targeting treatment with anti-HER2 therapies in Table 3 [103••,104,105,106,107,108,109,110,111,112,113, 114•, 115]. Represent a total of 17 patients, with a marked response, including 4 cases of CR (20%) and 9 PR (45%). Most of patients were treated with an anti-HER2 therapy at first line (12, 60%) in combination with a taxane (8, 66.7%), and a median PFS of 12 months. An unusual treatment with pyrotinib has been reported, with a PR but only 2 months of follow-up [116]. This case has not been included in Table 3.
Table 3.
Efficacy of targeted therapy against HER2
| First author | Year | Treatment modality | QT added | Effect | Line of treatment | PFS, months | OS, months |
|---|---|---|---|---|---|---|---|
| Karam [104] | 2008 | Trastuzumab (300 mg/m2) | PR | First line | 12 | NR | |
| Takahagi [105] | 2009 | Trastuzumab 2 mg/kg qw (LD 4 mg/kg) | Paclitaxel (80 mg/m2 qw)a | PR | First line | 6 | 15 |
| Hanawa [106] | 2011 | Trastuzumab 2 mg/kg (LD 4 mg/kg), 6 weeks on/2 weeks off | Paclitaxel (80 mg/m2 qw)b | PR | First line | 14 | NR |
| Wakabayashi [107] | 2012 | Trastuzumab 6 mg/kg q3w (LD 8 mg/kg) | CR | First line | >13 | NR | |
| Yoshimura [108] | 2013 | Trastuzumab 2 mg/kg qw (LD 4 mg/kg) | Paclitaxel | NR | Second line | 4 | 14 |
| Barth [109] | 2015 | Trastuzumab 6 mg/kg q3w (LD 8 mg/kg) | CR | First line | >12 | NR | |
| Zhang [110] | 2015 | Trastuzumab 6 mg/kg q3w | PR | Second line | >11 | NR | |
| Shin [111] | 2016 | Lapatinibc | Capecitabined | NR | Second line | NR | NR |
| T-DM1c | CR | Third line | 12 | ||||
| Watanabe [112] | 2016 | Trastuzumab 6 mg/kg q3w (LD 8 mg/kg)e | PR | First line | 5 | >17 | |
| Trastuzumab 6 mg/kg (LD 8 mg/kg) and pertuzumab 420 mg/m2 (LD 840 mg/m2) q3we | Docetaxel (75 mg/m2 q3w) | PR | Second Line | 12 | |||
| Ichiyama [113] | 2017 | Trastuzumab 2 mg/kg qw (LD 4 mg/kg) | Paclitaxel (80 mg/m2 qw) | PR | First line | >30 | NR |
| Lu [103••] | 2019 | Trastuzumab 2 mg/kg qw | Paclitaxel (80 mg/m2) and cisplatin (30 mg/m2) qw | CR | First line | 17 | NR |
| Trastuzumab 360 mg q3wf | Paclitaxel (80 mg/m2 qw) | SD | Second line | 5 | NR | ||
| Lapatinibf | Capecitabine | SD | Third line | 5 | |||
| Sekiguchi [114•] | 2020 | Trastuzumab 2 mg/kg qw | Paclitaxel 80 mg/m2 qw | PR | First line | 12 | 30 |
| Trastuzumab 2 mg/kg qw | Paclitaxel 80 mg/m2 qw | PD | First line | 2 | 6 | ||
| Trastuzumab 2 mg/kg qw | Paclitaxel 80 mg/m2 qw | PD | First line | 4 | 13 | ||
| Trastuzumab 2 mg/kg qw | Paclitaxel 80 mg/m2 qw | PD | First line | 3 | 20 | ||
| Kimura [115] | 2020 | Trastuzumab (biosimilar) 2 mg/kg q2w | SD | Second line | >6 | 13 |
QT, chemotherapy; PFS, progression free survival; OS, overall survival; LD, loaded dose; qm, once a month; qw, once a week; q3w, once every 3 weeks; NR, not reported; CR, complete response; PR, partial response; SD, stable disease
aIn the first cycle, chemotherapeutic agent was docetaxel. Paclitaxel was added because there was no response with docetaxel
bPaclitaxel was added in the second cycle, because there was no response
cThese regimens belong to the same patient
dAdded when lapatinib dose was reduced because hepatic toxicity
eThese regimens belong to the same patient
fThese regimens belong to the same patient
Even though the evidence of anti-HER2 efficacy in this entity is not robust, due to its low incidence, determination of HER2 expression should be performed in all cases of mEMPD.
Overexpression of androgen receptors and estrogen have been described in 53.6–100% and 0–19.44%, respectively, being able to coexist in 16.67% of cases [117–120]. The first case of EMPD treated with a complete hormonal blockade was a male patient who received chlormadinone acetate daily and leuprorelin acetate subcutaneous monthly, presenting a paradoxical response, with progression on skin lesions and PR in lymph nodes [121]. In another case, a man with a scrotal mEMPD, which expressed estrogen receptor alfa, and a metastatic prostate cancer, was treated with tamoxifen and bicalutamide concomitantly, with PR for 6 months [122].
Another male patient with mEMPD with overexpression of estrogen and progesterone receptors (30.8% and 1.1% of tumor cells with an Allred score of 5/8 and 4/8 respectively) was treated with first-line tamoxifen. It resulted in a PR which endures after 23 months of ongoing treatment [123].
Inhibition of the PI3K/AKT/mTOR pathway has also been explored in a case reported by Yin et al. The patient presented a HER2 negative EMPD with neuroendocrine features and upregulated PI3K/AKT/mTOR pathway due to a mutation in AMER1 (an inhibitor of PI3K phosphorylation). The combination of anlotinib (a multikinase inhibitor) and tislelizumab (an antibody which minimizes the binding to FcγR on macrophages in order to limit antibody-dependent phagocytosis) rendered the patient a significantly improved DFS of 8 months in an investigational context [124].
Other Therapies
Systemic immunotherapy has not been specifically studied in EMPD. Moreover, classic biomarker predictors of response with immune checkpoint inhibitors (ICIs) are usually lacking in EMPD [125, 126]. Low rates of PD-L1 expression have also been reported [127]. Nevertheless, PD-L1 has demonstrated to be a good predictor in very few settings, while in most cases, it fails to accurately anticipate a tumor response. A case of a patient with mEMPD who was treated with ipilimumab and nivolumab was reported in 2021. The patients achieved a PR which endured 5 months even though the treatment had to be stopped due to immunotherapy-related hepatitis [128].
Treatment with ICIs must be evaluated in mEMPD to a greater extent and more studies are warranted. An ongoing study (NCT02834013) is exploring the use of nivolumab plus ipilimumab in rare tumors, including EMPD.
There are other rare cases, with reported responses to agents such as apatinib [129].
Serum Biomarkers
CEA is a biomarker that is usually elevated in patients with mEMPD, while in those with localized disease typically remains in normal range. It can be used to evaluate the response to treatments in metastatic setting [130]. Cytokeratin 19 fragment 21-1 (CYFRA 21-1) has been reported as a sensitive marker for EMPD that may surpass CEA [131, 132].
Conclusions
We present an overview on the diagnosis and management of EMPD, both localized and metastatic. Given the low incidence of this disease, there are no randomized clinical trials and virtually all knowledge comes from the publication of retrospective cases and reports. However, some general recommendation can be given for the management of patients with EMPD.
The treatment of choice for localized disease endures to be surgery, where Mohs technique may be preferred in order to reduce recurrences.
Discarding the regional lymph node involvement, especially in cases of invasive EMPD or with foci of adenocarcinoma, is key to reduce distance recurrences. In this sense, PET and ultrasound can be useful tools to detect the possible presence of affected regional nodes. Since the performance of a lymphadenectomy has not demonstrated to improve patient survival, performing a SLNB to decide on a possible lymphadenectomy is something that must be individualized.
In those cases, in which surgery is not possible, imiquimod, photodynamic therapy, or radiotherapy should be considered alternative. Radiotherapy could be also used as an adjuvant treatment in case of margins affected, locoregional lymph node involvement, multifocal disease, or presence of adenocarcinoma.
In regard to the treatment of metastatic disease, multiple QT regimens have been used, none clearly superior to others. However, the most commonly used drugs have been taxanes, platins, and fluoropyrimidines, either as monotherapy or in various combinations. Anthracyclines may also be active. In a same way to chemotherapy treatment, there are only few reported cases treated with anti-HER2 treatment and androgen and/or estrogen blockade. However, long-lasting responses have been observed with these treatments. This fact, together with the scarcity of therapeutic options, makes us recommend that the overexpression of HER2, androgen, and estrogen receptors should be performed. The use of ICIs should be carefully assessed given the exceptional number of cases existing.
Finally, the use of some biomarker, such as CEA and CYFRA 21-1, could be used to monitoring the response to treatment.
Case Report
We present the case of a 75-year-old woman with no personal history of interest, who was being monitored for Paget’s disease of the vulva. The patient consulted in December 2017 for worsening of pruritus in the vulvo-vaginal area. A physical examination showed a vulvar neoformation in vaginal introit, with growth into the vagina and superficial extension to the reset of the vulva in the form of Paget’s disease.
A thoraco-abdominal-pelvic CT scan is performed in January 2018, describing pathological-looking adenopathies in the right external iliac-femoral chain of at least 5.5 × 2.6 cm, as well as suspicious ipsilateral inguinal adenopathies of 2.9 × 1.6 cm. There was also a nodular thickening in the left vaginal dome and a slight skin thickening in the vulvar region.
A biopsy of the vaginal introit is performed, showing an infiltration by a carcinoma compatible with primary vulvar. A biopsy of the inguinal adenopathies is also performed, showing large cell infiltrates with vesicular nuclei and macronucleoli forming solid nests, morphology corresponding to a poorly differentiated adenocarcinoma. The immunohistochemical profile (positivity for CK7, EMA, androgen receptors, and CEA; negativity for CK20, estrogen and progesterone receptors, and GCDFP15) orientates to adenocarcinoma metastases of vulvar origin.
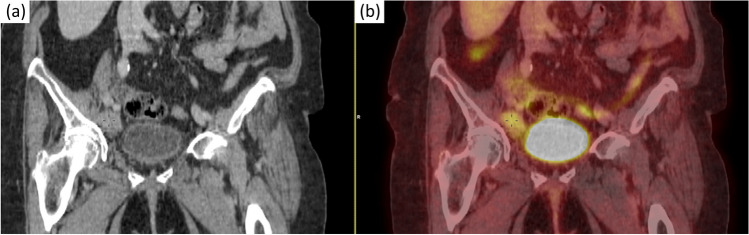
Given these findings, a PET-CT is performed in February 2018, which shows uptake in the following: right hemivulva (SUVmax 3.8), left vaginal dome (2.8 × 2.1 cm, SUVmax 6.6), right inguinal adenopathies (2.7 × 1.7 cm, SUVmax 2.6), external iliac chain adenopathies (6.2 × 3.1 cm, SUV max 3.4), right common iliac bifurcation adenopathies (1.9 × 1.2 cm, SUV max 2.6) (Fig. 2).
Fig. 2.
a TAC image with nodal affection. b PET image with nodal affection
With the diagnosis of vulvar mEMPD, due to lymph node involvement, it was decided to start a first-line treatment of chemotherapy with carboplatin AUC-4 plus paclitaxel 135 mg/m2 every 3 weeks. The doses were reduced with respect to the classic schemes of ovarian cancer due to the fragility of the patient.
The patient began treatment in March 2018. After 3 cycles, in the reassessment CT scan, a PR at the lymph node level was observed and it was maintained during 3 more cycles.
It was decided, in a multidisciplinary session, to administer consolidation radiotherapy. The administered treatment consisted of 50.4 Gy (1.8 Gy/fraction) on the vulvar area and pelvic and inguinal lymph node chains, with concomitant boost up to 61.6 Gy (2.2 Gy/fraction) on macroscopic lymph nodes and implant in vaginal vault. The treatment was administered in 28 sessions. In the first CT scan after radiotherapy, the patient maintained a PR. However, in February 2019, she presents hepatic progression.
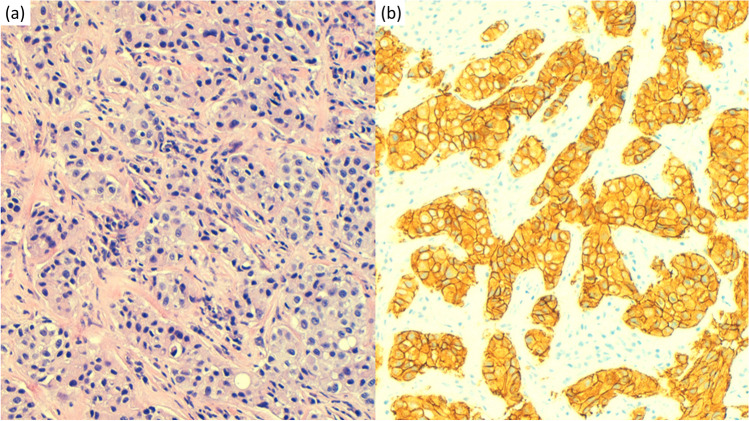
At this time, determination of HER2 is requested, resulting positive by immunohistochemistry with a value of 3+ (Fig. 3). It is then decided to initiate a second line of treatment with trastuzumab at a dose of 600 mg subcutaneously every 21 days, and paclitaxel 80 mg/m2 on days 1, 8, and 15 every 21 days. Paclitaxel was suspended from the 2nd cycle due to grade 2 neurotoxicity. The patient continued exclusively with trastuzumab 600 mg subcutaneously every 21 days, receiving two more cycles. The patient presents hepatic and nodal progression in July 2019. At this point, the patient moves to another state, with loss to follow-up.
Fig. 3.
a Paget disease (hematoxylin and eosin): nests of tumoral cells with pale cytoplasm infiltrating the vulvar dermis. b HER-2 immunohistochemistry (HercepTest, Agilent) scored as positive 3+
Acknowledgements
To the patients and their families
Authors’ Contribution
All authors contributed to the study conception and design.
• Jesús Chamorro Pérez: data curation, writing—original draft
• Alfonso Cortés Salgado: writing—review and editing
• Belen Pérez-Mies: writing—review and editing, visualization
• José Antonio Domínguez Rullán: writing—review and editing
• Odile Ajuria-Illarramendi: writing—review and editing, visualization
• Eva María Guerra Alia: writing—review and editing
• Juan José Serrano Domingo: conceptualization, data curation, writing—original draft, supervision
Declarations
Ethics Approval and Consent to Participate
As it is a narrative review, this article was not reviewed by local ethics committee. Due to the patient’s death, a relative has given written informed consent to the publication of the case details.
Competing Interests
The authors declare no competing interests.
Footnotes
Many of the authors’ names were reversed (i.e., surname listed before given name) and should be corrected.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/11/2023
A Correction to this paper has been published: 10.1007/s11912-023-01456-8
Contributor Information
Jesús Chamorro Pérez, Email: jchamorro@salud.madrid.org.
Alfonso Cortes Salgado, Email: alfonso.cortes@salud.madrid.org.
Belén Pérez-Mies, Email: bperezm@salud.madrid.org.
Jose Antonio Domínguez Rullán, Email: joseantonio.dominguez@salud.madrid.org.
Odile Ajuria-Illarramendi, Email: odile.ajuria@salud.madrid.org.
Eva María Guerra Alia, Email: evamaria.guerra@salud.madrid.org.
Juan José Serrano Domingo, Email: juanjose.serrano@salud.madrid.org.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Saraiya M, Watson M, Wu X, et al. Incidence of in situ and invasive vulvar cancer in the US, 1998-2003. Cancer. 2008;113(10):2865–2872. doi: 10.1002/cncr.23759. [DOI] [PubMed] [Google Scholar]
- 3.Paget J. Classic articles in colonic and rectal surgery: Sir James Paget, 1814-1899, on disease of the mammary areola preceeding cancer of the mammary gland. Dis Colon Rectum. 1980;23(4):280–281. doi: 10.1007/BF02587101. [DOI] [PubMed] [Google Scholar]
- 4.Crocker HR. Paget’s disease affecting the scrotum and penis. Trans Pathol Soc London. 1889;40:187–191. [Google Scholar]
- 5.Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21(5):581–590. doi: 10.1111/j.1468-3083.2007.02154.x. [DOI] [PubMed] [Google Scholar]
- 6.Karam A, Dorigo O. Treatment outcomes in a large cohort of patients with invasive extramammary Paget’s disease. Gynecol Oncol. 2012;125(2):346–351. doi: 10.1016/j.ygyno.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Curtin JP, Rubin SC, Jones WB, Hoskins WJ, Lewis JL. Paget’s disease of the vulva. Gynecol Oncol. 1990;39(3):374–377. doi: 10.1016/0090-8258(90)90269-q. [DOI] [PubMed] [Google Scholar]
- 8.Billings SD, Roth LM. Pseudoinvasive, nodular extramammary Paget’s disease of the vulva. Arch Pathol Lab Med. 1998;122(5):471–474. [PubMed] [Google Scholar]
- 9.Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58(8):871–879. doi: 10.1111/ijd.14328. [DOI] [PubMed] [Google Scholar]
- 10.Kazakov DV, Spagnolo DV, Kacerovska D, Michal M. Lesions of anogenital mammary-like glands: An update. Adv Anat Pathol. 2011;18(1):1–28. doi: 10.1097/PAP.0b013e318202eba5. [DOI] [PubMed] [Google Scholar]
- 11.Parker LP, Parker JR, Bodurka-Bevers D, et al. Paget’s disease of the vulva: pathology, pattern of involvement, and prognosis. Gynecol Oncol. 2000;77(1):183–189. doi: 10.1006/gyno.2000.5741. [DOI] [PubMed] [Google Scholar]
- 12.Fanning J, Lambert HCL, Hale TM, Morris PC, Schuerch C. Paget’s disease of the vulva: Prevalence of associated vulvar adenocarcinoma, invasive Paget’s disease, and recurrence after surgical excision. Am J Obstet Gynecol. 1999;180(1 Pt 1):24–27. doi: 10.1016/s0002-9378(99)70143-2. [DOI] [PubMed] [Google Scholar]
- 13.Feuer GA, Shevchuk M, Calanog A. Vulvar Paget’s disease: the need to exclude an invasive lesion. Gynecol Oncol. 1990;38(1):81–89. doi: 10.1016/0090-8258(90)90016-e. [DOI] [PubMed] [Google Scholar]
- 14.Pierie JPEN, Choudry U, Muzikansky A, Finkelstein DM, Ott MJ. Prognosis and management of extramammary Paget’s disease and the association with secondary malignancies. J Am Coll Surg. 2003;196(1):45–50. doi: 10.1016/s1072-7515(02)01619-8. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd V, Davidson EJ, Davies-Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112(3):273–279. doi: 10.1111/j.1471-0528.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18(6):543–553. doi: 10.1080/14737140.2018.1457955. [DOI] [PubMed] [Google Scholar]
- 17.Goldblum JR, Hart WR. Perianal Paget’s disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22(2):170–179. doi: 10.1097/00000478-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Nowak MA, Guerriere-Kovach P, Pathan A, Campbell TE, Deppisch LM. Perianal Paget’s disease: distinguishing primary and secondary lesions using immunohistochemical studies including gross cystic disease fluid protein-15 and cytokeratin 20 expression. Arch Pathol Lab Med. 1998;122(12):1077–1081. [PubMed] [Google Scholar]
- 19.Goldblum JR, Hart WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol. 1997;21(10):1178–1187. doi: 10.1097/00000478-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Yamada-Kanazawa S, Tasaki Y, Kajihara I, Sakamoto R, Maeda-Otsuka S, Ihn H. The expression of EpCAM in extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8(1):20–23. doi: 10.5582/irdr.2019.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urata K, Kajihara I, Myangat TM, et al. Overexpression of cyclin-dependent kinase 4 protein in extramammary Paget’s disease. J Dermatol. 2019;46(5):444–448. doi: 10.1111/1346-8138.14858. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura S, Yanagi T, Maeda T, Ujiie H. Cyclin-dependent kinase 4/6 inhibitors suppress tumor growth in extramammary Paget’s disease. Cancer Sci. 2022;113(2):802–807. doi: 10.1111/cas.15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18(1):1–9. doi: 10.1186/s12885-018-4257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83(3):234–239. doi: 10.1016/j.jdermsci.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40(5):473–478. doi: 10.1111/ced.12690. [DOI] [PubMed] [Google Scholar]
- 26.Ogata D, Kiyohara Y, Yoshikawa S, Tsuchida T. Usefulness of sentinel lymph node biopsy for prognostic prediction in extramammary Paget’s disease. Eur J Dermatology. 2016;26(3):254–259. doi: 10.1684/ejd.2016.2744. [DOI] [PubMed] [Google Scholar]
- 27.Aoyagi S, Sato-Matsumura KC, Shimizu H. Staging and assessment of lymph node involvement by 18F-fluorodeoxyglucose-positron emission tomography in invasive extramammary Paget’s disease. Dermatol Surg. 2005;31(5):595–598. doi: 10.1111/j.1524-4725.2005.31172. [DOI] [PubMed] [Google Scholar]
- 28.Bin CS, Yun M, Lee M-G, Chung KY. Variable patterns of positron emission tomography in the assessment of patients with extramammary Paget’s disease. J Am Acad Dermatol. 2005;52(2):353–355. doi: 10.1016/j.jaad.2004.10.864. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Ye DW, Yao XD, et al. Clinicopathological characteristics, management and outcome of metastatic penoscrotal extramammary Paget’s disease. Br J Dermatol. 2009;161(3):577–582. doi: 10.1111/j.1365-2133.2009.09203.x. [DOI] [PubMed] [Google Scholar]
- 30.Treglia G, Giovannini E, Bertagna F, Giovanella L, Malaggese M. An unusual case of metastatic extramammary Paget’s disease of the vulva identified by 18F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol. 2013;32(6):402–403. doi: 10.1016/j.remn.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Li ZG, Qin XJ. Extensive invasive extramammary paget disease evaluated by F-18 FDG PET/CT: a case report. Medicine (Baltimore). 2015;94(3):e371. doi: 10.1097/MD.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian Y, Wu HB, Li DL, Li HS, Zhou WL, Wang QS. Utility of 18 F-FDG PET/CT in the diagnosis and staging of extramammary Paget’s disease. Nucl Med Commun. 2015;36(9):892–897. doi: 10.1097/MNM.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 33.Fujiwara M, Suzuki T, Senoo A, Fukamizu H, Tokura Y. Evaluation of positron emission tomography imaging to detect lymph node metastases in patients with extramammary Paget’s disease. J Dermatol. 2017;44(8):939–943. doi: 10.1111/1346-8138.13833. [DOI] [PubMed] [Google Scholar]
- 34.Hatta N, Morita R, Yamada M, et al. Sentinel lymph node biopsy in patients with extramammary Paget’s disease. Dermatologic Surg. 2004;30(10):1329–1334. doi: 10.1111/j.1524-4725.2004.30377.x. [DOI] [PubMed] [Google Scholar]
- 35.Fujisawa Y, Yoshino K, Kiyohara Y, et al. The role of sentinel lymph node biopsy in the management of invasive extramammary Paget’s disease: multi-center, retrospective study of 151 patients. J Dermatol Sci. 2015;79(1):38–42. doi: 10.1016/j.jdermsci.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Omodaka T, Kiyohara Y, Uematsu T, Mori K, Okuyama R. Preoperative ultrasound evaluation of lymph nodes for extramammary Paget’s disease in the genital area. J Dermatol. 2019;46(4):361–363. doi: 10.1111/1346-8138.14796. [DOI] [PubMed] [Google Scholar]
- 37.Niikura H, Yoshida H, Ito K, et al. Paget’s disease of the vulva: clinicopathologic study of type 1 cases treated at a single institution. Int J Gynecol Cancer. 2006;16(3):1212–1215. doi: 10.1111/j.1525-1438.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 38.Tsutsumida A, Yamamoto Y, Minakawa H, Yoshida T, Kokubu I, Sugihara T. Indications for lymph node dissection in the treatment of extramammary Paget’s disease. Dermatol Surg. 2003;29(1):21–24. doi: 10.1046/j.1524-4725.2003.29001.x. [DOI] [PubMed] [Google Scholar]
- 39.Fishman DA, Chambers SK, Schwartz PE, Kohorn EI, Chambers JT. Extramammary Paget’s disease of the vulva. Gynecol Oncol. 1995;56(2):266–270. doi: 10.1006/gyno.1995.1044. [DOI] [PubMed] [Google Scholar]
- 40.Tebes S, Cardosi R, Hoffman M. Paget’s disease of the vulva. Am J Obstet Gynecol. 2002; 187(2):281–3; discussion 283-4. DOI: 10.1067/mob.2002.125700 [DOI] [PubMed]
- 41.Zollo JD, Zeitouni NC. The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br J Dermatol. 2000;142(1):59–65. doi: 10.1046/j.1365-2133.2000.03242.x. [DOI] [PubMed] [Google Scholar]
- 42.Stacy D, Burrell MO, Franklin EW. Extramammary Paget’s disease of the vulva and anus: use of intraoperative frozen-section margins. Am J Obstet Gynecol. 1986;155(3):519–522. doi: 10.1016/0002-9378(86)90270-x. [DOI] [PubMed] [Google Scholar]
- 43.Jensen SL, Sjølin KE, Shokouh-Amiri MH, Hagen K, Harling H. Paget’s disease of the anal margin. Br J Surg. 1988;75(11):1089–1092. doi: 10.1002/bjs.1800751113. [DOI] [PubMed] [Google Scholar]
- 44.Kodama S, Kaneko T, Saito M, Yoshiya N, Honma S, Tanaka K. A clinicopathologic study of 30 patients with Paget’s disease of the vulva. Gynecol Oncol. 1995;56(1):63–70. doi: 10.1006/gyno.1995.1010. [DOI] [PubMed] [Google Scholar]
- 45.Sarmiento JM, Wolff BG, Burgart LJ, Frizelle FA, Ilstrup DM. Paget’s disease of the perianal region--an aggressive disease? Dis Colon Rectum. 1997;40(10):1187–1194. doi: 10.1007/BF02055165. [DOI] [PubMed] [Google Scholar]
- 46.Hendi A, Brodland DG, Zitelli JA. Extramammary Paget’s disease: surgical treatment with Mohs micrographic surgery. J Am Acad Dermatol. 2004;51(5):767–773. doi: 10.1016/j.jaad.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 47.O’Connor WJ, Lim KK, Zalla MJ, et al. Comparison of mohs micrographic surgery and wide excision for extramammary Paget’s disease. Dermatol Surg. 2003;29(7):723–727. doi: 10.1046/j.1524-4725.2003.29184.x. [DOI] [PubMed] [Google Scholar]
- 48.Kim SJ, Thompson AK, Zubair AS, et al. Surgical treatment and outcomes of patients with extramammary Paget disease: a cohort study. Dermatol Surg. 2017;43(5):708–714. doi: 10.1097/DSS.0000000000001051. [DOI] [PubMed] [Google Scholar]
- 49.Liau MQM, Yang SS, Tan KB, Aw CWD. Topical imiquimod in the treatment of extramammary Paget’s disease: a 10 year retrospective analysis in an Asian tertiary centre. Dermatol Ther. 2016;29(6):459–462. doi: 10.1111/dth.12394. [DOI] [PubMed] [Google Scholar]
- 50.Wang LC, Blanchard A, Judge DE, Lorincz AA, Medenica MM, Busbey S. Successful treatment of recurrent extramammary Paget’s disease of the vulva with topical imiquimod 5% cream. J Am Acad Dermatol. 2003;49(4):769–771. doi: 10.1067/s0190-9622(03)02107-8. [DOI] [PubMed] [Google Scholar]
- 51.Hatch KD, Davis JR. Complete resolution of paget disease of the vulva with imiquimod cream. J Low Genit Tract Dis. 2008;12(2):90–94. doi: 10.1097/LGT.0b013e31815a58a5. [DOI] [PubMed] [Google Scholar]
- 52.Denehy T, Taylor RR, Mc Weeney DT, Gregori CABJ. Successful immune modulation therapy with topical imiquimod 5% cream in the management of recurrent extramammary Paget’s disease of the vulva. Gynecol Oncol. 2008;101(1):S88–S89. [Google Scholar]
- 53.Sendagorta E, Herranz P, Feito M, et al. Successful treatment of three cases of primary extramammary Paget’s disease of the vulva with Imiquimod - proposal of a therapeutic schedule. J Eur Acad Dermatology Venereol. 2010;24(4):490–492. doi: 10.1111/j.1468-3083.2009.03451.x. [DOI] [PubMed] [Google Scholar]
- 54.Machida H, Moeini A, Roman LD, Matsuo K. Effects of imiquimod on vulvar Paget’s disease: a systematic review of literature. Gynecol Oncol. 2015;139(1):165–171. doi: 10.1016/j.ygyno.2015.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cowan RA, Black DR, Hoang LN, et al. A pilot study of topical imiquimod therapy for the treatment of recurrent extramammary Paget’s disease. Gynecol Oncol. 2016;142(1):139–143. doi: 10.1016/j.ygyno.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawada M, Kato J, Yamashita T, et al. Imiquimod 5% cream as a therapeutic option for extramammary Paget’s disease. J Dermatol. 2018;45(2):216–219. doi: 10.1111/1346-8138.14117. [DOI] [PubMed] [Google Scholar]
- 57.Dogan A, Hilal Z, Krentel H, et al. Paget’s disease of the vulva treated with imiquimod: case report and systematic review of the literature. Gynecol Obstet Invest. 2017;82(1):1–7. doi: 10.1159/000449158. [DOI] [PubMed] [Google Scholar]
- 58.van der Linden M, van Hees CL, van Beurden M, et al. The Paget Trial: topical 5% imiquimod cream for noninvasive vulvar Paget disease. Am J Obstet Gynecol. 2022;227(2):250.e1–250.e8. doi: 10.1016/j.ajog.2022.04.012. [DOI] [PubMed] [Google Scholar]
- 59.Vicentini C, Carpentier O, Lecomte F, Thecua E, Mortier L, Mordon SR. Treatment of a vulvar Paget’s disease by photodynamic therapy with a new light emitting fabric based device. Lasers Surg Med. 2017;49(2):177–180. doi: 10.1002/lsm.22631. [DOI] [PubMed] [Google Scholar]
- 60.Shieh S, Dee AS, Cheney RT, Frawley NP, Zeitouni NC, Oseroff AR. Photodynamic therapy for the treatment of extramammary Paget’s disease. Br J Dermatol. 2002;146(6):1000–1005. doi: 10.1046/j.1365-2133.2002.04801.x. [DOI] [PubMed] [Google Scholar]
- 61.Rioli DI, Samimi M, Beneton N, et al. Efficacy and tolerance of photodynamic therapy for vulvar Paget’s disease: a multicentric retrospective study. Eur J Dermatology. 2018;28(3):351–355. doi: 10.1684/ejd.2018.3289. [DOI] [PubMed] [Google Scholar]
- 62.Tolia M, Tsoukalas N, Sofoudis C, et al. Primary extramammary invasive Paget’s vulvar disease: what is the standard, what are the challenges and what is the future for radiotherapy? BMC Cancer. 2016;16(1):10–17. doi: 10.1186/s12885-016-2622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tagliaferri L, Casà C, Macchia G, et al. The role of radiotherapy in extramammary Paget disease: a systematic review. Int J Gynecol Cancer. 2018;28(4):829–839. doi: 10.1097/IGC.0000000000001237. [DOI] [PubMed] [Google Scholar]
- 64.Piedbois P, Breau JL, Morere JF, Israel L. Sweat gland carcinoma with bone and visceral metastases. Prolonged complete remission lasting 16 months as a result of chemotherapy. Cancer. 1987;60(2):170–172. doi: 10.1002/1097-0142(19870715)60:2<170::AID-CNCR2820600208>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 65.Balducci L, Athar M, Smith GF, Khansur T, McKenzie D, Crawford ED. Metastatic extramammary Paget’s disease: dramatic response to combined modality treatment. J Surg Oncol. 1988;38(1):38–44. doi: 10.1002/jso.2930380111. [DOI] [PubMed] [Google Scholar]
- 66.Yokoyama Y, Mabuchi M, Kawabata I. Metastatic vulvar Paget’s disease responding to combination chemotherapy: case report. Jpn J Clin Oncol. 1990;20(4):426–430. [PubMed] [Google Scholar]
- 67.Yamazaki N, Yamamoto A, Wada T, Ishikawa M, Moriya Y, Nakanishi Y. A case of metastatic extramammary Paget’s disease that responded to combination chemotherapy. J Dermatol. 1999;26:311–316. doi: 10.1111/j.1346-8138.1999.tb03477.x. [DOI] [PubMed] [Google Scholar]
- 68.Oashi K, Tsutsumida A, Namikawa K, et al. Combination chemotherapy for metastatic extramammary Paget disease. Br J Dermatol. 2014;170(6):1354–1357. doi: 10.1111/bjd.12788. [DOI] [PubMed] [Google Scholar]
- 69.Matsushita S, Yonekura K, Mera K, Kawai K, Kanekura T. Successful treatment of metastatic extramammary Paget’s disease with S-1 and docetaxel combination chemotherapy. J Dermatol. 2011;38(10):996–998. doi: 10.1111/j.1346-8138.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- 70.Oguchi S, Kaneko M, Uhara H, Saida T. Docetaxel induced durable response in advanced extramammary Paget’s disease: a case report. J Dermatol. 2002;29(1):33–37. doi: 10.1111/j.1346-8138.2002.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 71.Voigt H, Basserrmann R, Nafhrath W. Cytoreductive combination chemotherapy for regionally advanced unresectable extramammary Paget carcinoma. Cancer. 1992;70(3):704–708. doi: 10.1002/1097-0142(19920801)70:3<704::AID-CNCR2820700327>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 72.Kariya K, Tsuji T, Schwartz RA. Trial of low-dose 5-fluorouracil/cisplatin therapy for advanced extramammary Paget’s disease. Dermatologic Surg. 2004;30(2):341–344. doi: 10.1111/j.1524-4725.2004.30093.x. [DOI] [PubMed] [Google Scholar]
- 73.Tokuda Y, Arakura F, Uhara H. Combination chemotherapy of low-dose 5-fluorouracil and cisplatin for advanced extramammary Paget’s disease. Int J Clin Oncol. 2015;20(1):194–197. doi: 10.1007/s10147-014-0686-2. [DOI] [PubMed] [Google Scholar]
- 74.Kato H, Watanabe S, Kariya K, Nakamura M, Morita A. Efficacy of low-dose 5-fluorouracil/cisplatin therapy for invasive extramammary Paget’s disease. J Dermatol. 2018;45(5):560–563. doi: 10.1111/1346-8138.14247. [DOI] [PubMed] [Google Scholar]
- 75.Chen K, Liang H, Peng J, Zheng Y. Successful treatment of metastatic extramammary Paget’s disease with pemetrexed monotherapy systemically and 5-fluorouracil topically. Indian J Dermatol Venereol Leprol. 2019;85(1):56–59. doi: 10.4103/ijdvl.IJDVL_171_17. [DOI] [PubMed] [Google Scholar]
- 76.Huang GS, Juretzka M, Ciaravino G, Kohler S, Teng NNH. Liposomal doxorubicin for treatment of metastatic chemorefractory vulvar adenocarcinoma. Gynecol Oncol. 2002;87(3):313–318. doi: 10.1006/gyno.2002.6830. [DOI] [PubMed] [Google Scholar]
- 77.Tauveron V, Body G, Machet L, Lenain H, Ouldamer L, Lorette G. Prolonged remission of Paget disease of the vulva after chemotherapy for breast carcinoma. Br J Dermatol. 2014;170(5):1199–1200. doi: 10.1111/bjd.12825. [DOI] [PubMed] [Google Scholar]
- 78.Fujisawa Y, Umebayashi Y, Otsuka F. Metastatic extramammary Paget’s disease successfully controlled with tumour dormancy therapy using docetaxel. Br J Dermatol. 2006;154(2):375–376. doi: 10.1111/j.1365-2133.2005.07046.x. [DOI] [PubMed] [Google Scholar]
- 79.Nakamori R, Omoto Y, Yamanaka K, Habe K, Kurokawa I, Mizutani H. Complete remission of advanced extramammary Paget’s disease treated with docetaxel: a case report. Clin Exp Dermatol. 2012;37(2):194–195. doi: 10.1111/j.1365-2230.2011.04204.x. [DOI] [PubMed] [Google Scholar]
- 80.Yoshino K, Fujisawa Y, Kiyohara Y, et al. Usefulness of docetaxel as first-line chemotherapy for metastatic extramammary Paget’s disease. J Dermatol. 2016;43(6):633–637. doi: 10.1111/1346-8138.13200. [DOI] [PubMed] [Google Scholar]
- 81.Hegarty PK, Suh J, Fisher MB, et al. Penoscrotal extramammary Paget’s disease: the University of Texas M. D. Anderson Cancer Center contemporary experience. J Urol. 2011;186(1):97–102. doi: 10.1016/j.juro.2011.02.2685. [DOI] [PubMed] [Google Scholar]
- 82.Moretto P, Nair VJ, El Hallani S, et al. Management of penoscrotal extramammary paget disease: case series and review of the literature. Curr Oncol. 2013;20(4):311–320. doi: 10.3747/co.20.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogata D, Hokama Y, Tsuchida T. Successful treatment of bilateral multiple lymph node metastases in extramammary Paget’s disease with surgery and sequential chemotherapy of S-1 and docetaxel. J Dermatol. 2015;42(12):1193–1194. doi: 10.1111/1346-8138.13089. [DOI] [PubMed] [Google Scholar]
- 84.Egashira S, Kajihara I, Kanemaru H, et al. Achieved good response of S-1 and docetaxel combination chemotherapy in two patients with metastatic extramammary Paget’s disease. J Dermatol. 2017;44(5):e103–e104. doi: 10.1111/1346-8138.13693. [DOI] [PubMed] [Google Scholar]
- 85.Kato J, Hida T, Yamashita T, et al. Successful TS-1 monotherapy as the second-line treatment for advanced extramammary Paget’s disease: a report of two cases. J Dermatol. 2018;45(1):80–82. doi: 10.1111/1346-8138.14017. [DOI] [PubMed] [Google Scholar]
- 86.Mikoshiba Y, Uhara H, Kubo H, Okuyama R. S-1 induced a durable response in metastatic extramammary Paget’s disease. J Dermatol. 2013;40(8):664–665. doi: 10.1111/1346-8138.12177. [DOI] [PubMed] [Google Scholar]
- 87.Fukuda K, Hirai I, Nakamura Y, et al. S-1 in combination with docetaxel as salvage therapy for patients with metastatic extramammary Paget’s disease. Eur J Dermatology. 2020;30(3):301–302. doi: 10.1684/ejd.2020.3782. [DOI] [PubMed] [Google Scholar]
- 88.Hirai I, Tanese K, Nakamura Y, Ishii M, Kawakami Y, Funakoshi T. Combination cisplatin-epirubicin-paclitaxel therapy for metastatic extramammary Paget’s disease. Oncologist. 2019;24(6):e394–e396. doi: 10.1634/theoncologist.2018-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lammie GA, Barnes DM, Millis RR, Gullick WJ. An immunohistochemical study of the presence of c-erbB-2 protein in Paget’s disease of the nipple. Histopathology. 1989;15(5):505–514. doi: 10.1111/j.1365-2559.1989.tb01610.x. [DOI] [PubMed] [Google Scholar]
- 90.Wolber RA, Dupuis BA, Wick MR. Expression of c-erbB-2 oncoprotein in mammary and extramammary Paget’s disease. Am J Clin Pathol. 1991;96(2):243–247. doi: 10.1093/ajcp/96.2.243. [DOI] [PubMed] [Google Scholar]
- 91.Ogawa T, Nagashima Y, Wada H, et al. Extramammary Paget’s disease: analysis of growth signal pathway from the human epidermal growth factor receptor 2 protein. Hum Pathol. 2005;36(12):1273–1280. doi: 10.1016/j.humpath.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 92.Takata M, Fujimoto A, Aoki H, Hatta N, Ooi A, Takehara K. erbB-2 overexpression but no activation of β-catenin gene in extramammary Paget’s disease. J Invest Dermatol. 1999;113(2):258–262. doi: 10.1046/j.1523-1747.1999.00634.x. [DOI] [PubMed] [Google Scholar]
- 93.Tanskanen M, Jahkola T, Asko-Seljavaara S, Jalkanen J, Isola J. HER2 oncogene amplification in extramammary Paget’s disease. Histopathology. 2003;42(6):575–579. doi: 10.1046/j.1365-2559.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- 94.Brummer O, Stegner HE, Böhmer G, Kühnle H, Petry KU. HER-2/neu expression in Paget disease of the vulva and the female breast. Gynecol Oncol. 2004;95(2):336–340. doi: 10.1016/j.ygyno.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 95.Reich O, Liegl B, Tamussino K, Regauer S. HER2 overexpression and HER2 oncogene amplification in recurrent vulvar Paget’s disease. Mod Pathol. 2005;18(3):354–357. doi: 10.1038/modpathol.3800243. [DOI] [PubMed] [Google Scholar]
- 96.Bianco MK, Vasef MA. HER-2 gene amplification in Paget disease of the nipple and extramammary site: a chromogenic in situ hybridization study. Diagnostic Mol Pathol. 2006;15(3):131–135. doi: 10.1097/01.pdm.0000213456.30151.5b. [DOI] [PubMed] [Google Scholar]
- 97.Plaza JA, Torres-Cabala C, Ivan D, Prieto VG. HER-2/neu expression in extramammary Paget disease: a clinicopathologic and immunohistochemistry study of 47 cases with and without underlying malignancy. J Cutan Pathol. 2009;36(7):729–733. doi: 10.1111/j.1600-0560.2008.01148.x. [DOI] [PubMed] [Google Scholar]
- 98.Richter CE, Hui P, Buza N, et al. HER-2/NEU overexpression in vulvar Paget disease: the Yale experience. J Clin Pathol. 2010;63(6):544–547. doi: 10.1136/jcp.2010.077446. [DOI] [PubMed] [Google Scholar]
- 99.Miyamoto A, Akasaka K, Oikawa H, Akasaka T, Masuda T, Maesawa C. Immunohistochemical study of HER2 and TUBB3 proteins in extramammary paget disease. Am J Dermatopathol. 2010;32(6):578–585. doi: 10.1097/DAD.0b013e3181cd35e0. [DOI] [PubMed] [Google Scholar]
- 100.Hikita T, Ohtsuki Y, Maeda T, Furihata M. Immunohistochemical and fluorescence in situ hybridization studies on noninvasive and invasive extramammary Paget’s disease. Int J Surg Pathol. 2012;20(5):441–448. doi: 10.1177/1066896912444159. [DOI] [PubMed] [Google Scholar]
- 101.Tanaka R, Sasajima Y, Tsuda H, et al. Human epidermal growth factor receptor 2 protein overexpression and gene amplification in extramammary Paget disease. Br J Dermatol. 2013;168(6):1259–1266. doi: 10.1111/bjd.12249. [DOI] [PubMed] [Google Scholar]
- 102.Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8(10):13233–13240. [PMC free article] [PubMed] [Google Scholar]
- 103.Lu X, Zhang P, Zhu Y, Ye D. Human epidermal growth factor receptor 2 amplification as a biomarker for treatment in patients with lymph node-metastatic penoscrotal extramammary Paget’s disease. Oncol Lett. 2019;17(3):2677–2686. doi: 10.3892/ol.2019.9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karam A, Berek JS, Stenson A, Rao JY, Dorigo O. HER-2/neu targeting for recurrent vulvar Paget’s disease. A case report and literature review. Gynecol Oncol. 2008;111(3):568–571. doi: 10.1016/j.ygyno.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 105.Takahagi S, Noda H, Kamegashira A, et al. Metastatic extramammary Paget’s disease treated with paclitaxel and trastuzumab combination chemotherapy. J Dermatol. 2009;36(8):457–461. doi: 10.1111/j.1346-8138.2009.00676.x. [DOI] [PubMed] [Google Scholar]
- 106.Hanawa F, Inozume T, Harada K, Kawamura T, Shibagaki N, Shimada S. A case of metastatic extramammary Paget’s disease responding to trastuzumab plus paclitaxel combination therapy. Case Rep Dermatol. 2011;3(3):223–227. doi: 10.1159/000333002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wakabayashi S, Togawa Y, Yoneyama K, Suehiro K, Kambe N, Matsue H. Dramatic clinical response of relapsed metastatic extramammary Paget’s disease to trastuzumab monotherapy. Case Rep Dermatol Med. 2012;2012:401362. doi: 10.1155/2012/401362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoshimura N, Arihiro K, Takahagi S, Hide M. An autopsy case of metastatic extramammary Paget’s disease treated with multimodality treatment including anti-HER2 therapy: what is the clinical and pathological significance of trastuzumab to the patient? Mod Chemother. 2013;02(4):66–68. doi: 10.4236/mc.2013.24008. [DOI] [Google Scholar]
- 109.Barth P, Al-Saleem ED, Edwards KW, Millis SZ, Wong YN, Geynisman DM. Metastatic extramammary Paget’s disease of scrotum responds completely to single agent trastuzumab in a hemodialysis patient: case report, molecular profiling and brief review of the literature. Case Rep Oncol Med. 2015;2015:895151. doi: 10.1155/2015/895151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang X, Jin W, Zhu H, Yu H. Extramammary Paget’s disease in two brothers. Indian J Dermatol. 2015;60(4):423. doi: 10.4103/0019-5154.160541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shin DS, Sherry T, Kallen ME, Wong S, Drakaki A. Human epidermal growth factor receptor 2 (HER-2/neu)-directed therapy for rare metastatic epithelial tumors with HER-2 amplification. Case Rep Oncol. 2016;9(2):298–304. doi: 10.1159/000445827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Watanabe S, Takeda M, Takahama T, et al. Successful human epidermal growth receptor 2-targeted therapy beyond disease progression for extramammary Paget’s disease. Invest New Drugs. 2016;34(3):394–396. doi: 10.1007/s10637-016-0329-8. [DOI] [PubMed] [Google Scholar]
- 113.Ichiyama T, Gomi D, Fukushima T, et al. Successful and long-term response to trastuzumab plus paclitaxel combination therapy in human epidermal growth factor receptor 2-positive extramammary Paget’s disease: a case report and review of the literature. Mol Clin Oncol. 2017;7(5):763–766. doi: 10.3892/mco.2017.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sekiguchi N, Kubota S, Noguchi T, et al. Experiences of trastuzumab plus paclitaxel combination therapy in metastatic human epidermal growth factor receptor 2-positive extramammary Paget’s disease: four cases and a review. J Dermatol. 2020;47(11):1276–1279. doi: 10.1111/1346-8138.15515. [DOI] [PubMed] [Google Scholar]
- 115.Kimura T, Akamatsu Y, Kajihara I, Fukushima S, Ihn H. Case of metastatic extramammary Paget’s disease treated with trastuzumab-biosimilar monotherapy after S-1 and docetaxel combination chemotherapy. J DermatolJ Dermatol. 2020;41(1):e1–e2. doi: 10.1111/1346-8138.15096. [DOI] [PubMed] [Google Scholar]
- 116.Guo J, Jiao X, Wu Y, Qin B, Liu K, Zang Y. Response to pyrotinib in a Chinese patient with bone-metastatic scrotal Paget’s disease harboring triple uncommon HER2 mutation: a case report. Onco Targets Ther. 2020;13:6289–6293. doi: 10.2147/OTT.S244814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Leon ED, Carcangiu ML, Prieto VG, et al. Extramammary Paget disease is characterized by the consistent lack of estrogen and progesterone receptors but frequently expresses androgen receptor. Am J Clin Pathol. 2000;113(4):572–575. doi: 10.1309/P756-XXCB-TV71-U4XV. [DOI] [PubMed] [Google Scholar]
- 118.Liegl B, Horn LC, Moinfar F. Androgen receptors are frequently expressed in mammary and extramammary Paget’s disease. Mod Pathol. 2005;18(10):1283–1288. doi: 10.1038/modpathol.3800437. [DOI] [PubMed] [Google Scholar]
- 119.Azmahani A, Nakamura Y, Ozawa Y, et al. Androgen receptor, androgen-producing enzymes and their transcription factors in extramammary Paget disease. Hum Pathol. 2015;46(11):1662–1669. doi: 10.1016/j.humpath.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 120.Zhou S, Zhong W, Mai R, Zhang G. Mammary and extramammary Paget’s disease presented different expression pattern of steroid hormone receptors. Biomed Res Int. 2017;2017:3768247. doi: 10.1155/2017/3768247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shirasaki F, Takata M, Ishii T, Fujimoto A, Hatta NTK. Total Androgen blockade therapy for an advanced case of extramammary Paget’s carcinoma. Skin Cancer. 2001;16(2):247–250. doi: 10.5227/skincancer.16.247. [DOI] [Google Scholar]
- 122.Iijima M, Uhara H, Ide Y, et al. Estrogen-receptor-alpha-positive extramammary Paget’s disease treated with hormonal therapy. Dermatology. 2006;213(2):144–146. doi: 10.1159/000093854. [DOI] [PubMed] [Google Scholar]
- 123.Isomoto K, Haratani K, Watanabe S, Takeda M, Iwasa T, Nakagawa K. Successful treatment of a case of hormone receptor – positive metastatic extramammary Paget disease with tamoxifen. Invest New Drugs. 2022;40(1):194–197. doi: 10.1007/s10637-021-01168-5. [DOI] [PubMed] [Google Scholar]
- 124.Yin X, Li X, Li M, et al. Treatment of metastatic primary extramammary Paget disease with combination anlotinib and tislelizumab: a case report and review of the literature. Front Med (Lausanne). 2022;9:891958. doi: 10.3389/fmed.2022.891958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers. 2019;11(6):754. doi: 10.3390/cancers11060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karpathiou G, Chauleur C, Hathroubi S, Habougit C, Peoc’h M. Expression of CD3, PD-L1 and CTLA-4 in mammary and extra-mammary Paget disease. Cancer Immunol Immunother. 2018;67(8):1297–1303. doi: 10.1007/s00262-018-2189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3 , B7-H4 , PD-L1 , PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10(58):6152–6167. doi: 10.18632/oncotarget.27247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guercio BJ, Iyer G, Kidwai WZ, et al. Treatment of metastatic extramammary Paget disease with combination ipilimumab and nivolumab: a case report. Case Rep Oncol. 2021;14(1):430–438. doi: 10.1159/000514345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang YN, Chen Y, Gao F, Chen N, Liu JY. Advanced scrotal extramammary Paget’s disease treated with apatinib: a case report. Clin Genitourin Cancer. 2018;16(2):e339–e342. doi: 10.1016/j.clgc.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 130.Umemura H, Yamasaki O, Kaji T, Otsuka M, Asagoe K, Iwatsuki K. Serum carcinoembryonic antigen level as a marker for advanced stage and chemotherapeutic response in extramammary Paget’s disease. Acta Derm Venereol. 2018;98(7):706–707. doi: 10.2340/00015555-2948. [DOI] [PubMed] [Google Scholar]
- 131.Kato J, Sumikawa Y, Hida T, et al. Serum cytokeratin 19 fragment 21-1 is a useful tumor marker for the assessment of extramammary Paget’s disease. J Dermatol. 2017;44(6):666–670. doi: 10.1111/1346-8138.13760. [DOI] [PubMed] [Google Scholar]
- 132.Onishi M, Maeda F, Akasaka T, Amano H. Monitoring serum cytokeratin 19 fragment 21-1 to determine the efficacy of docetaxel chemotherapy in advanced extramammary Paget’s disease. J Dermatol. 2019;46(3):e83–e84. doi: 10.1111/1346-8138.14627. [DOI] [PubMed] [Google Scholar]