Abstract
Pancreatic ductal adenocarcinoma (PDAC) is not sensitive to immune checkpoint blockade therapy, and negative feedback of tumor immune evasion might be partly responsible. We isolated CD8+ T cells and cultured them in vitro. Proteomics analysis was performed to compare changes in Panc02 cell lines cultured with conditioned medium, and leucine-rich repeat kinase 2 (LRRK2) was identified as a differential gene. LRRK2 expression was related to CD8+ T cell spatial distribution in PDAC clinical samples and upregulated by CD8+ T cells via interferon gamma (IFN-γ) simulation in vitro. Knockdown or pharmacological inhibition of LRRK2 activated an anti-pancreatic cancer immune response in mice, which meant that LRRK2 acted as an immunosuppressive gene. Mechanistically, LRRK2 phosphorylated PD-L1 at T210 to inhibit its ubiquitination-mediated proteasomal degradation. LRRK2 inhibition attenuated PD-1/PD-L1 blockade-mediated, T cell-induced upregulation of LRRK2/PD-L1, thus sensitizing the mice to anti-PD-L1 therapy. In addition, adenosylcobalamin, the activated form of vitamin B12, which was found to be a broad-spectrum inhibitor of LRRK2, could inhibit LRRK2 in vivo and sensitize PDAC to immunotherapy as well, which potentially endows LRRK2 inhibition with clinical translational value. Therefore, PD-L1 blockade combined with LRRK2 inhibition could be a novel therapy strategy for PDAC.
Keywords: LRRK2, PD-L1, PDAC, immunotherapy inhibitor
Graphical abstract

Bai and colleagues found that LRRK2 could be in the negative feedback loop for PDAC immune evasion. LRRK2 phosphorylated PD-L1 at T210 to inhibit its ubiquitination-mediated proteasomal degradation and thus promote tumor progression. LRRK2 inhibition or supplementation with adenosylcobalamin could sensitize PDAC to anti-PD1/PD-L1 immunotherapy.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers, ranking seventh in terms of estimated deaths worldwide.1,2 Although systematic treatment has been in clinical use, the numbers of new cases and deaths are still increasing.3,4 Programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1) blockade (e.g., durvalumab) is a promising therapy for some types of cancers;5,6 however, the response rate to durvalumab was not good in a PDAC clinical trial.7 The underlying reason is complicated. A discrepancy in CD8+ T cell infiltration in PDAC has been found that limits the scope of treatment. Previous work found that the subset of “immune inflamed” PDAC favored anti-PD therapy.8,9,10 However, a negative feedback loop might exist in this subset when tumors interact with reactivated CD8+ T cells, which is called “adaptive immune resistance,”11,12 where inflammatory cytokines, including interferon gamma (IFN-γ), play a critical role.13,14,15,16 The balance between immune evasion and immunogenic cell death determines the outcome of immunotherapy in the tumor microenvironment. Appropriate combination therapy could switch this balance and reshape the immune microenvironment.
Overexpression of PD-L1 in tumor cells can be induced not only by endogenous abnormally activated pathways, such as epidermal growth factor receptor (EGFR), MET proto-oncogene, receptor tyrosine kinase (MET), the nuclear factor κB (NF-κB) pathway, and the Hippo pathway, but also the exogenous immune environment.17 CD8+ T cells can constitutively upregulate PD-L1 levels in tumor cells via IFN-γ stimulation, and this endows PD-L1 with different roles in immunotherapy.11 On one hand, PD-L1 might be a biomarker of response of anti-PD therapy.18 High expression of CD8+ T cell-derived PD-L1 might imply a status of immune activation and provide a target for anti-PD therapy.13 On the other hand, tumor-associated PD-L1 promotes T cell apoptosis, inhibits T cell proliferation, and changes the cytokine expression profile of T cells.19 PD-1/PD-L1 blockade-mediated, T cell-induced upregulation of PD-L1 impairs immunotherapy.20 PD-L1 itself is viewed as an immunosuppressive gene and takes part in a negative feedback loop for immune evasion to exhaust cytotoxic T cells, which results in adaptive immune resistance.20 In addition, post-translational modification of PD-L1 affects the blocking effects of monoclonal antibodies (mAbs). Hung et al. found that steric hindrance by glycosylation of PD-L1 could inhibit mAb recognition and binding.21 Therefore, exogenous blockage of PD-L1 might not be enough for immunotherapy, and an endogenous decrease in glycosylation or reducing the total PD-L1 level could sensitize tumors to anti-PD therapy.22 Regarding this theory, our team has found previously that the MET, never in mitosis gene A-related kinase 2 (NEK2), tumor necrosis factor receptor 2 (TNFR2), and ubiquitin-specific peptidase 8 (USP8) can maintain PD-L1 expression at the translational or post-translational level to exert immunosuppressive effects, and the combination strategy can sensitize tumors to anti-PD therapy in murine models.23,24,25,26 These proteins maintain PD-L1 levels and were not induced by exogenous immune environment. We thought that precisely targeting key genes in the negative feedback loop could be a good combination strategy. Here, using high-throughput proteomics to identify proteins whose expression changed significantly in the presence of CD8+ T cells, we found leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2), which was significantly upregulated when cultured with CD8+ T cell conditioned medium.
LRRK2 is a serine/threonine-protein kinase that phosphorylates a wide range of proteins involved in multiple processes, such as neuronal plasticity, autophagy, and vesicle trafficking.27 Previous studies have found that LRRK2 is closely associated with immune-related disease such as Parkinson’s disease, Crohn’s disease, and leprosy. Zimprich et al.28 found that G2019S mutation enhances the kinase activity of LRRK2 and then causes autosomal-dominant parkinsonism with pleomorphic pathology. Takagawa et al.29 found that the risk polymorphism rs11564258 upregulates LRRK2 expression and, thus, suppresses autophagy and enhanced Dectin-1-induced immunity in colitis. Interestingly, further research indicated that LRRK2 can be upregulated in the inflammatory environment of Parkinson’s disease and Crohn’s disease, where IFN-γ is the critical cytokine.30,31,32,33 These studies suggest that overexpression of LRRK2 could result in occurrence of certain diseases and that an inflammatory environment could continually upregulate LRRK2, which accelerates disease progression. Studies of LRRK2 have focused on autoimmune disease, whereas very few studies have discussed the role of LRRK2 in tumors, being limited to non-immune roles in renal and thyroid cancer.34,35 The function of LRRK2 in tumor immunity has been rarely explored. Does LRRK2 play a role in immune evasion? Is LRRK2 activated in the tumor microenvironment, and does it promote cancer progression? The present study aimed to investigate the role of LRRK2 in pancreatic cancer immunotherapy.
Overall, in this work, we found that LRRK2 is upregulated in PDAC and has an immune-suppressive function by maintaining PD-L1 levels via post-translational modification. LRRK2 could be upregulated in a CD8+ T cell-enriched environment and played a role in the negative feedback loop for immune evasion. LRRK2 inhibition attenuated PD-1/PD-L1 blockade-mediated, T cell-induced upregulation of LRRK2/PD-L1, thus sensitizing tumors to anti-PD therapy.
Results
Profile of CD8+ T cell distribution and general PD-L1 expression in PDAC
Using multiplex immunohistochemistry and a tissue microarray, a large sample of the immune microenvironment in PDAC could be assessed. Although the tissue microarray could not reflect the overall percentage of CD8+ T cells and the absolute PD-L1 protein level, at least the diversity among patients and different regions, even between different areas close to each other, could be identified in one PDAC sample. The percentage of CD8+ T cells in PDAC samples ranged from 0.02%–26.34%, and a considerable number of relatively CD8+ T cell-enriched patients existed clinically (Figure S1A). This could be used as the foundation for research and clinical application of PD-1/PD-L1 blockade therapy in pancreatic cancer (Figure S1A).
LRRK2 was upregulated, especially in relatively “CD8+ T cell-enriched” PDAC
To identify novel targets that might function in the negative feedback loop of immune evasion when treated with immunotherapy, we isolated CD8+ T cells from mice and cultured them in RPMI 1640 complete medium containing anti-CD3 beads, anti-CD28 beads, and interleukin-2 (IL-2) for 3 days. CD8+ T cell conditioned medium was then added to Panc02 cell lines in vitro, while only RPMI 1640 complete medium containing anti-CD3 beads, anti-CD28 beads, and IL-2 at the same concentration was added for the negative control group. In this proteomics analysis, we found that several classic IFN-induced genes were significantly upregulated under treatment with CD8+ T cell conditioned medium, such as Stat1, Stat2, Tap2, H2-K, H2-D, H2-T23, and Cd74 (Figure 1A; Table S1). To better reflect clinical relevance, we used The Cancer Genome Atlas (TCGA) database to perform co-expression analysis and found that five genes (Lrrk2, Cd74, Glipr2, Cyld, and Itgb7) were significantly positively associated with CD8a (Pearson correlation coefficient (PCC) > 0.5) (Table S2). To reach clinical translational value, we searched the database for small molecular compounds and mAbs and found that a couple of inhibitors of LRRK2 were found in a previous study of Parkinson’s disease, such as the canonical inhibitor GSK2578215A; the novel inhibitor DNL201, which is in clinical trial; and the broad-spectrum inhibitor adenosylcobalamin.36,37,38 These inhibitors gave LRRK2 potential clinical translational value and intrigued us for further study. Western blotting confirmed that LRRK2 could be upregulated by CD8+ T cell conditioned medium (CM) (Figure S2A). The database of the immune microenvironment showed that LRRK2 was positively related to CD8+ T cells, the results of which seemed to be most significant in PDAC (Figure S2B). Co-expression analysis also revealed a strong positive correlation among CD8A, CD8B, and LRRK2 (Figure S2C). Western blotting of paired samples also revealed this correlation (Figure S2D). To determine how CD8+ T cells were regulated by LRRK2, we tested secretion of classical cytokines by CD8+ T cells and found that IFN-γ upregulated LRRK2 in cell lines and that this effect could be restored by anti-IFN-γ mAbs (Figures S2E–S2G). Co-expression analysis of CD8A, CD8B, IFN-γ, and LRRK2 and western blotting of paired PDAC tissue proved this correlation (Figures S2C and S2D). To further analyze LRRK2 expression in PDAC, immunohistochemistry of PDAC paraffin sections and western blotting of frozen PDAC tissue were performed. Significantly higher expression of LRRK2 in tumors compared with normal tissue was observed (Figures 1B–1D and S2D), which revealed that LRRK2 might act as an oncogene in PDAC. Additionally, LRRK2 levels were enriched in CD8+ T cell-enriched areas of PDAC tumors and in patients with PDAC, according to multiplex immunohistochemistry (Figures 1F–1H). A positive relationship between the percentage of CD8+ T cells and the mean fluorescence intensity of total LRRK2 in tumor cells was found using a tissue microarray (Figure 1E). Although high LRRK2 expression was not related to overall survival in the TCGA database, relative bad prognoses in CD8+ T cell-enriched patients were found, indicating that LRRK2 might have an immunosuppressive function (Figure S3A). What’s more, LRRK2 was positively related to some immune modulators in the database, which meant that LRRK2 might regulate some modulators to exert an immunosuppressive function (Figure S3B). Collectively, LRRK2 was upregulated in PDAC. In addition, the LRRK2 level was higher in CD8+ T cell-enriched PDAC and might have functions in the immune microenvironment.
Figure 1.
LRRK2 was upregulated especially in CD8 enriched PDAC
(A) After adding CD8+ T cell CM to Panc02 cell lines for 24 hours (h), cells were collected, and proteomics analysis was performed. Heatmap for differential genes are showed (log fold change > 1.3 or log fold change < 0.7, adjusted p value <0.05). (B) LRRK2 expression was measured in paired tumor and normal pancreatic tissues by IHC staining; shown are representative images. Scale bars, 75 μm (100×) and 50 μm (200×). (C) Statistics results of H score for IHC (n = 8). (D) Statistics results of normalized gray value (LRRK2/GAPDH) for western blotting. (E) Statistics results of multiplex Immunohistochemistry (mIHC) staining of CD8a and LRRK2 in a tissue microarray (n = 138). (F and G) mIHC was used to figure out the relationship between LRRK2 and CD8+ T cell distribution (DAPI, blue; PanCK, green; CD8a, red; LRRK2, yellow). The LRRK2 and CD8+ T cell localization relationship is shown. (H) mIHC and a tissue microarray were used to present the discrepancy of CD8+ T cell distribution and LRRK2 expression among patients (n = 138).
LRRK2 deficiency activated the anti-pancreatic cancer immune response
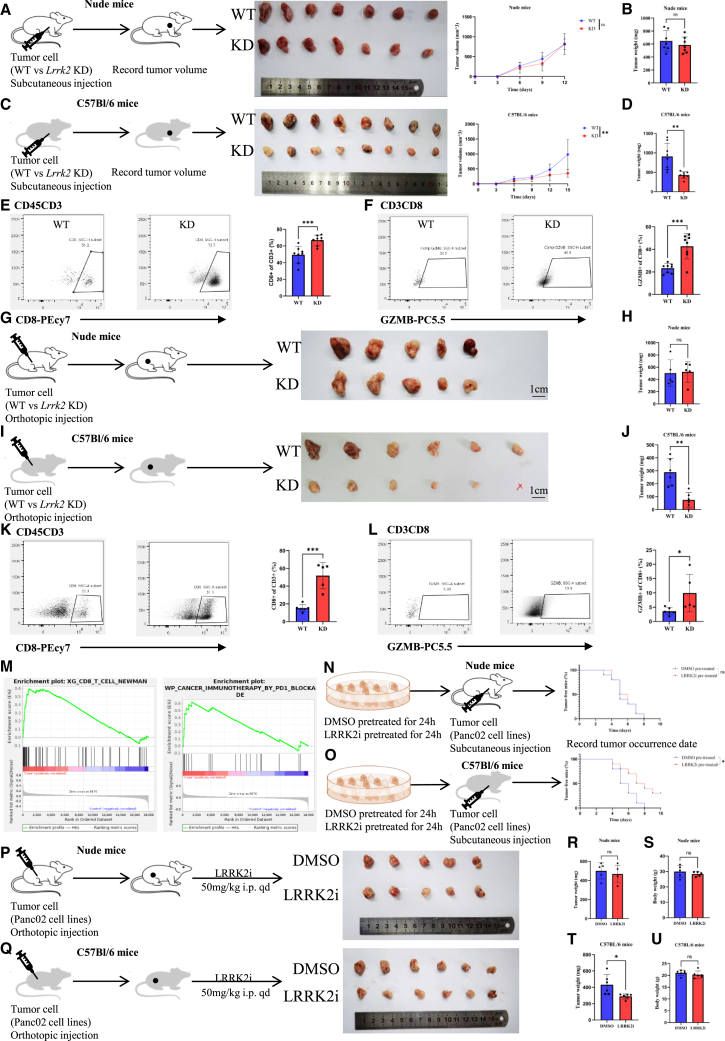
Because previous studies of LRRK2 focused on its role in macrophages, multiplex immunohistochemistry was used to identify the distribution of LRRK2 in PDAC. LRRK2 was mainly expressed on tumor cells regarding intensity and quantity (Figures S4A and S34B). Consequently, this research focused on the role of LRRK2 in pancreatic tumor cells. To assess LRRK2’s function in vivo, a Lrrk2 knockdown (KD) Panc02 cell line was generated to construct a subcutaneous injection mouse model. No growth difference between wild-type and Lrrk2 KD tumors in immunodeficient nude mice was observed (Figures 2A and 2B). However, a significantly decreased tumor growth rate and tumor volume were observed in immunocompetent C57Bl/6 mice with Lrrk2 KD tumors, which revealed that LRRK2 was related to immune response (Figures 2C and 2D). Flow cytometry was performed to detected changes in the immune microenvironment, and the percentage of CD8+ T cells was significantly increased in Lrrk2 KD cell-derived tumors (Figure 2E). Moreover, the level of granzyme B, a lethality index of CD8+ T cells, also increased (Figure 2F). These results were confirmed in an orthotopic tumor model, which was closer to the real immune microenvironment in PDAC, indicating that LRRK2 on tumor cells suppressed the immune response (Figures 2G–2L). In addition, RNA sequencing of Lrrk2 KD tumors versus Lrrk2 wild-type (WT) tumors from the orthotopic model was performed. Gene set enrichment analysis (GSEA) showed a significant upregulation of genes involved in CD8+ T cells and immunotherapy by PD-1 blockade (Figure 2M). Taken together, knocking down Lrrk2 in tumors reshaped the PDAC immune microenvironment and inhibited tumor growth in an immune-dependent manner. To further investigate whether LRRK2 inhibition was of transformation value, GSK2578215A, a classic inhibitor of LRRK2, was used in vitro and in vivo. Panc02 cells with or without pretreatment of GSK2578215A were injected subcutaneously into C57Bl/6 and nude mice, respectively. Pretreatment with GSK2578215A decreased the incidence and occurrence of tumors in immunocompetent C57Bl/6 mice, while this phenomenon was not significant in immunodeficient nude mice (Figures 2N and 2O). In addition, two groups of orthotopic tumor-bearing mice were treated with GSK2578215A or dimethyl sulfoxide (DMSO). Consistent with the KD model, inhibition of LRRK2 significantly decreased the tumor weight in C57Bl/6 mice but not in nude mice, while their body weight did not change significantly during treatment (Figures 2P–2U). Because LRRK2 was expressed in macrophages and cancer-associated fibroblasts (CAFs) in relatively small quantities, we tested whether LRRK2 inhibition enhanced the anti-pancreatic cancer immune response mainly by targeting tumor cells. Clodronate liposomes (CLs) were used to depleted macrophages, while all-trans-retinoic acid (ATRA) was used to quiet pancreatic satellite cells (PSCs). Macrophage depletion or PSC inactivation did not undermine the LRRK2 inhibitor (LRRK2i) effect on tumor suppression (Figures S5A–S5C). What’s more, LRRK2i did not inhibit PSC proliferation (Figures S5D and S5E). In addition, when PSC cell lines were cocultured with CD8+ T cells in vitro, LRRK2i could not improve the function and proliferation of CD8+ T cells (Figure S5F). Further, GSK2578215A did not suppress Lrrk2-KD cell-derived tumor progression compared with Lrrk2-WT cell-derived tumors (Figure S5G). Above all, GSK2578215A mainly targeted LRRK2 in tumor cells to exert an immune activation effect. To test how GSK2579215A affected CD8+ T cells, we found that, when only CD8+ T cells were treated with GSK2579215A, GSK2579215A did not affect CD8+ T cells function or proliferation in vitro (Figure S6A), but when CD8+ T cells and Panc02 cells were cocultured with GSK2579215A, significant increases in granzyme B (GZMB)+ T cells and Ki67+ T cells were found (Figure S6B). This suggested that GSK2579215A itself could not directly affect CD8+ T cell function to activate the immune response but prevented tumor cells from immune clearance. Immune checkpoints on tumor cells might explain this phenomenon. To further investigate how the function of CD8+ T cells was enhanced, we assessed a couple of immune checkpoints and found that PD-L1 levels were most significantly changed when LRRK2 was knocked down (Figure S6C). In addition, KD or kinase inhibition of LRRK2 decreased PD-L1 levels in vivo (Figures S6D–S6F). Because tumor-associated PD-L1 promotes T cell apoptosis, inhibits T cell proliferation, changes the cytokine expression profile of T cells, and subsequently results in exhaustion, decreased tumor-derived PD-L1 might partly explain the reason why LRRK2 deficiency could activate the immune response. Moreover, knocking out Cd274 in Panc02 cell lines could largely attenuate the reduction of tumor weight resulting from inhibition of LRRK2 (Figures S6G–S6I). These results suggested that LRRK2 deficiency enhanced the anti-pancreatic cancer immune response mainly via the PD-1/PD-L1 pathway.
Figure 2.
LRRK2 deficiency activated the anti-pancreatic cancer immune response
(A and C) Schematic protocols displaying that WT and LRRK2 KD Panc02 cells were separately and subcutaneously injected into immunocompetent and immunodeficient mice; a growth curve reflects tumor growth speed. (B and D) The weight of tumors from immunocompetent (n = 8) and immunodeficient mice (n = 7) was determined at the endpoint (n = 7). (E and F) Representative images and statistical results of percentages of CD8+ T cells and GZMB+ cells (n = 8). (G and I) Schematic protocols displaying that WT and LRRK2-KD Panc02 cells were separately and orthotopically injected into immunocompetent and immunodeficient mice. (H and J) The weight of tumors from immunocompetent (n = 6) and immunodeficient mice (n = 5) was determined at the endpoint (n = 6). (K and L) Representative images and statistical results of percentages of CD8+ T cells and GZMB+ cells (n = 6). (M) Significant differential genes in RNA-seq were enriched, as shown by GSEA. (N and O) Schematic protocols of Panc02 cancer cells, with or without pretreatment with the LRRK2 inhibitor GSK2578215A (1 μM, 24 h), separately and subcutaneously injected into immunocompetent and immunodeficient mice (n = 10). (P and Q) Schematic protocols displaying that Panc02 cell lines were orthotopically injected into immunocompetent and immunodeficient mice, and then the mice were divided into two groups for treatment with GSK2578215A or DMSO. (R–U) The tumor weight and body weight of immunocompetent (n = 6) and immunodeficient mice (n = 5) were determined at the endpoint (n = 6).
LRRK2 was positively related to, and directly combined with, PD-L1
We further assessed the correlation between LRRK2 and PD-L1. The TCGA database revealed a potential positive relationship between LRRK2 and CD274 (Figure S2C), and western blotting of paired clinical tissue samples supported this (Figure S2D). The tissue microarray provided large samples for correlation analysis, and a significant relationship between LRRK2 and PD-L1 was found (Figures 3A and 3B). Multiplex immunohistochemistry of PDAC paraffin sections and cell immune florescence confirmed that LRRK2 colocalized with PD-L1 (Figures 3C–3F). In addition, co-immunoprecipitation confirmed that LRRK2 could bind to PD-L1 (Figure S7A). Furthermore, LRRK2 colocalized with PD-L1 in the endoplasmic reticulum, which was marked by calnexin (Figure 3G). A Duolink assay revealed that the direct combination of LRRK2 and PD-L1 increased when treated with IFN-γ and decreased when Lrrk2 and Cd274 were knocked down (Figures 3H and 3I). These results suggested that LRRK2 might directly modify PD-L1.
Figure 3.
LRRK2 was positively related to, and directly combined with, PD-L1
(A and B) mIHC was used to figure out the relationship between LRRK2 and PD-L1 expression in a PDAC tissue microarray. Statistics results are shown. (C) mIHC staining for LRRK2 and PD-L1 (DAPI, blue; PD-L1, red; LRRK2, green). The colocalization area is marked in yellow. (D–F) Representative immunofluorescence (IF) images and statistical results of LRRK2 and PD-L1 colocalization in pancreatic cancer cell lines. (G) Representative IF images of LRRK2, PD-L1, and calnexin (DAPI, 405; LRRK2, 488; PD-L1, 555; calnexin, 647). (H and I) Representative IF images and statistical results of the combination signal of LRRK2 and PD-L1 (Duolink signal, 555).
LRRK2 inhibits ubiquitination-mediated proteasomal degradation of PD-L1 by phosphorylating the T210 residue
LRRK2 was positively related with, and could bind to, PD-L1; therefore, we performed functional assays to further investigate the mechanism. KD and inhibition of LRRK2 decreased the total protein level and membrane PD-L1 level in human and mouse PDAC cell lines (Figure 4A). Interestingly, KD of Lrrk2 did not affect the Cd274 transcriptional level, suggesting that LRRK2 regulated PD-L1 expression via post-translational modification (Figures S8A–S8D), which was suggested previously by their colocalization in the endoplasmic reticulum (Figure 3H). We also found that LRRK2 deficiency did not decrease the major histocompatibility complex (MHC) class 1 level because IFN-γ could upregulate the antigen presentation process (Figures S8E–S8K). To ensure that LRRK2 could post-translational modify PD-L1, exogenous WT PD-L1 was transfected into Cd274 knockout Panc02 cell lines, in which Cd274 could be transcriptionally regulated only by the cytomegalovirus (CMV) promoter, and GSK2578215A could also downregulate the PD-L1 level (Figure S8L). IFN-γ was used to treat WT PD-L1-transfected Cd274 knockout cell lines, and the PD-L1 level increased, accompanied by upregulation of LRRK2, which could be attenuated using GSK2579215A (Figure S8L). These results revealed that, although LRRK2 could be upregulated by IFN-γ, LRRK2 maintenance of the PD-L1 level was independent of the canonical IFN-γ-STAT1-IRF1-PD-L1 transcriptional axis. KD of Lrrk2 did not change the classic PD-L1 transcriptional regulator and autophagy pathway, which indicated that LRRK2 interacted with PD-L1 in a more direct and precise manner (Figure S6C). An in vitro kinase assay revealed that WT LRRK2 could phosphorylate PD-L1 while D1994A LRRK2, a kinase-dead mutant, could not (Figure 4C). Overexpression of WT LRRK2 significantly upregulated the PD-L1 level in the KPC (KrasLSL-G12D; Trp53LSL-R172H; Pdx1-Cre) cell line while kinase-dead LRRK2 (D1994S) could not (Figure S8M). Together with the evidence that the LRRK2 kinase-specific inhibitor GSK2579215A could decrease the PD-L1 level, we could figure out that LRRK2 maintained the PD-L1 level in a kinase-dependent manner. Moreover, decreasing the PD-L1 level could be reversed using the proteasome degradation inhibitor MG132 (Figure 4D). Additionally, the ubiquitination level of PD-L1 was upregulated when LRRK2 was inhibited or knocked down (Figure 4E). Indeed, KD or inhibition of LRRK2 decreased the half-life of PD-L1, which could be restored using MG132 (Figures 4H–4K). These results suggested that LRRK2 maintained the PD-L1 protein level in a kinase-dependent manner and protected PD-L1 from ubiquitination-mediated proteasomal degradation.
Figure 4.
LRRK2 inhibited ubiquitination-mediated proteasomal degradation of PD-L1 by phosphorylating the T210 residue
(A) Western blot analysis of PD-L1 expression in pancreatic cancer cell lines after treatment with an LRRK2 inhibitor (1 μM, 24 h) and Lrrk2 KD. A representative image is shown (n = 3 independent experiments). (B) Flow cytometry of membrane PD-L1 expression in pancreatic cancer cell lines after treatment with an LRRK2 inhibitor (1 μM, 24 h) and Lrrk2 KD. Statistical analysis is shown. (C) In vitro kinase assay and western blot analysis of p-threonine expression of recombinant PD-L1 WT and Lrrk2 WT (active) or D1994A (kinase dead) protein. A representative image is shown (n = 3 independent experiments). (D) Western blot analysis of PD-L1 expression in Panc02 Lrrk2 WT or Lrrk2 KD cells treated with an LRRK2 inhibitor (1 μM, 24 h) after treatment with MG132 (5 μM, 24 h). A representative image is shown (n = 3 independent experiments). (E) Ubiquitination assay of PD-L1 in Lrrk2 WT or Lrrk2 KD cells treated with an LRRK2 inhibitor (1 μM, 24 h), subjected to anti-PD-L1 immunoprecipitation (IP) and anti-ubiquitin western blot analysis. A representative image is shown (n = 3 independent experiments). (F) Ubiquitination assay of PD-L1 in FLAG-PD-L1 WT and T210A or T210D-transfected 293T cells subjected to anti-FLAG IP and anti-ubiquitin western blot analysis. A representative image is shown (n = 3 independent experiments). (G) Ubiquitination assay of PD-L1 in FLAG-PD-L1 WT and T209A- or T209D-transfected Panc02 Cd274 knockout (KO) cells subjected to anti-PD-L1 IP and anti-ubiquitin western blot analysis. A representative image is shown (n = 3 independent experiments). (H and I) Stability analysis of PD-L1 in Lrrk2 WT and Lrrk2 KD Panc02 cells treated with cycloheximide (CHX; 50 μg/mL). A representative image is shown (n = 3 independent experiments). The intensity of PD-L1 protein expression was quantified using a densitometer. (J and K) Stability analysis of PD-L1 in Panc02 cells treated with an LRRK2 inhibitor (1 μM, 24 h) after treatment with CHX (50 μg/mL). A representative image is shown (n = 3 independent experiments). The intensity of PD-L1 protein expression was quantified using a densitometer.
A LRRK2 phosphorylation binding motif (F/YxT) was identified around the T210 residue (Mus musculus T209) in the glycosylation-rich region of PD-L1 (N200 and N219) (Figure S9).22,24,39 Co-immunoprecipitation and mass spectrometry analysis found that the T210 residue could be phosphorylated in 293T cells, whereas, when the cells were treated with GSK2579215A, phosphorylation of T210 was not detected by mass spectrometry (Figure S10). Consistent with the results using HEK293T cells, the same treatment in Panc02 cells largely reduced the abundance of phosphorylation of the T209 residue together with decreased abundance of glycosylation of the N199 and N218 residues (Figures S11–S13). Constitutively phosphorylated (T210D/T209D) and non-phosphorylated (T210A/T209A) mutants were constructed to further investigate the function of phosphorylation of T210 or T209. The T210D mutation increased PD-L1 expression while the T210A mutation decreased it, which could be restored by MG132 treatment (Figures S14A–S14C). Moreover, the T210A mutation resulted in a higher ubiquitination level of PD-L1, while the T210D mutation had the opposite effect (Figure 4F). The results for the mouse T209 residue were consistent with those for human T210 (Figures 4G and S14D–S14F). Additionally, GSK2579215A could not reduce the levels of T209A/T209D PD-L1 to the same extent as WT PD-L1 (Figure S14G), indicating that LRRK2 maintenance of PD-L1 levels was mainly dependent on T209 phosphorylation. WT, T209A, and T209D PD-L1, together with the vector control, were lentivirally transfected into Cd274 knockout Panc02 cell lines, and the stable cell lines were then orthotopically injected into C57Bl/6 mice. Overexpression of PD-L1 could increase the tumor weight, whereas the weight of the T209A-mutated tumors was lower than that of the WT and T209D-mutated tumors, consistent with their PD-L1 levels (Figures S14H and S14I). Collectively, LRRK2 inhibits ubiquitination-mediated proteasomal degradation of PD-L1 via phosphorylation of T210.
LRRK2 deficiency sensitized PDAC tumors to PD-1/PD-L1-targeted immunotherapy
LRRK2 maintained PD-L1 stability; therefore, we hypothesized that a combination therapy comprising LRRK2 inhibition and PD-L1 blockade would sensitize PDAC to immunotherapy. Lrrk2 KD tumor-bearing mice were sensitive to anti-PD-L1 mAb treatment, as assessed by the tumor volume and survival rate (Figures 5A–5D). Consistent with the results for Lrrk2 KD, the LRRK2 kinase inhibitor GSK2579215A combined with the anti-PD-L1 mAb significantly decreased tumor growth and extended mouse survival (Figures 5E–5H). Hematoxylin and eosin staining of important organs and biochemical detection revealed that the combination therapy did not cause damage to the mice and excluded that the inhibitor itself affected the status of tumor-bearing mice (Figure S15). Through immunohisochemistry, we found that the combination therapy could significantly increase CD8+ T cell infiltration and lethality compared with either monotreatment, indicating that combination therapy could further reactive immune microenvironment and sensitize immunotherapy (Figures 5I–5K). The mechanism of the sensitization effect was associated with LRRK2 and induction of CD8+ T cells. CD8+ T cells could upregulate PD-L1 and LRRK2 in vitro (Figures S2E–S2G). The anti-PD-L1 mAb could increase the number and activity of CD8+ T cells and thus upregulate LRRK2 and PD-L1 expression in C57Bl/6 mice but not in nude mice (Figure S16A). By contrast, CD8 depletion downregulated LRRK2 and PD-L1 (Figure S16B). GSK2579215A combined with the PD-L1 mAb could attenuate CD8+ T cell-induced upregulation of PD-L1 to sensitize PD-1/PD-L1 blockade (Figure S16C). From a perspective of clinical translation, GSK2579215A was a kinase-specific inhibitor of LRRK2 and was effective in vivo; however, it is not in clinical use, and its low water solubility limits its future translational value. Adenosylcobalamin, the activated form of vitamin B12, which is used to treat pernicious anemia and is a broad-spectrum inhibitor of LRRK2, was taken into consideration. Adenosylcobalamin has high water solubility and could inhibit LRRK2 on tumor tissue, which could be detected by western blotting (Figure S17A). Adenosylcobalamin combined with the anti-PD-L1 mAb increased CD8+ T cell infiltration and function and was shown to be safe, with no significant changes in body weight or spleen weight (Figures S17B–S17G). Overall, PD-L1 blockade combined with LRRK2 inhibition or vitamin B12 supplementation is a promising therapy for PDAC.
Figure 5.
LRRK2 deficiency sensitized PDAC tumors to PD-1/PD-L1 targeted immunotherapy
(A) Schematic protocols displaying that WT and Lrrk2-KD Panc02 cells were separately and orthotopically injected into immunocompetent and immunodeficient mice. Tumor-bearing mice were treated with anti-PD-L1 mAb (200 μg/mouse). (B and C) The tumor weight and body weight of immunocompetent mice (n = 6) were determined at the endpoint (n = 6). (D) Kaplan-Meier curve for tumor-bearing mice receiving treatment (n = 10). (E) Schematic protocol of the combination of anti-PD-L1 mAb and LRRK2 inhibitor therapy. (F and G) Tumor weight and body weight of mice treated with anti-PD-L1 mAb (200 μg/mouse), LRRK2 inhibitor (50 mg/kg), or their combination (n = 5) were determined at the endpoint. (H) Kaplan-Meier curve for tumor-bearing mice receiving treatment (n = 10). (I–K) Representative images and quantification of CD8+ cells and GZMB+ cells. Scale bar, 75 μm. (L) Schematic protocol of the combination of anti-PD-L1 mAb and Adocbl therapy. (M) Tumor weight of mice treated with anti-PD-L1 mAb (200 μg/mouse), Adocbl (10 mg/kg), or their combination (n = 6).
The anti-pancreatic cancer effect of LRRK2 inhibition is dependent on CD8+ T cells
To further investigate whether the anti-pancreatic cancer effect of LRRK2 inhibition is dependent on CD8+ T cells, an anti-CD8 mAb was used in vivo. CD8 deletion could attenuate the decreased tumor weight resulting from KD of Lrrk2 or the combination of GSK2579215A and PD-L1 mAb (Figures 6A–6C and 6H–6J). Flow cytometry analysis confirmed that CD8+ T cells were almost depleted (Figures 6D, 6E, 6K, and 6L). A T cell-mediated tumor cell destruction assay revealed that LRRK2 deficiency could enhance the lethal effect of CD8+ T cells, which was more significant when combined with the PD-L1 mAb, consistent with the results in vivo (Figures 6F, 6G, 6M, and 6N).
Figure 6.
The anti-pancreatic cancer effect of LRRK2 inhibition was dependent on CD8+ T cells
(A) Schematic protocols displaying that WT and Lrrk2 KD Panc02 cells were separately and orthotopically injected into immunocompetent mice. Tumor-bearing mice were treated with anti-CD8 mAb (100 μg/mouse). (B and C) The tumor weight and body weight of immunocompetent mice (n = 6) were determined at the endpoint. (D and E) Representative flow cytometry and quantification of CD8 staining of splenocytes to confirm immune cell depletion (n = 3). (F and G) Representative images and statistical result of a T cell-mediated cancer cell killing assay. Panc02 cells with Lrrk2 KD were cocultured with activated T cells for 48 h and subjected to crystal violet staining. (H) Schematic protocol of the combination of anti-PD-L1 mAb and LRRK2 inhibitor therapy and anti-CD8 mAb treatment. (I and J) Tumor weight and body weight of mice treated with a combination of anti-PD-L1 mAb (200 μg/mouse) and LRRK2 inhibitor (50 mg/kg) or CD8 depletion (n = 6). (K and L) Representative flow cytometry and quantification of CD8 staining of splenocytes to confirm immune cell depletion (n = 3). (M and N) Representative images and statistical result of a T cell-mediated cancer cell killing assay. Panc02 cells pretreated with an LRRK2 inhibitor were cocultured with activated T cells for 48 h and subjected to crystal violet staining.
In conclusion, LRRK2 is upregulated in PDAC, and exerts an immunosuppressive effect by maintaining PD-L1 levels. LRRK2 inhibits ubiquitination-mediated proteasomal degradation of PD-L1 by phosphorylating the T210 residue. LRRK2 could be upregulated by CD8+ T cells via IFN-γ and took part in the negative feedback loop for tumor immune evasion. LRRK2 inhibition attenuated PD-1/PD-L1 blockade-mediated, T cell-induced, upregulation of LRRK2/PD-L1, thus sensitizing tumors to anti-PD therapy.
Discussion
PD-1/PD-L1 blockade has become the most successful immunotherapy in many types of cancer; however, it is not so sensitive in PDAC. Researchers have attributed this inefficiency to the so called “immune desert” of PDAC; indeed, many studies, including ours, found a discrepancy in CD8+ T cell infiltration among patients with PDAC.8,9,10,40 For the subset of PDAC that does show a strong inflammatory response, including accumulation of antigen-specific CD8+ T cells, the mechanism of immune escape is worthy of investigation. One potential reason is that a negative feedback loop exists in pancreatic cancer cells when exposed to CD8+ T cells. In our study, LRRK2 was upregulated in PDAC and played a role in immunosuppression. Interestingly, LRRK2 could be induced by CD8+ T cells via IFN-γ infiltration. As is universally acknowledged, IFN-γ exerts opposing effects in immunity. On one hand, IFN-γ polarizes T helper (Th) cells toward a Th1 cell phenotype, promotes tumor recognition by the MHC class 1 pathway, induces tumor cell apoptosis, and inhibits tumor proliferation.41,42 On the other hand, IFN-γ could upregulate the expression of immune-suppressive genes, such as that encoding PD-L1, via transcriptional regulation, suppressing the function of CD8+ T cells.16,43 Thus, IFN-γ is a double-edged sword that limits its clinical application. Previous research tried to address the adaptive immune resistance induced by IFN-γ by inhibiting the transcriptional activity of signal transducer and activator of transcription 1 (STAT1). Huang et al.15 found that cell-dependent kinase (CDK)1/2/5 inhibition blocked Jun proto-oncogene, AP-1 transcription factor subunit (JUN)-dependent STAT1 expression and activation. Liu et al.14 found that expression of heat shock protein 90 (HSP90) inhibited PD-L1 via the IFN-γ-STAT1-IRF1-PD-L1 transcriptional axis. Cerezo et al.20 found that eukaryotic translation initiation factor 4 gamma 1 (EIF4F) maintained IFN-γ-induced PD-L1 by upregulating translation of STAT1. However, because STAT1 also has an anti-cancer effect, transcriptional inhibition of the IFN-γ-STAT1-IRF1-PD-L1 axis is not precise and might cause side effects.44 In this research, we found that LRRK2 could be induced by IFN-γ and maintained PD-L1 levels via post-transcriptional modification. In this event, IFN-γ could upregulate PD-L1 expression via a non-canonical transcriptional pathway. In other words, LRRK2 inhibition could attenuate IFN-γ induced upregulation of PD-L1 and at the same time did not affect the anti-tumor effect of IFN-γ. This might offer a solution to address resistance of IFN-γ in immunotherapy.
Immune clearance and tolerance are dynamic processes. When immune tolerance is established, even anergic CD8+ T cells can secrete detectable IFN-γ, which might continually upregulate PD-L1.45 Treatment using an anti-PD-L1 mAb can normalize immunity and reactivate exhausted CD8+ T cells. Most solid tumors lack the costimulatory molecules B7-1 and B7-2, which downregulate the antigen presentation process so that the tumors are not rejected by the initial accumulation of CD8+ T cells, which would favor establishment of immune tolerance.9,10,46 The balance between immune evasion and immunogenic cell death determines the outcome of immunotherapy in the tumor microenvironment. Therefore, appropriate combination therapy could switch this balance and reshape the immune microenvironment. In this study, we found that, like PD-L1, LRRK2 was upregulated in the CD8+ T cell-enriched microenvironment and contributes to the negative feedback loop in tumor immune evasion. We found that PD-L1 blockade upregulated expression of the immunosuppressive protein LRRK2 and PD-L1, which might cause adaptive immune resistance. LRRK2 inhibition attenuated PD-1/PD-L1 blockade-mediated, T cell-induced upregulation of LRRK2/PD-L1, sensitizing tumors to anti-PD therapy. Therefore, a combination of LRRK2 inhibition and PD-L1 blockade is promising for treatment of PDAC.
Although LRRK2 is viewed as star molecule to treat Parkinson’s disease, inhibitors of LRRK2 are still in clinical trials, restricting clinical transformation in the short term. However, adenosylcobalamin has been found to be a broad-spectrum inhibitor of LRRK2.38
Adenosylcobalamin, the active form of vitamin B12, is water soluble and safe. Adenosylcobalamin is used to correct or prevent pernicious anemia caused by chemotherapy.47,48 Coincidentally, clinical use of PD-1/PD-L1 blockade immunotherapy in PDAC is only for patients who have failed to respond to chemotherapy.49 Thus, the combination of immunochemotherapy and adenosylcobalamin would likely have a benefit without causing harm.
Collectively, LRRK2 is an immunosuppressive protein in PDAC by maintaining PD-L1 levels via post-translational modification. LRRK2 could be upregulated by CD8+ T cells via IFN-γ stimulation and is in a negative feedback loop for cancer immune evasion. Thus, LRRK2 is a potential target for immunotherapy. The combination of LRRK2 inhibition with anti-PD therapy could sensitize tumors to anti-PD therapy. In the future, further research will focus on LRRK2 translational application in PDAC and extend its pan-cancer applicability.
There are limitations to this research. First, since there is no acceptable database of the expression profile or prognosis which compared the treatment of immunotherapy with placebo in PDAC, we could not confirm whether high expression of LRRK2 correlates with poor prognosis in patients with PDAC treated with immunotherapy. Second, CD8+ T cells stimulate tumors in different ways, and this research only provided one possible explanation of negative feedback in tumor immune evasion and supplied one solution. Additionally, combination therapy comprising PD-L1 blockade and LRRK2 inhibition (or supplementation with adenosylcobalamin) is proposed for the first time, which needs further validation in different types of tumors and models.
Materials and methods
Cell culture
The Panc02, SW1990, HEK293T, and HEK293FT cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). The KPC cell line, derived from spontaneous tumors in a KrasLSL-G12D; Trp53LSL-R172H; Pdx1-Cre mouse model, was a kind gift from the laboratory of Prof. Raghu Kalluri (MD Anderson Cancer Center, Houston, TX, USA). All of these cell lines were cultured in D10 medium (Dulbecco’s modified Eagle’s medium [DMEM] high glucose [Thermo Fisher Scientific, Waltham, MA, USA], 10% fetal bovine serum (Thermo Fisher Scientific), and 1% penicillin and streptomycin [Cienry, CR-15140]) at 37°C with 5% CO2. Mycoplasma was detected at regular intervals using PCR and eliminated using tiamulin fumarate and minocycline hydrochloride for the sake of quality and repeatability. Recombinant human IFN-γ (MedChemExpress [MCE], Monmouth Junction, NJ, USA; HY-P70610), recombinant mouse IFN-γ (MCE, HY-P70667), recombinant mouse IL-2 (MCE, HY-P7077), recombinant mouse TNF-α (MCE, HY-P70571), the LRRK2 inhibitor GSK2578215A (Selleck, Houston, TX, USA; S7664), adenosylcobalamin (MCE, HY-112790), MG132 (Beyotime Biotechnology, Jiangsu, China; S1748), cycloheximide (Sigma, St. Louis, MO, USA; 239763-M), the anti-IFN-γ mAb (Bio X Cell, Lebanon, NH, USA; BE0312), and the anti-PD-L1 mAb (Bio X Cell, 10F.9G2-CP168) were added to the medium at the concentrations mentioned above.
Plasmids and cell transfection
LRRK2 short hairpin RNA plasmids and control shRNA Plasmid-A were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA; sc45750 [m], sc45380 [h] and sc-108060 [control-A]). The lentiviral mammalian expression template plasmid pSLenti-EF1-EGFP-P2A-Puro-CMV-MCS-3×FLAG-Posttranscriptional Regulatory Element (WPRE) (GL107) and the CD274 coding sequence (CDS) template were purchased from Obio Technology (Shanghai, China). The lentiviral CRISPR knockout plasmid Lenticrispr-v2-gfp was purchased from Addgene (Watertown, MA, USA). The lentiviral ovalbumin (OVA) antigen overexpression plasmid pLV3-CMV-OVAL(chicken)-CopGFP-Puro was purchased from Addgene. The mammalian expression plasmids pPuro3.1(+)_Strep-Lrrk2-Myc and pPuro3.1(+)_Strep-Lrrk2(D1994S)-Myc were purchased from Addgene. The small guide RNA for mouse Cd274 (5′-CTGGATCCACGGAAATTCTC-3′) was cloned into Lenticrispr-v2-gfp using Golden Gate Assembly (New England Biolabs, Ipswich, MA, USA; E1602L). Point mutations of human CD274 were created using Infusion Cloning (Takara, Dalian, China; 638947) and the following primers:
T210A-FORWARD, 5′-CTACTGCGCATTTAGGAGATTAGATCCTGAGGAAA-3′;
T210A-REVERSE, 5′-CTAAATGCGCAGTAGAAAATCTCATTAGTTGTT-3′;
T210D-FORWARD, 5′-CTACTGCGACTTTAGGAGATTAGATCCTGAGGAAA-3′;
T210D-REVERSE, 5′-CTAAAGTCGCAGTAGAAAATCTCATTAGTTGTT-3′.
Primers for the point mutations of mouse Cd274 were as follows:
T209A-FORWARD, 5′-CTACTGTGCGTTTTGGAGATCACAGCCAGGG-3′;
T209A-REVERSE, 5′-CAAAACGCACAGTAGAAAACATCATTCGCTGTG-3′;
T209D-FORWARD, 5′-CTACTGTGACTTTTGGAGATCACAGCCAGGG-3′;
T209D-REVERSE, 5′-CAAAAGTCACAGTAGAAAACATCATTCGCTGTG-3′.
For transient transfection, when the cell confluence reached 50% in 6-well plates, 2 μg of plasmids was mixed with 500 μL of jetPRIME buffer (Polyplus, Illkirch, France; 101000001) with 4 μL of jetPRIME reagent for 15 min and then added to serum-containing medium. For lentivirus production, 4 μg of targeted plasmids, 2 μg of plasmid psPAX2, and 1 μg of plasmid pMD2.G were mixed and then added to HEK293FT cells in a 10-cm dish. 48 h after changing the medium, the supernatant was collected, filtered through a 0.45-μm Stericup filter unit (Millipore, Billerica, MA, USA) and concentrated using PEG8000 (Beyotime Biotechnology, ST483). To generate a stable cell line, lentivirus was transfected at a multiplicity of infection (MOI) of less than 0.4, and transfected cells were selected using puromycin (InvivoGen, San Diego, CA, USA) at a certain concentration that was tested to be the lowest to kill all untransfected cells. To achieve a Cd274 knockout subclone, the lenticrispr plasmid was transiently transfected and sorted into single cells by cytometry using GFP in 96-well plates. Transient and stable transfection was verified using western blotting.
Animal care
C57BL/6-Tg (TcraTcrb)1100Mjb/J OT-I mice were bred in the Experimental Animal Center, Huajiachi Campus, Zhejiang University. C57BL/6 mice and nude mice were purchased from Hangzhou Ziyuan Experimental Animal Technology (Hangzhou, China). Mice were raised in the Experimental Animal Center, First Affiliated Hospital, School of Medicine, Zhejiang University. All animal experiments were approved by the ethics committee of the First Affiliated Hospital, School of Medicine, Zhejiang University. Animal suffering was minimized to guarantee animal welfare during the study.
Animal experiments
Six-week-old male mice were challenged with 10,000 Panc02 cells in phosphate-buffered saline (PBS) in all experiments, and each experiment included a control group. For the subcutaneous tumor model, Panc02 cells in 100 μL of PBS were injected into the right flanks of mice. The injection site and depth were controlled similarly to eliminate the effect of blood supply. The tumor volume was calculated by measuring the longest diameter and the perpendicular short diameter. Tumor growth conditions were recorded every 3 days using calipers. The formula to calculate the tumor volume was as follows: 1/2 × length × width2. For the orthotopic tumor model, Panc02 cells in 25 μL of PBS were injected into the pancreas under anesthesia. For treatment, we calculated the weight of tumor-bearing mice to generate a randomization sequence with no blinding. Treatment doses used in the study were as follows: GSK2578215A (Selleck, S7664, 1 mg/mouse, intraperitoneal [i.p.], quaque die (qd)), PD-L1 mAb (Bio X Cell, 10F.9G2-CP168, 200 μg/mouse, i.p., quaque omni die (qod)), anti-CD8 mAb (Bio X Cell, YTS 169.4, 100 μg/mouse, i.p., qod), CLs (Yeasen, 40337ES08, 200 μl/mouse, i.p., once), ATRA (MCE, HY-14649, 0.5 mg/mouse, i.p., qd), and adenosylcobalamin (MCE, HY-112790, 200 μg/mouse, i.p., qd). Detailed treatment information and sample sizes (n > 5) are shown in the figures or figure legends. Two weeks after injection, the mice were sacrificed, and the tumors were excised for weighing and further analysis. All mice that were alive were included in data analysis. Each tumor was divided into three pieces for flow cytometry, western blotting, and immunohistochemistry. Regarding the survival experiments, Panc02 cells in 25 μL of PBS were injected into the pancreas under anesthesia. Forty tumor-bearing mice were divided randomly into four groups (n = 10). The time of death of each mouse was recorded.
Human tissues
Human PDAC tissue and adjacent normal tissues specimens were obtained from the Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University. The protocol was approved by the institutional review board at the First Affiliated Hospital, School of Medicine, Zhejiang University. Paraffin-embedded PDAC tissue array slides comprising 156 samples from patients were created by Wuhan Servicebio Technology (Wuhan, China) using PDAC tissue specimens from the First Affiliated Hospital, School of Medicine, Zhejiang University.
Flow cytometry
For flow cytometry analysis, tissues were prepared as a single-cell suspension in advance, while cell lines did not need such preparation. Tissues were first cut up and then placed in DMEM with 2% fetal bovine serum (FBS), collagenase IV (Thermo Fisher Scientific, 17104019, 1 mg/mL), DNase (Sigma, D5025, 10 μg/mL) + dispase (Gibco, Grand Island, NY, USA; 17105041, 0.6 mg/mL), and CaCl2 (Sigma, 21115, 3 mM) and shaken at 200 rpm for 30 min at 37°C. Digestion was stopped by adding D10 medium, and tissues were filtered through 70-μm cell strainers (Sigma, CLS431751-50EA). Erythrocytes were lysed using lysis buffer (BD Biosciences, San Jose, CA, USA; 555899), and samples were stained using a LIVE/DEAD Fixable Violet Dead Cell Staining Kit (Thermo Fisher Scientific, L34960, BV421). After blocking using TruStain FcX antibody (BioLegend, San Diego, CA, USA; anti-mouse CD16/32), cell surface markers were stained. To stain intracellular markers, samples were fixed and permeabilized using the eBioscience Forkhead Box O3 (FOXP3)/Transcription Factor Staining Buffer Set (Thermo Fisher Scientific, 00-5523-00). Antibodies used for flow cytometry were as follows: fluorescein isothiocyanate (FITC) anti-mouse CD3 antibodies (BioLegend, 100203), Brilliant Violet 785 anti-mouse CD45 antibodies (BioLegend, 103111), phycoerythrin (PE)/cyanine-7 (Cy7) anti-mouse CD8a antibodies (BioLegend, 100722), peridinin chlorophyll protein complex (PerCP)/cyanine-5.5 anti-human/mouse GZMB recombinant antibodies (BioLegend, 372212), PE anti-mouse CD274 antibodies (BioLegend, 124308), allophycocyanin (APC) anti-mouse CD274 antibodies (BioLegend, 124312), PE anti-mouse CD274 antibodies (BioLegend, 124308), PE anti-human CD274 antibodies (BioLegend, 329706), PerCP/Cyanine-5.5 anti-mouse MHC class 1 determinant H-2Kb/H-2Db antibody (BioLegend, 124620), Brilliant Violet 605 anti-mouse IFN-γ antibody (BioLegend, 505839), PE anti-mouse perforin antibody (BioLegend, 154306), Brilliant Violet 510 anti-mouse TNF-α antibody (BioLegend, 506339), and APC anti-mouse Ki-67 antibody (BioLegend, 652406). All samples were detected using a Fortessa flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), and data were analyzed using FlowJo software (Becton Dickinson, v.10.8.1).
Western blotting and immunoprecipitation
Cells and tissues were lysed using radioimmunoprecipitation assay (RIPA) buffer (Beyotime Biotechnology, P0013B) containing a protease inhibitor cocktail (Bimake, Houston, TX, USA; B14001) and a phosphatase inhibitor cocktail (Bimake, B15001) for 30 min on ice and centrifuged at 1,4000 × g for 20 min. The supernatant was retained, and the protein concentration was determined using the bicinchoninic acid (BCA) reagent (Beyotime Biotechnology, P0012). Then, 4× loading buffer and double distilled H2O (ddH2O) was used to dilute the protein samples to the same concentration. After heat denaturation at 100°C, the samples were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred electrophoretically to a polyvinylidene fluoride membrane (Millipore). After blocking using 5% skim milk for 1 h, the membrane was incubated with the corresponding primary antibodies overnight. The primary antibodies used for western blotting were as follows: anti-LRRK2 (Abcam, Cambridge, MA, USA; ab133474, 1:5,000), anti-LRRK2 (phospho-S935) (Abcam, ab133450, 1:1,000), anti-LRRK2 (Novus Biologicals, St. Louis, MO, USA; NB300, 1:1,000), anti-PD-L1 (Abcam, ab213480, 1:1,000), anti-PD-L1 (Cell Signaling Technology, Danvers, MA, USA; 13684, 1:1,000), anti-PD-L1 (Proteintech, Rosemont, IL, USA; 1:2,000, 14-5983-82), anti-FLAG (Sigma, F9291, 1:5,000), anti-vinculin (Abcam, ab219649, 1:5,000), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam, ab8245, 1:2,000), anti-IFN-γ (Abcam, ab267369, 1:1,000), anti-myc tag (Abcam, ab32, 1:1,000), anti-GFP (Proteintech, 50430-2-AP, 1:2,000), and anti-ubiquitin (CST, P4D1, 1:1,000). The next day, the membrane was washed using Tris-buffered saline-Tween 20 (TBST) and then incubated at 4°C for 4 h with secondary antibodies (Beyotime Biotechnology, A0192 and A0218). The signals were amplified using an EzWay DAB Western Blot Kit (KOMA BIOTECH, Seoul, Korea) and detected using ChemiScopeTouch (Clinx Science Instruments, Shanghai, China). For immunoprecipitation, cell supernatants gathered after treatment were incubated with corresponding primary antibodies for 4 h and then with protein A/G magnetic beads (Bimake, B23202) for 2 h. Samples were rinsed with washing buffer (lysis buffer diluted with PBS to 10%) to remove nonspecific binding, eluted from the beads, and subjected to western blotting.
Quantitative reverse transcriptase PCR (qRT-PCR) analysis
Total cell RNA was isolated using the FastPure Cell/Tissue Total RNA Isolation Kit V2 (Vazyme, Nanjing, China; RC112-01), and then reverse transcribed using a PrimeScript RT reagent kit (RR047A, Takara). The resulting cDNA was used as the template for the quantitative real-time PCR step of the qRT-PCR protocol using TB Green Premix Ex Taq II (RR820A, Takara) and corresponding primers in a real-time PCR machine (7500 Fast Real-Time PCR System; Applied Biosystems, Foster City, CA, USA). Primers used in this research were are follows: human ACTB forward, 5′-CATGTACGTTGCTATCCAGGC-3′; human ACTB reverse, 5′-CTCCTTAATGTCACGCACGAT-3′; human LRRK2 forward, 5′-GAGCACGCCTCCAAGTTATTT-3′; human LRRK2 reverse, 5′-ACTGGCATTATGAACTGTTAGCA-3′; human CD274 forward, 5′-TGGCATTTGCTGAACGCATTT-3′; human CD274 reverse, 5′-TGGCATTTGCTGAACGCATTT-3′; mouse Actb forward, 5′-GTGACGTTGACATCCGTAAAGA-3′; mouse Actb reverse, 5′-GCCGGACTCATCGTACTCC-3′; mouse Lrrk2 forward, 5′-AGCCTTGGATCTCCTCCTAGA-3′; mouse Lrrk2 reverse, 5′-ACGTACTCAGCAGTATCGTGTAA-3′; mouse Cd274 forward, 5′-GCTCCAAAGGACTTGTACGTG-3′; mouse Cd274 reverse, 5′-TGATCTGAAGGGCAGCATTTC-3′. The relative expression of LRRK2/Lrrk2 or CD274/Cd274 was normalized to that of ACTB/Actb (encoding β-actin) using the 2−ΔΔCt method.50
Immunohistochemistry
Tissues from mouse tumors were incubated in 10% neutral buffered formalin and embedded in paraffin. Tissues were sliced into 4-μm-thick sections using a rotary microtome (HistoCore MULTICUT; Leica, Wetzlar, Germany) and then baked for 60 min at 68°C. After deparaffinizing, antigens were retrieved and blocked using 3% BSA. Slices were incubated with corresponding primary antibodies at 4°C overnight. The primary antibodies used were as follows: anti-CD8a (CST, 60168, 1:400), anti-GZMB (CST, E5V2L, 1:200), and anti-PD-L1 (Proteintech, 14-5983-82, 1:1,000). The next day, the samples were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h. Signals were amplified using the diaminobenzidine (DAB) chromogen kit (BDB2004, Biocare, Redditch, UK). Nuclei were stained using hematoxylin for 8 s. Images were captured using ImageScope software (Leica Biosystems). Quantitative analysis of differential expression was carried out using ImageJ (NIH, Bethesda, MD, USA).
Immunofluorescence and Duolink assay
Cells were cultured in 8-well chambered slides with #1.5 high-performance cover glass (Cellvis, Mountain View, CA, USA; C8-1.5H-N). When attached, the cells were fixed at room temperature for 10 min using 4% paraformaldehyde. After rinsing with PBS, the cells were permeabilized for 15 min using 0.5% Triton X-100 (Solarbio, Beijing, China; T8200), and blocked with 3% BSA. Primary antibodies from different species were mixed and incubated with cells overnight. The primary antibodies used were as follows: anti-LRRK2 (Novus Biologicals; NB300; 1:100, rabbit) and anti-PD-L1 (Thermo Fisher Scientific, M1H1, 1:100, mouse). The next day, the cells were incubated with a mixture of Alexa Fluor 488-conjugated anti-mouse immunoglobulin G (IgG; CST, 4408, 1:200) and Alexa Fluor555-conjugated anti-rabbit IgG (CST, 4413, 1:200) for 1 h at room temperature. Hoechst 33258 (Servicebio, G1001) was used for staining nuclei. Images were captured using a confocal laser-scanning microscope (Leica, TCS SP8 Coherent anti-Stokes Raman Scattering (CARS)). ImageJ was used to quantitatively analyze protein colocalization. A Duolink assay was performed, following the manufacturer’s guidelines (Sigma, DUO92101). The primary antibodies were the same as those used for cell immunofluorescence. Signals were counted for quantitative analysis.
In vitro kinase assay
Recombinant human PD-L1 (active; Abcam, ab167713) was incubated with recombinant human LRRK2 (WT) (Thermo Fisher Scientific, PV4873) and recombinant human LRRK2 (D1994A, kinase dead) separately. Then, 200 μM of ATP (9804S, CST) was added to kinase buffer (9802S, CST) and incubated at 30°C for 30 min. Next, 4× loading buffer was added, and samples were heated at 100°C for 5 min to terminate the kinase reaction. The results of the kinase assay were detected by western blotting using an anti-phospho-threonine antibody (CST, 42H4) to detect LRRK2-mediated phosphorylation of PD-L1.
CD8+ T cell isolation and cytotoxic T lymphocyte killing assay
Primary CD8+ OT-I T cells were extracted from mouse spleens. Spleen cells were mechanically separated through a 40-μM filter, and red blood cells were removed using lysis buffer ((BioLegend). Recombinant mouse IL-2 (MCE, HY-P7077, 100 IU/mL), anti-CD3e (BioLegend, 100340, 1 μg/mL), and anti-CD28 (BioLegend, 102116, 0.5 μg/mL) were added to RPMI 1640 complete medium (GE Healthcare Life Sciences, Chicago, IL, USA; SH30027.0) to culture T cells. After 24 h, CD8+ T cells were isolated using a CD8a+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany; 130-104-075). Primary T cells were incubated with biotin-CD8a cocktail and then incubated with anti-biotin microbeads. An LS column in the magnetic field of a suitable magnetic-activated cell sorting (MACS) separator was used to enrich CD8a+ T cells. Enriched cells were verified by cell cytometry. Panc02 cells were incubated in advance to adhere to 96-well plates. The ratio of enriched cells to Panc02 cells was 10:1. Two days later, the plates were rinsed with PBS three times to remove T cells. The remaining Panc02 cells were fixed with 4% paraformaldehyde. 0.5% crystal violet was used for staining, which was dissolved using acetic acid before quantification using a spectrophotometer at 570 nm. Tumor-associated CD8 cells were isolated from tissue of the orthotopic tumor model. Single cells were isolated in the same way as for flow cytometry. CD8+ T cells were isolated using a CD8a+ T cell isolation kit (Miltenyi Biotec, 130-104-075) and cultured in RPMI 1640 complete medium added to recombinant mouse IL-2 (MCE, HY-P7077, 100 IU/mL).
Multiplex immunohistochemistry (mIHC)
The Opal Polaris 7-Color Manual IHC Kit (NEL861001KT) was used to perform multiplex IHC. Each tissue section or tissue microarray was baked in the oven at 65°C for 1 h, dewaxed using xylene, and rehydrated through a graded series of ethanol solutions: (100%, 95%, 85%, and 75%). After rehydration, the slides were fixed in 10% neutral buffered formalin for 20 min. For antigen retrieval, each slide was placed vertically in an Opal slide processing jar filled with AR6 buffer and microwaved for 45 s at 100% power and 15 min at 25% power. When the slides had cooled to room temperature, tissue sections were covered with blocking buffer for 10 min at room temperature and then incubated with primary antibodies to detect the target protein. After 1 h, the slides were washed with TBST three times, and Opal Polymer HRP Ms+Rb was used to cover each slide, followed by incubation for 10 min. The Opal fluorophore was diluted for signal amplification. Blocking, primary antibody incubation, introduction of Opal Polymer HRP, and signal amplification were repeated until all targets of interest had been detected. After the last cycle, 4′,6-diamidino-2-phenylindole (DAPI) working solution was applied for 5 min at room temperature. Each slide was then covered with mounting medium and a coverslip. Antibody information and the corresponding coupled tyramide signal amplification (TSA) colors (Opal numbers) are provided below. For tissue sections, LRRK2 (Novus Biologicals, NB300, 1:200, Opal 570), PD-L1 (Thermo Fisher Scientific, M1H1, 1:100, Opal 620), CD8 (Abcam, ab237710, 1:400, Opal 690), and pan-cytokeratin (PanCK; Abcam, ab86734, Opal Polaris 780) antibodies were employed. For the tissue microarray, LRRK2 (Novus Biologicals, NB300, 1:200, Opal 570), PD-L1 (Thermo Fisher Scientific, M1H1, 1:100, Opal 620), CD8 (Abcam, ab237710, 1:400, Opal 690), and PanCK (Abcam, ab86734, Opal 520, Opal Polaris 780) antibodies were used. A Vectra Polaris slide scanner (PerkinElmer, Waltham, MA, USA) was used for scanning each slide. The fixed resolution was 0.25 μm (40 ×). InForm (v.2.5; Akoya Biosciences, Menlo Park, CA, USA) was used for follow-up quantitative analysis. First, 12 fields were used for machine learning. Cells were adaptively segmented by the nuclear and cell membrane markers PanCK and CD8a. Then several cells were manually classified into tumor cells, CD8+ T cells, or CAFs by markers, size, and shape so that the machine could be trained to recognize each type of cell. When phenotyping was finished, a .csv file containing the numbers of each type of cell and the mean florescence intensity (MFI) of each type of cell was created. When training was completed, batch analysis was performed to analyze each field among the tissue microarrays in bulk. Severely handicapped fields in the tissue microarray were abandoned, and 138 patients’ tissues were analyzed. In this research, the percentage of CD8+ T cells among all cells was used to represent the density of CD8+ T cells in tumor tissue, while the entire tumor cells’ MFI of LRRK2 and PD-L1 revealed their relative expression, which was used for correlation analysis. Colocalization analysis was performed using InForm, where the colocalization area was marked in yellow.
RNA sequencing (RNA-seq)
All samples were pretreated and analyzed by the Biomedical Big Data Center of the First Affiliated Hospital, School of Medicine, Zhejiang University. In brief, total RNA extraction, RNA qualification, double-stranded cDNA synthesis, adaptor addition, fragment selection and PCR, library quality assessment, and sequencing (Illumina, San Diego, CA, USA) were carried out. The raw data that passed quality control were subjected to differential expression and functional analysis.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis
For digestion, the protein solution was reduced with 5 mM dithiothreitol and alkylated with 11 mM iodoacetamide for 15 min at room temperature in the dark. The protein sample was then diluted by adding 100 mM NH4HCO3 to obtain a urea concentration of less than 2 M. Finally, trypsin was added at a 1:50 trypsin-to-protein mass ratio for the first digestion overnight. The tryptic peptides were dissolved in 0.1% formic acid (solvent A) and directly loaded onto a laboratory-prepared reverse-phase analytical column (15 cm, 75 μm inside diameter (i.d.)) packed with 1.9 μm of Reprosil-Pur C18 beads (Dr. Maisch, Ammerbuch, Germany). The gradient comprised an increase from 3% to 8% solvent B (0.1% formic acid in 98% acetonitrile) over 3 min; 8%–20% in 37 min; 20%–30% in 12 min and climbing to 80% in 4 min, then holding at 80% for the last 4 min, all at a constant flow rate of 300 nL/min on an UltiMate 3000 nanoLC system (Thermo Fisher Scientific). The peptides were subjected to a nanospray ionization (NSI) source, followed MS/MS in an Orbitrap Exploris 480 (Thermo Fisher Scientific) coupled online to the Ultra Performance Liquid Chromatography (UPLC). The electrospray voltage applied was 2.0 kV. The m/z scan range was 400–1,200 for a full scan, and intact peptides were detected in the Orbitrap at a resolution of 60,000. Peptides were then selected for MS/MS using a normalized collision energy (NCE) setting of 27 and the fragments were detected in the Orbitrap at a resolution of 15,000. A data-dependent procedure that alternated between one MS scan followed by 20 MS/MS scans with 30-s dynamic exclusion was used. Automatic gain control (AGC) was set at 5E4. The compensation voltage for field asymmetric waveform ion mobility spectrometry (FAIMS) was set to −45 V, −65 V. The resulting MS/MS data were processed using the MaxQuant search engine (v.1.6.15.0).51 MS/MS spectra were searched against the Proteome Discoverer 2.5 database (Thermo Fisher Scientific) concatenated with a reverse decoy database. The mass tolerance for precursor ions was set as 20 ppm in the first search and 5 ppm in the main search, and the mass tolerance for fragment ions was set as 0.02 Da. Carbamidomethyl on Cys was specified as a fixed modification, and oxidation on Met was specified as a variable modification. Label-free quantification (LFQ) was used, the false discovery rate (FDR) was adjusted to less than 1%, and the minimum score for peptides was set to greater than 40. The resulting MS/MS data were processed using Proteome Discoverer software v.2.5 (Thermo Fisher Scientific). MS/MS spectra were searched against the Discoverer 2.5 database using the SEQUEST algorithm. Trypsin (full) was specified as the cleavage enzyme, allowing up to two missing cleavages. The minimum peptide length was six amino acids with a maximum of five modifications per peptide. The mass tolerance for precursor ions was set as 10 ppm, and the mass tolerance for fragment ions was set as 0.02 Da. Carbamidomethyl on Cys was specified as a fixed modification. The oxidation of Met (M) and acetyl, met-loss, and met-loss+acetyl of protein N termini were set as dynamic modifications. Proteins and peptide-spectrum matches (PSMs) were filtered with a maximum FDR of 1%.
Quantification and statistical analysis
GraphPad Prism software (GraphPad, La Jolla, CA, USA; v.7.0) was used for the statistical analyses. Data from biological replicates are presented as the mean ± SD. For comparison of differences between two groups, two-sided Student’s t tests were used, while differences among three or more groups were analyzed using one-way analysis of variance (ANOVA). Spearman’s rank correlation was used to compare two variables. The Kaplan-Meier method and the Gehan-Breslow-Wilcoxon test were used to analyze the differences between survival curves. Throughout the study, p < 0.05 was considered statistically significant.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81871925 and 82071867 to X.B. and U20A20378 and 81830089 to T.L.), the Development Program of Zhejiang Province (2020C03117 to X.B. and 2019C03019 to T.L.), the National Natural Science Foundation Basic Science Center of China (Study of Tumor Material and Energy Dynamics) (82188102 to T.L.), and the National Key Research and Development Program (2019YFC1316000 and U20A20378 to T.L.).
We would like to thank Junlei Zhang, Yongtao Ji, and Jiangchao Wu for technical support during the experiments of flow cytometry and mIHC. We would like to thank Minghua Sun, Yi Wang, Junyu Qiu, and Lei Ni for technical support during sample processing and assistance with mouse experiments. We are grateful for excellent technical support (mass spectrometry, Pengda Zou and Ziqian Wan) from the core facility, Central Laboratory, First Affiliated Hospital, Zhejiang University School of Medicine. We would like to thank Rujia Zhen, Jianhui Wang, Wenjue Xu, and the Biomedical Big Data Center of the First Affiliated Hospital, School of Medicine, Zhejiang University for assistance with RNA-seq analysis.
Author contributions
X.Z. and K.S. designed the research. K.S., X.Z., M.L., L.H., and S.W. supported the methodology of this research. K.S., X.Z., M.L., H.Y., J.X., M.L., Y.C., H.Z., X.L., Y.H., J.Z., and J.L. performed the experiments. K.S., X.Z., Z.H., J.T., J.S., C.G., S.Z., and J.H. visualized the results. X.B. and T.L. supervised this research. K.S., X.Z., and X.B. wrote and revised the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.07.021.
Contributor Information
Tingbo Liang, Email: liangtingbo@zju.edu.cn.
Xueli Bai, Email: shirleybai@zju.edu.cn.
Supplemental information
Data and code availability
Figures S2B, S2C, S3A, and S3B and Table S2 were generated from the TCGA databases, GTEx by GEPIA2 (http://gepia2.cancer-pku.cn), kmplot (http://kmplot.com/analysis/), and TISIDB (http://cis.hku.hk/TISIDB). The datasets used and analyzed during the current study are available from the corresponding authors upon reasonable request.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA. Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Grossberg A.J., Chu L.C., Deig C.R., Fishman E.K., Hwang W.L., Maitra A., Marks D.L., Mehta A., Nabavizadeh N., Simeone D.M., et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA. Cancer J. Clin. 2020;70:375–403. doi: 10.3322/caac.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizrahi J.D., Surana R., Valle J.W., Shroff R.T. Pancreatic cancer. Lancet (London, England) 2020;395:2008–2020. doi: 10.1016/s0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 5.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., Yokoi T., Chiappori A., Lee K.H., de Wit M., et al. PACIFIC Investigators Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 6.Massard C., Gordon M.S., Sharma S., Rafii S., Wainberg Z.A., Luke J., Curiel T.J., Colon-Otero G., Hamid O., Sanborn R.E., et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J. Clin. Oncol. 2016;34:3119–3125. doi: 10.1200/jco.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Reilly E.M., Oh D.Y., Dhani N., Renouf D.J., Lee M.A., Sun W., Fisher G., Hezel A., Chang S.C., Vlahovic G., et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1431–1438. doi: 10.1001/jamaoncol.2019.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey P., Chang D.K., Forget M.A., Lucas F.A.S., Alvarez H.A., Haymaker C., Chattopadhyay C., Kim S.H., Ekmekcioglu S., Grimm E.A., et al. Exploiting the neoantigen landscape for immunotherapy of pancreatic ductal adenocarcinoma. Sci. Rep. 2016;6:35848. doi: 10.1038/srep35848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balli D., Rech A.J., Stanger B.Z., Vonderheide R.H. Immune Cytolytic Activity Stratifies Molecular Subsets of Human Pancreatic Cancer. Clin. Cancer Res. 2017;23:3129–3138. doi: 10.1158/1078-0432.Ccr-16-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P.L., Roh W., Reuben A., Cooper Z.A., Spencer C.N., Prieto P.A., Miller J.P., Bassett R.L., Gopalakrishnan V., Wani K., et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov. 2016;6:827–837. doi: 10.1158/2159-8290.Cd-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benci J.L., Xu B., Qiu Y., Wu T.J., Dada H., Twyman-Saint Victor C., Cucolo L., Lee D.S.M., Pauken K.E., Huang A.C., et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167:1540–1554.e12. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prat A., Navarro A., Paré L., Reguart N., Galván P., Pascual T., Martínez A., Nuciforo P., Comerma L., Alos L., et al. Immune-Related Gene Expression Profiling After PD-1 Blockade in Non-Small Cell Lung Carcinoma, Head and Neck Squamous Cell Carcinoma, and Melanoma. Cancer Res. 2017;77:3540–3550. doi: 10.1158/0008-5472.Can-16-3556. [DOI] [PubMed] [Google Scholar]
- 13.Vesely M.D., Zhang T., Chen L. Resistance Mechanisms to Anti-PD Cancer Immunotherapy. Annu. Rev. Immunol. 2022;40:45–74. doi: 10.1146/annurev-immunol-070621-030155. [DOI] [PubMed] [Google Scholar]
- 14.Liu K., Huang J., Liu J., Li C., Kroemer G., Tang D., Kang R. HSP90 Mediates IFNγ-Induced Adaptive Resistance to Anti-PD-1 Immunotherapy. Cancer Res. 2022;82:2003–2018. doi: 10.1158/0008-5472.Can-21-3917. [DOI] [PubMed] [Google Scholar]
- 15.Huang J., Chen P., Liu K., Liu J., Zhou B., Wu R., Peng Q., Liu Z.X., Li C., Kroemer G., et al. CDK1/2/5 inhibition overcomes IFNG-mediated adaptive immune resistance in pancreatic cancer. Gut. 2021;70:890–899. doi: 10.1136/gutjnl-2019-320441. [DOI] [PubMed] [Google Scholar]
- 16.Spranger S., Spaapen R.M., Zha Y., Williams J., Meng Y., Ha T.T., Gajewski T.F. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl. Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi H., Hsu J.M., Yang W.H., Hung M.C. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat. Rev. Clin. Oncol. 2022;19:287–305. doi: 10.1038/s41571-022-00601-9. [DOI] [PubMed] [Google Scholar]
- 18.Koemans W.J., Chalabi M., van Sandick J.W., van Dieren J.M., Kodach L.L. Beyond the PD-L1 horizon: In search for a good biomarker to predict success of immunotherapy in gastric and esophageal adenocarcinoma. Cancer Lett. 2019;442:279–286. doi: 10.1016/j.canlet.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 20.Cerezo M., Guemiri R., Druillennec S., Girault I., Malka-Mahieu H., Shen S., Allard D., Martineau S., Welsch C., Agoussi S., et al. Translational control of tumor immune escape via the eIF4F-STAT1-PD-L1 axis in melanoma. Nat. Med. 2018;24:1877–1886. doi: 10.1038/s41591-018-0217-1. [DOI] [PubMed] [Google Scholar]
- 21.Li C.W., Lim S.O., Chung E.M., Kim Y.S., Park A.H., Yao J., Cha J.H., Xia W., Chan L.C., Kim T., et al. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer cell. 2018;33:187–201.e10. doi: 10.1016/j.ccell.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C.W., Lim S.O., Xia W., Lee H.H., Chan L.C., Kuo C.W., Khoo K.H., Chang S.S., Cha J.H., Kim T., et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X., Huang X., Xu J., Li E., Lao M., Tang T., Zhang G., Guo C., Zhang X., Chen W., et al. NEK2 inhibition triggers anti-pancreatic cancer immunity by targeting PD-L1. Nat. Commun. 2021;12:4536. doi: 10.1038/s41467-021-24769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li E., Huang X., Zhang G., Liang T. Combinational blockade of MET and PD-L1 improves pancreatic cancer immunotherapeutic efficacy. J. Exp. Clin. Cancer Res. 2021;40:279. doi: 10.1186/s13046-021-02055-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X., Lao M., Xu J., Duan Y., Yang H., Li M., Ying H., He L., Sun K., Guo C., et al. Combination cancer immunotherapy targeting TNFR2 and PD-1/PD-L1 signaling reduces immunosuppressive effects in the microenvironment of pancreatic tumors. J. Immunother. Cancer. 2022;10:e003982. doi: 10.1136/jitc-2021-003982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan J.L., Wang B., Wu M.L., Li J., Gong R.M., Song L.N., Zhang H.S., Zhu G.Q., Chen S.P., Cai J.L., et al. MTDH antisense oligonucleotides reshape the immunosuppressive tumor microenvironment to sensitize Hepatocellular Carcinoma to immune checkpoint blockade therapy. Cancer Lett. 2022;541:215750. doi: 10.1016/j.canlet.2022.215750. [DOI] [PubMed] [Google Scholar]
- 27.Greggio E., Jain S., Kingsbury A., Bandopadhyay R., Lewis P., Kaganovich A., van der Brug M.P., Beilina A., Blackinton J., Thomas K.J., et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R.J., Calne D.B., et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Takagawa T., Kitani A., Fuss I., Levine B., Brant S.R., Peter I., Tajima M., Nakamura S., Strober W. An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis. Sci. Transl. Med. 2018;10:eaan8162. doi: 10.1126/scitranslmed.aan8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuss M., Adamopoulou E., Kahle P.J. Interferon-γ induces leucine-rich repeat kinase LRRK2 via extracellular signal-regulated kinase ERK5 in macrophages. J. Neurochem. 2014;129:980–987. doi: 10.1111/jnc.12668. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadi Rastegar D., Hughes L.P., Perera G., Keshiya S., Zhong S., Gao J., Halliday G.M., Schüle B., Dzamko N. Effect of LRRK2 protein and activity on stimulated cytokines in human monocytes and macrophages. NPJ Parkinsons Dis. 2022;8:34. doi: 10.1038/s41531-022-00297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panagiotakopoulou V., Ivanyuk D., De Cicco S., Haq W., Arsić A., Yu C., Messelodi D., Oldrati M., Schöndorf D.C., Perez M.J., et al. Interferon-γ signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat. Commun. 2020;11:5163. doi: 10.1038/s41467-020-18755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardet A., Benita Y., Li C., Sands B.E., Ballester I., Stevens C., Korzenik J.R., Rioux J.D., Daly M.J., Xavier R.J., Podolsky D.K. LRRK2 is involved in the IFN-gamma response and host response to pathogens. J. Immunol. 2010;185:5577–5585. doi: 10.4049/jimmunol.1000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Looyenga B.D., Furge K.A., Dykema K.J., Koeman J., Swiatek P.J., Giordano T.J., West A.B., Resau J.H., Teh B.T., MacKeigan J.P. Chromosomal amplification of leucine-rich repeat kinase-2 (LRRK2) is required for oncogenic MET signaling in papillary renal and thyroid carcinomas. Proc. Natl. Acad. Sci. USA. 2011;108:1439–1444. doi: 10.1073/pnas.1012500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Z.C., Chen X.J., Zhou Q., Gong X.H., Chen X., Wu W.J. Downregulated LRRK2 gene expression inhibits proliferation and migration while promoting the apoptosis of thyroid cancer cells by inhibiting activation of the JNK signaling pathway. Int. J. Oncol. 2019;55:21–34. doi: 10.3892/ijo.2019.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reith A.D., Bamborough P., Jandu K., Andreotti D., Mensah L., Dossang P., Choi H.G., Deng X., Zhang J., Alessi D.R., Gray N.S. GSK2578215A; a potent and highly selective 2-arylmethyloxy-5-substitutent-N-arylbenzamide LRRK2 kinase inhibitor. Bioorg. Med. Chem. Lett. 2012;22:5625–5629. doi: 10.1016/j.bmcl.2012.06.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jennings D., Huntwork-Rodriguez S., Henry A.G., Sasaki J.C., Meisner R., Diaz D., Solanoy H., Wang X., Negrou E., Bondar V.V., et al. Preclinical and clinical evaluation of the LRRK2 inhibitor DNL201 for Parkinson's disease. Sci. Transl. Med. 2022;14:eabj2658. doi: 10.1126/scitranslmed.abj2658. [DOI] [PubMed] [Google Scholar]
- 38.Schaffner A., Li X., Gomez-Llorente Y., Leandrou E., Memou A., Clemente N., Yao C., Afsari F., Zhi L., Pan N., et al. Vitamin B(12) modulates Parkinson's disease LRRK2 kinase activity through allosteric regulation and confers neuroprotection. Cell Res. 2019;29:313–329. doi: 10.1038/s41422-019-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pungaliya P.P., Bai Y., Lipinski K., Anand V.S., Sen S., Brown E.L., Bates B., Reinhart P.H., West A.B., Hirst W.D., Braithwaite S.P. Identification and characterization of a leucine-rich repeat kinase 2 (LRRK2) consensus phosphorylation motif. PloS one. 2010;5:e13672. doi: 10.1371/journal.pone.0013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunderson A.J., Kaneda M.M., Tsujikawa T., Nguyen A.V., Affara N.I., Ruffell B., Gorjestani S., Liudahl S.M., Truitt M., Olson P., et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 2016;6:270–285. doi: 10.1158/2159-8290.Cd-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benci J.L., Johnson L.R., Choa R., Xu Y., Qiu J., Zhou Z., Xu B., Ye D., Nathanson K.L., June C.H., et al. Opposing Functions of Interferon Coordinate Adaptive and Innate Immune Responses to Cancer Immune Checkpoint Blockade. Cell. 2019;178:933–948.e14. doi: 10.1016/j.cell.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn G.P., Koebel C.M., Schreiber R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 43.Taube J.M., Anders R.A., Young G.D., Xu H., Sharma R., McMiller T.L., Chen S., Klein A.P., Pardoll D.M., Topalian S.L., Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chin Y.E., Kitagawa M., Kuida K., Flavell R.A., Fu X.Y. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol. Cel. Biol. 1997;17:5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomura T., Sakaguchi S. Naturally arising CD25+CD4+ regulatory T cells in tumor immunity. Curr. Top. Microbiol. Immunol. 2005;293:287–302. doi: 10.1007/3-540-27702-1_13. [DOI] [PubMed] [Google Scholar]
- 46.Anderson R.C.E., Anderson D.E., Elder J.B., Brown M.D., Mandigo C.E., Parsa A.T., Goodman R.R., McKhann G.M., Sisti M.B., Bruce J.N. Lack of B7 expression, not human leukocyte antigen expression, facilitates immune evasion by human malignant gliomas. Neurosurgery. 2007;60:1129–1136. doi: 10.1227/01.Neu.0000255460.91892.91944. [DOI] [PubMed] [Google Scholar]
- 47.Ceresoli G.L., Zucali P.A., Favaretto A.G., Grossi F., Bidoli P., Del Conte G., Ceribelli A., Bearz A., Morenghi E., Cavina R., et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J. Clin. Oncol. 2006;24:1443–1448. doi: 10.1200/jco.2005.04.3190. [DOI] [PubMed] [Google Scholar]
- 48.Dali-Youcef N., Andrès E. An update on cobalamin deficiency in adults. QJM : monthly J. Assoc. Physicians. 2009;102:17–28. doi: 10.1093/qjmed/hcn138. [DOI] [PubMed] [Google Scholar]
- 49.Tempero M.A., Malafa M.P., Al-Hawary M., Behrman S.W., Benson A.B., Cardin D.B., Chiorean E.G., Chung V., Czito B., Del Chiaro M., et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 50.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 51.Tyanova S., Temu T., Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016;11:2301–2319. doi: 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Figures S2B, S2C, S3A, and S3B and Table S2 were generated from the TCGA databases, GTEx by GEPIA2 (http://gepia2.cancer-pku.cn), kmplot (http://kmplot.com/analysis/), and TISIDB (http://cis.hku.hk/TISIDB). The datasets used and analyzed during the current study are available from the corresponding authors upon reasonable request.