Abstract
Objective:
Examine drivers of durable viral suppression (DVS) disparities among people with HIV (PWH) using quantitative intersectional approaches.
Design:
Retrospective cohort analysis from electronic health records informed by intersectionality to better capture the concept of interlocking and interacting systems of oppression.
Methods:
We analyzed data of PWH seen at a LGBTQ federally qualified health center in Chicago (2012-2019) with ≥3 viral loads. We identified PWH who achieved DVS using latent trajectory analysis and examined disparities using three intersectional approaches: Adding interactions, latent class analysis (LCA), and qualitative comparative analysis (QCA). Findings were compared to main effects only regression.
Results:
Among 5,967 PWH, 90% showed viral trajectories consistent with DVS. Main effects regression showed that substance use (OR: 0.56, 0.46-0.68) and socio-economic status like being unhoused (OR: 0.39, 0.29-0.53), but not sexual orientation or gender identity (SOGI) were associated with DVS.
Adding interactions, we found that race and ethnicity modified the association between insurance and DVS (p for interaction <0.05). With LCA, we uncovered four social position categories influenced by SOGI with varying rates of DVS. For example, the transgender women-majority class had worse DVS rates versus the class of mostly non-poor white cisgender gay men (82% vs 95%). QCA showed that combinations, rather than single factors alone, were important for achieving DVS. Combinations vary with marginalized populations (e.g., Black gay/lesbian transgender women) having distinct sufficient combinations compared to historically privileged groups (e.g., white cisgender gay men).
Conclusion:
Social factors likely interact to produce DVS disparities. Intersectionality-informed analysis uncover nuance that can inform solutions.
Keywords: HIV, intersectionality, health disparities, sexual and gender minorities, viral suppression
I. Introduction
Achieving durable viral suppression (DVS) is an important treatment milestone for people with human immunodeficiency virus (PWH).[1] DVS translates to benefits such as lower risk for HIV-associated chronic conditions and population benefits since virally suppressed individuals are highly unlikely to transmit HIV.[2-5] Achieving DVS is a key strategy in the US Ending the HIV Epidemic plan.[6] Unfortunately, the achievement of one-time viral suppression in the United States remain sub-optimal at ~65%.[7,8] Importantly, one-time viral suppression disparities by race-ethnicity, gender, socio-economic status (SES), and other demographic variables exist.[9,10]
These disparities are largely due to social factors. Poor viral suppression is associated with society-inflicted adverse conditions like having depression, living in economically-deprived areas, or facing difficulty in meeting daily needs.[9,11,12] HIV viral suppression disparities are produced by the complex interactions of health and societal systems with intersectional social position and identities among PWH populations.[9,13] Despite this underlying theory, research typically focused on isolating the role of a single social determinant of health (SDOH) or examining disparities through a single axis of identity (e.g., just race).[13,14] In turn, policy recommendations often target singular issues or population groups.
There is an increasing call within public health to apply intersectionality in health research.[13-15] An intersectional approach attempts to address the complexity of how interlocking social determinants and systems of oppression produce disparities seen at the population level, and investigates the unique impacts of these interacting forces on each person holding multiply-constituted social identities.[16,17] Applying this approach better attends to the fact that many PWH hold multiple marginalized identities (e.g., Hispanic bisexual cisgender women) and PWH often face multiple adverse socio-economic conditions caused by historical, generational, and present day stigma and oppression.[9,13,18,19] By embracing this complexity, new insights may be gained which can guide development of interventions to address persistent health disparities.[14]
Here, we conducted an intersectionality-informed quantitative analysis to investigate the association of SDOH-related factors with DVS. We drew extensively from the work of health scholars engaged in integrating quantitative methods with intersectionality[20-22]. Our goal is to uncover new insights to inform improvement in policy and implementation of the HIV elimination plan for the US.[6,23] We demonstrate intersectionality-informed analysis using electronic health records (EHR) data that can be used for guiding HIV elimination globally.[24]
II. Methods
a. Participants and procedures
We used the EHR of Howard Brown Health (HBH), a federally qualified health center that specializes in lesbian, gay, bisexual, transgender, and queer (LGBTQ+) health with nine locations in Chicago. We included data from adult (age ≥18 years) PWH seen from 2012-2019. Due to our outcome, we excluded those with <3 viral loads (VL). The protocol and waiver of informed consent was approved by the HBH and Northwestern University institutional review boards.
b. Durable viral suppression
Our main outcome of interest was achievement of DVS during observation. DVS can be measured deterministically or through modeling.[25-28] A deterministic approach (e.g., “≥3 suppressed viral loads six months apart”), however, may miss cases where follow-ups are irregular in frequency and spacing such as in EHR data. To overcome this, we used latent trajectory analysis (LTA), which models the repeated VL and identify subgroups based on similarities of trajectories.[29,30]
Using the best-fitting model, we examined the identified trajectories graphically and qualitatively assigned individuals to the those who achieved or did not achieve DVS. (Supplemental Digital Content (SDC) 1) As a sensitivity analysis, we analyzed an alternative definition where DVS is achieved if ≥50% of observed VL were <100copies/mL and their last VL is <100copies/mL.
c. Covariates
Data on age, sexual orientation and gender identity (SOGI), SDOH (insurance, self-identified race-ethnicity, housing status, and poverty status) and comorbidities (mental health diagnosis, substance use diagnosis, hypertension (HTN) and diabetes mellitus (DM)) were obtained from the EHR. (Supplemental Table 1, SDC2 for diagnosis definitions)
d. Overview of Statistical Analysis
We utilized three intersectionality-informed approaches: regression with interaction terms, latent class analysis with regression, and qualitative comparative analysis.[22,31]
We view “main effects” only regression as insufficient for intersectional analysis. To demonstrate issues of this non-intersectional approach, we compared findings from “main effects” regression to selected intersectional methods.[21] Candidate covariates of the base “main effects” models include: age, SOGI, SES indicators (poverty, housing, insurance), and co-morbidities (diabetes mellitus, hypertension, mental health disorder, substance use disorder).
All analyses were implemented in R 4.1/RStudio[32,33] with the following packages for certain methods: lcmm for LTA,[34] poLCA for latent class analysis (LCA),[35] and qca for qualitative comparative analysis (QCA).[36] For regression-based methods, missing data (Supplemental Table 2, SDC2) were imputed using missForest.[37] Implementation details are found in SDC1.
e. Regression-based: Adding interaction terms for exploring heterogenous associations
Regression is used to estimate if an exposure is associated with an outcome after adjusting for potential confounders. This assumes that a social factor has equal impact across identity groups – an assumption which intersectionality asks us to reconsider.
We generated base “main effects only” regression models the association of DVS with SDOH variables (housing, insurance, poverty, mental health disorder, substance use disorder). Age, year of first VL, demographics, and hypertension or diabetes status were included if they were confounders. (Supplemental Figure 1, SDC 2 for directed acyclic graphs (DAG)). Here, demographic variables are indicators of marginalization related to a demographic category (e.g., race & ethnicity as indicators of racialization and racism).
We then generated models with an additional interaction term between a demographic variable and an SDOH variable. Models that showed better fit (p-value <0.10 based on Type III ANOVA) than the corresponding base model were then examined further for interpretation. To facilitate interpretability, we limited this to 2x2 interaction terms and calculated predicted differences across intersectional categories.
f. Regression-based: Latent Class Analysis for Assessing Disparities
Regression is also used to assess disparities after adjustment for allowable confounders. Usually, the analysis would treat other demographic variables as confounders with no interactions. However, this conceptualization runs against the principle of interlocking and interacting systems of oppression that lies at the core of intersectionality. Instead, we conceptualized a latent social position co-constituted by race & ethnicity, SOGI, and SES. (Supplemental Figure 1C, SDC2) Interactions can also be used to implement this operationalization, but data sparsity can limit application. In our sample, even with just cross-tabulation of sexual orientation, gender identity, and race & ethnicity already produced a lot of categories with very few (≤4) individuals (Supplemental Table 3, SDC2).
Instead, we used LCA to generate a latent multiply-constituted social position variable while retaining the full sample. Our LCA model includes race-ethnicity, SOGI, and SES indicators. This operationalizes how various systems of oppression like racialization, transphobia, etc., combine and dictate a person’s ability to access health-related resources.[38] By rejecting the seemingly more facile single dimension interpretability, we combined several variables in a way better aligned with intersectionality. Descriptive class labels were developed based on summary statistics in consultation with HBH staff.
To assess disparities, we ran a model with the categorical “social position” adjusting also for age and year of first VL and a second model that includes comorbidities. Results were compared to a non-intersectional approach where race & ethnicity and SOGI were treated as independent variables (DAG in Supplemental Figure 1B, SDC 2).
g. Qualitative Comparative analysis
Intersectionality-informed analysis requires the characterization of how “interlocking systems of oppression” contribute to disparities. We posit that intersectional social position affects SDOH via differentials in social systems of oppression, which in turn collectively impacts DVS disparities. [21,39] Regression-based methods, however, are more useful for quantifying single factor-to-outcome associations. QCA offers an alternative approach to identify combinations that act jointly to produce outcomes.
The QCA algorithm takes in a set of variables and uses set theory to identify which variables (singly or in combination) are highly associated with the outcome.[40] (SDC1 for details and this paper for a tutorial[41]) We analyzed which SDOH combinations are associated with DVS for a specific intersectional group. For example, the QCA algorithm will identify the combination “having housing and having insurance” as a combination associated with DVS if ≥80% of Hispanic bisexual men with HIV with this combination were able to achieve DVS.
We used crisp set QCA to identify sufficient combinations of five modifiable SDOH factors (poverty, housing, insurance, mental health disorder, substance use disorder) associated with DVS. Since QCA can handle small samples, we ran QCA on intersectional subgroups based on SOGI and race & ethnicity with n≥30. (Supplemental Table 4, SDC 2)
III. Results
a. Overview of Cohort
We analyzed data from 5,967 PWH who were on average 43 (SD: 13) years old at time of the first VL test. Most were assigned male at birth (82.4%) and currently identified man/male (88.6%). Seventy-nine percent (79%) were gay/lesbian and 10% were heterosexual. Around 4% were transgender. Thirty-nine percent (39%) were non-Hispanic white, 34% were non-Hispanic Black, 22% were Hispanic, and 14% were other race & ethnicities. Nearly half (46%) were diagnosed with HIV before 2012. Compared to the excluded, the analytic sample was older, had fewer people assigned female at birth, more men, lower white, and had higher rates of having permanent housing. (Supplemental Table 5, SDC2).
b. Durable viral suppression
From LTA, 89.3% of the sample were DVS achievers based on two trajectories: durable 1 consistently had low VL (63.1%) and durable 2 (26.2%) started at high VL and rapidly achieved <100 copies/mL by year 2. (Supplemental Figure 2, SDC2) The other two patterns were considered non-durable suppressors due to longer decline of viral load trajectory (not suppressed 1, 5.2%) or the trajectory did not breach <100copies/mL threshold (not suppressed 2, 5.5%). There was good separation among the patterns (mean posterior membership probabilities > 0.85). (Supplemental Table 6, SDC2) To simplify subsequent analyses, we collapsed the four trajectories into a binary variable (DVS vs non-DVS).
In unadjusted analyses, there were notable differences in terms of race and ethnicity, SOGI, and SDOH between DVS and non-DVS individuals (Table 1). Validating our binary classification, nearly all (94%) of DVS achievers had VL <100 copies/mL on their last test and 91% met the alternative DVS definition. Meanwhile, only 35.8% had <100 copies/mL in the non-DVS group and 17% met the alternative DVS definition.
Table 1.
Comparison of People who achieved Durable Viral Suppression (DVS) to those who did not achieve DVS (non-DVS), Howard Brown Health, 2012-2019 (n=5,967)
| No Durable Viral Suppression |
Durable Viral Suppression |
|
|---|---|---|
| n | 659 | 5308 |
| Age at baseline (mean (SD)) | 39.15 (11.11) | 43.43 (12.62) |
| Not assigned male at birth (%) | 121 (18.4) | 935 (17.6) |
| Gender identity (%) | ||
| cisgender man | 559 (85.6) | 4637 (88.4) |
| cisgender woman | 32 ( 4.9) | 268 ( 5.1) |
| transgender woman | 60 ( 9.2) | 291 ( 5.5) |
| transgender man, genderqueer, or else1 | 2 ( 0.3) | 51 ( 1.0) |
| Sexual Orientation (%) | ||
| Bisexual | 62 (9.8) | 344 (6.8) |
| Gay or lesbian | 480 (76.2) | 4006 (79.7) |
| Heterosexual | 73 (11.6) | 540 (10.7) |
| Queer or else2 | 15 (2.4) | 139 (2.8) |
| Race/ethnicity (%) | ||
| Black, non-Hispanic | 304 (47.2) | 1691 (32.5) |
| Hispanic | 128 (19.9) | 1166 (22.4) |
| Others, non-Hispanic3 | 33 (5.1) | 258 (5.0) |
| white, non-Hispanic | 179 (27.8) | 2089 (40.1) |
| Insurance class (%) | ||
| Private | 141 (21.4) | 2538 (47.8) |
| Government | 328 (49.8) | 1761 (33.2) |
| Uninsured or others4 | 190 (28.8) | 1009 (19.0) |
| Housing status at baseline (%) | ||
| Unhoused | 79 (12.0) | 196 (3.7) |
| Non-permanent/Institution | 109 (17.9) | 550 (11.2) |
| Permanent | 421 (63.9) | 4164 (78.4) |
| Poverty category (%) | ||
| ≤100% FPL | 478 (72.5) | 2887 (54.4) |
| >100 to ≤200% FPL | 93 (14.1) | 869 (16.4) |
| >200% FPL | 88 (13.4) | 1548 (29.2) |
| Comorbidities | ||
| Mental health disorder (%) | 321 (48.7) | 2241 (42.2) |
| Substance abuse disorder (%) | 239 (36.3) | 1261 (23.8) |
| Diabetes or Hypertension (%) | 27 (4.1) | 361 (6.8) |
| HIV-related variables | ||
| Year of HIV onset (median [IQR]) | 2012 [2008, 2015] | 2012 [2007, 2016] |
| HIV onset before 2012 (%) | 293 (44.5) | 2403 (46.4) |
| Year of First Viral load (median [IQR]) | 2014 [2012, 2016] | 2015 [2012, 2017] |
| Year of Last Viral load (median [IQR]) | 2019 [2018, 2019] | 2019 [2018, 2019] |
| Duration of first to last Viral load (year, median [IQR]) | 3.14 [1.83, 6.02] | 2.79 [1.47, 5.52] |
| Total Viral load tests (mean (SD)) | 7 [4, 11] | 8 [5, 13] |
| Number of Viral load <100 copies/mL (median [IQR]) | 2 [0, 4] | 7 [4, 11] |
| Proportion of total Viral load <100 copies (mean (SD)) | 28 (23) | 86 (19) |
| Viral load (copies/mL) (median [IQR])5 | ||
| First Viral Load | 37280 [34160, 100000] | 20 [20, 16922] |
| Last Viral Load in 1st year | 4440 [50, 53454] | 20.00 [20, 20] |
| Last Viral Load Ever | 2660 [32, 44750] | 20 [20, 20] |
| Viral Load <100 copies/mL (%) | ||
| First Viral Load | 75 (11.4) | 3172 (59.8) |
| Last Viral Load in 1st year | 203 (30.8) | 4918 (92.7) |
| Last Viral Load Ever | 236 (35.8) | 4988 (94.0) |
Note: FPL – federal poverty line, IQR – interquartile range, SD – standard deviation
Includes genderqueer, queer, nonbinary and all other responses except missing or refused(n=67)
Includes genderqueer, gender non-conforming and replied “something else” when asked about sexual orientation except missing or refused (n=308).
Other race and ethnicity variables include Asian, Pacific Islander, Multiracial, and Unspecified.
Other insurance categories include entries like walk-in, sliding scale, or grant.
c. Results from Regression: Adding interaction terms for exploring heterogenous associations
The base “main effects” only models suggested SDOH variables were significant predictors of being in the DVS group: experiencing poverty, lack of formal insurance, having no permanent housing, and having a substance use disorder were associated with lower odds of achieving DVS. (Table 2)
Table 2.
Association of Selected Socio-economic Status Indicators with Durable Viral Suppression, Howard Brown Health, 2012-2020
| Odds Ratio (95% Confidence Interval) | ||
|---|---|---|
| Variable | Complete case | Imputed Dataset |
| Poverty (ref: Above 200% FPL)1 | ||
| • Between 101 to 200% FPL | 0.58 (0.41 - 0.80)* | 0.56 (0.41 - 0.75)* |
| • 100% FPL and below | 0.36 (0.28 - 0.46)* | 0.37 (0.29 - 0.47)* |
| Housing status (ref: Has housing) | ||
| • Unhoused | 0.39 (0.29 - 0.53)* | 0.41 (0.31 - 0.55)* |
| Insurance (ref: Private insurance) | ||
| • Government insurance | 0.38 (0.3 - 0.49)* | 0.39 (0.31 - 0.50)* |
| • Uninsured | 0.38 (0.29 - 0.50)* | 0.40 (0.31 - 0.51)* |
| Mental Health (ref: No disorder)4 | ||
| • With disorder | 0.86 (0.71 - 1.04) | 0.86 (0.72 - 1.03) |
| Substance Use disorder (ref: No disorder) 4 | ||
| • With disorder | 0.56 (0.46 - 0.68)* | 0.58 (0.49 - 0.70)* |
Notes: FPL – federal poverty line
confidence interval does NOT cross null (1.0). Odds ratio were calculated using multivariable regression. Each socio-economic variable has a separate model.
Poverty models adjust for age, year of first viral load, race & ethnicity, sexual orientation, gender identity, poverty status. Housing and insurance are mediators, thus, were exclude from the model.
Housing model adjusts for variables in poverty model and housing status. Poverty is a confounder, but insurance is a mediator, so insurance is excluded from the model.
Insurance model adjusts for variables in poverty model as well as housing, poverty, and insurance status. Poverty and housing status are confounders, so they were included.
Mental health and substance use disorders use the same model which adjusts for age, year of first viral load, race & ethnicity, sexual orientation, gender identity, diabetes or hypertension, mental health disorder, substance use disorder, and poverty status. Insurance and housing were mediators of poverty so were excluded.
Our exploration of two-way interactions suggested potential differential associations according to race & ethnicity. We found a significant (p<0. 05) interaction between insurance and race ðnicity. Compared to White PWH with private insurance, Black PWH with private insurance had lower probability of DVS while Asian/other and Hispanic PWH with private insurance had comparable DVS probability. (Supplemental Figure 3, SDC 2). We also observed potential interactions (0.05 <p<0.10) between poverty and race & ethnicity, substance use and sexual orientation, and poverty and gender identity (imputed analysis only) (Supplemental Figure 4-6, SDC 2).
d. Results from Regression with latent class analysis
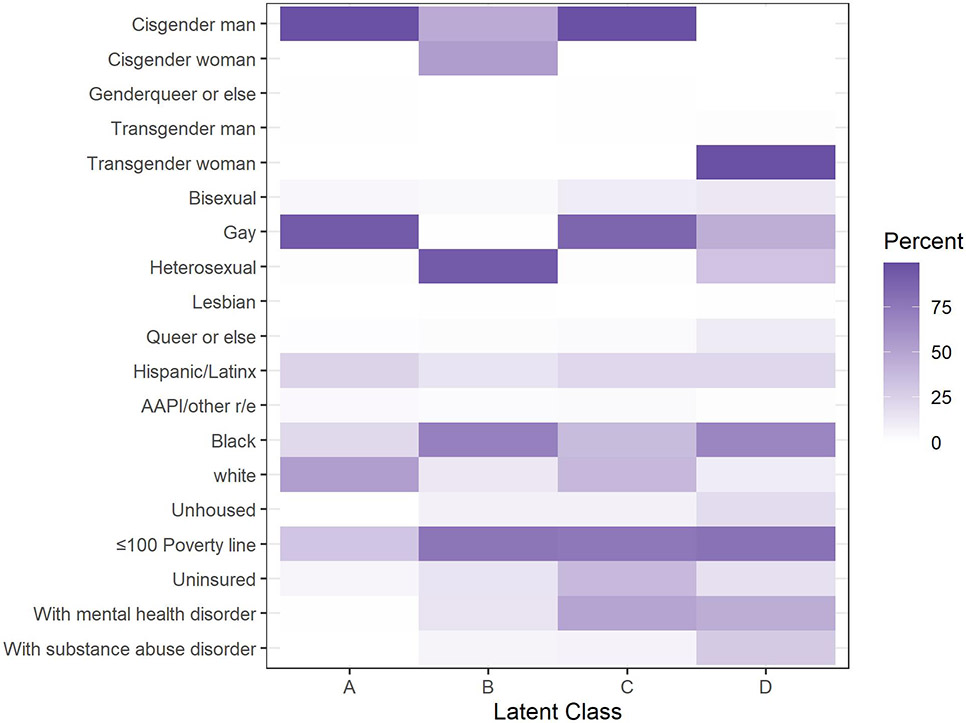
LCA using complete case data (n=5,186) revealed four social position groups: A (47%), B (8.3%), C (39%), and D (5.5%). Group A was majority cisgender men with mixed race & ethnicity categories and relatively lower rates of being poor and unhoused. Group B was majority Black PWH with a mix of cisgender men and women. The majority were poor and using government insurance. Like Group A, Group C was majority cisgender men of mixed race & ethnicity but had comparably worse SES (e.g., high uninsured). Group D captured nearly all the transgender women, was majority Black, and had a high percentage of unhoused people. (Figure 1, Supplemental Table 7, SDC2) While diagnosis of a mental health or substance use disorder was not included in the LCA model, there were also some differences across the groups with Group C and D showing relatively higher rates of mental health disorder and Group D showing relatively high rates of substance use disorder.
Figure 1. Distribution of Demographic Characteristics according to Social Position from Latent Class Analysis, Howard Brown Health, 2012-2019 (n=5,967).
Notes: Value represent proportion within a latent class that has that characteristic. For example, in Latent Class A >75% were cisgender men while for Class D 0% were cisgender men. Genderqueer or else also includes gender non-conforming and replied “something else” when asked about gender. Queer or else refers to Queer, Questioning, and Something else responses to sexual orientation. Other race and ethnicities (r/e) include Asian, Pacific Islander, (AAPI) Multiracial, and Unspecified.
The LCA groups showed significant differences in DVS on unadjusted analysis. Group A had the highest DVS rate (94.5%) followed by Group B (88.0%) then C (83.4%) and D (82.2%). In adjusted models, we saw that Groups B, C, and D had significantly lower odds of achieving DVS compared to Group A (Table 3B). These findings contrast to the base “main effects” model which suggested that Black race & ethnicity had lower odds of DVS, and that transgender man/genderqueer PWH had higher odds of DVS than cisgender men. (Table 3A).
Table 3.
Disparities in Durable Viral Suppression according to selected demographic categories, Howard Brown Health, 2012-2020
| A. Non-intersectional analysis (Odds Ratio, 95% Confidence interval) | ||||||
|---|---|---|---|---|---|---|
| Category | Complete Case | Imputed Data | ||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| A. Race & ethnicity (ref: White, non-Hispanic) | ||||||
| Hispanic | 0.97 (0.75 - 1.25) | 0.99 (0.77 - 1.29) | 0.99 (0.76 - 1.29) | 0.91 (0.71 - 1.15) | 0.91 (0.72 - 1.17) | 0.92 (0.72 - 1.18) |
| Asian/other, non-Hispanic1 | 1.22 (0.7 - 2.3) | 1.24 (0.71 - 2.35) | 1.15 (0.65 - 2.2) | 0.92 (0.62 - 1.42) | 0.93 (0.62 - 1.43) | 0.93 (0.62 - 1.45) |
| Black, non-Hispanic | 0.6 (0.48 – 0.73)* | 0.63 (0.51 – 0.79)* | 0.73 (0.58 – 0.93)* | 0.57 (0.46 – 0.69)* | 0.58 (0.47 – 0.71)* | 0.68 (0.54 – 0.84)* |
| B. Gender identity (ref: Cisgender man) | ||||||
| Cisgender woman | 0.76 (0.51 – 1.16) | 0.96 (0.59 – 1.6) | 1.05 (0.64 – 1.76) | 0.87 (0.61 – 1.3) | 1.04 (0.66 – 1.68) | 1.16 (0.73 – 1.86) |
| Transgender woman | 0.66 (0.48 - 0.92) | 0.76 (0.54 - 1.09) | 1.09 (0.76 - 1.57) | 0.72 (0.54 - 0.98) | 0.82 (0.59 - 1.14) | 1.12 (0.8 - 1.57) |
| Transgender man, genderqueer, or else2 | 5.57 (1.2 - 99)* | 5.3 (1.12 - 95)* | 6.34 (1.31 - 114)* | 3.58 (1.1 - 22)* | 3.5 (1.06 - 22)* | 3.74 - 23)* |
| C. Sexual orientation (ref: Heterosexual/Straight) | ||||||
| Bisexual | 0.9 (0.61 – 1.32) | 0.79 (0.52 – 1.21) | 0.84 (0.55 – 1.3) | 0.85 (0.59 – 1.23) | 0.76 (0.51 – 1.14) | 0.83 (0.56 – 1.24) |
| Gay or Lesbian | 1.37 (1.03 – 1.81)* | 1.07 (0.75 – 1.52) | 0.96 (0.67 – 1.36) | 1.2 (0.93 – 1.54) | 0.95 (0.68 – 1.31) | 0.86 (0.62 – 1.18) |
| Queer or else3 | 1.82 (0.99 – 3.56) | 1.49 (0.79 – 2.98) | 1.38 (0.73 – 2.8) | 1.57 (0.9 – 2.93) | 1.3 (0.73 – 2.48) | 1.21 (0.67 – 2.33) |
| B. Intersectional analysis with Latent class | ||||
|---|---|---|---|---|
| Category | Complete case | Imputed Data | ||
| Model 4 | Model 5 | Model 4 | Model 5 | |
| Reference: A (mixed r/e, mixed SES, gay, cis men) | ||||
| B (majority Black, low SES, cis, het men/women) | 0.37 (0.26 - 0.52)* | 0.45 (0.33 - 0.63)* | 0.46 (0.33 - 0.64)* | 0.45 (0.33 - 0.63)* |
| C (mixed r/e, low SES, bi/gay, cis men) | 0.31 (0.25 - 0.38)* | 0.41 (0.34 - 0.49)* | 0.38 (0.32 - 0.46)* | 0.41 (0.34 - 0.49)* |
| D (mixed r/e, low SES, mixed sexual orientation, trans women) | 0.32 (0.23 - 0.46)* | 0.43 (0.31 - 0.6)* | 0.40 (0.29 - 0.57)* | 0.43 (0.31 - 0.6)* |
Note:
confidence interval does NOT cross null (1.0). r/e – race & ethnicity, SES – socio-economic status, het – heterosexual, TG – transgender.DM – diabetes mellitus, HTN – hypertension, LCA – latent class analysis, r&e – race and ethnicity, SES – socio-economic status, SO – sexual orientation, TG- transgender.
Other race and ethnicity variables include Asian, Pacific Islander, Multiracial, and Unspecified.
Includes genderqueer, gender non-conforming or replied “something else” when asked about gender. Pooled with trans man due to low numbers.
Other sexual orientations include Queer, Questioning, and Something else responses
Each model adjusts for slightly different variables. Models 1, 2, and 4 adjusts only for confounders with model 2 assuming demographic variables as confounders of each other. Model 3 and 5 adjusts for mediators of demographic variables. Specific variables are specified as follows:
Model 1: adjusts only for age and year of first viral load. Three separate models, one for each demographic variable.
Model 2: One model that adjusts for age, year of first viral load, and demographic categories.
Model 3: One model that adjusts for variables in model 2 as well as mediator variables including housing, poverty, insurance, diabetes or hypertension status, mental health disorder, and substance use disorder.
Model 4: Similar to model 1 in that it adjusts for age and year of first viral load
Model 5: Similar to model 3 in that it adjusts for age and year of first viral load as well as mediators which include hypertension status, mental health disorder, and substance use disorder.
e. Results from Qualitative Comparative analysis
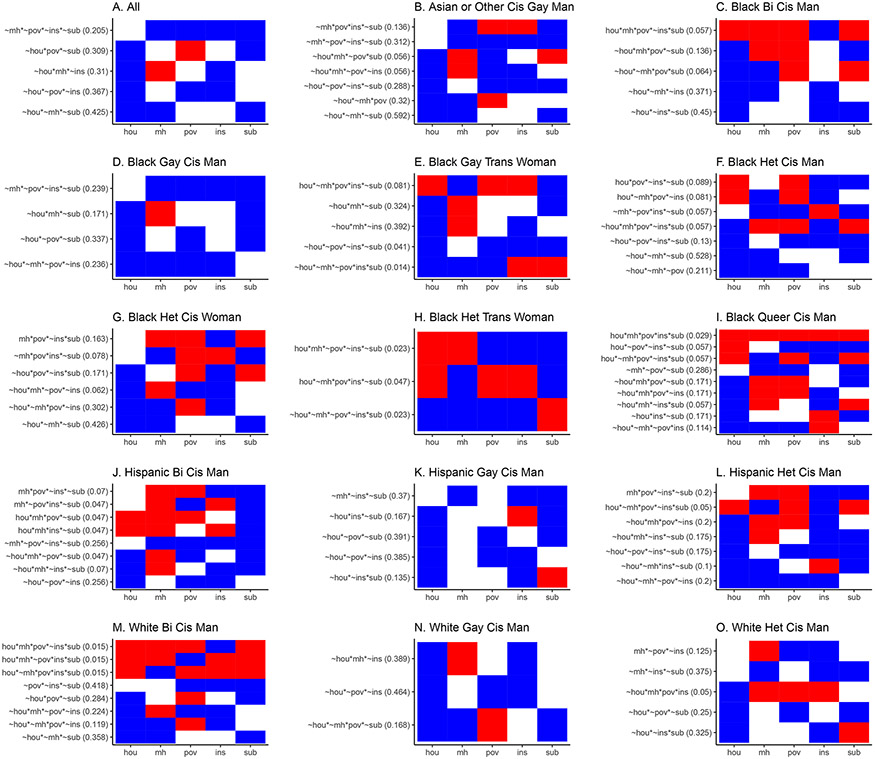
The QCA analysis identified combinations associated with DVS. In the whole sample analysis, we identified five sufficient combinations. (Figure 2A) Unlike regression where a factor would often have just one direction of association, QCA highlights how this varies depending on other factors. For example, the second-row combination in Figure 2A showed poor people (“red cell”) likely achieved DVS if they did not have housing or substance use problems (“blue cells”). Similarly, in the third-row combination, a person with a mental health disorder likely achieved DVS if they did not have housing or insurance problems. No single factor was identified as sufficient on its own for achieving DVS.
Figure 2. Combinations Associated with Achievement of Durable Viral Suppression, Howard Brown Health, 2012-2019 (n=4,787).
Notes:
1Abbreviations of factors: hou – no permanent housing, mh – has mental health disorder, pov – experiencing poverty, ins – not insured, sub – has substance use disorder. Addition of a tilde (“~”) creates the inverse of the factor (e.g., ~hou means has permanent housing, ~mh means does NOT have a mental health disorder). The asterisk (“*”) refers to the logic operator “AND” (e.g., ~hou*~mh means “has permanent housing” AND “has no mental health disorder”).
2Abbreviations of labels: bi -bisexual, cis – cisgender, gay – gay or lesbian, het – heterosexual, trans - transgender
3Each sub-figure (e.g., 2A, 2B) contains the combinations identified to be associated with DVS. Row names reflect sufficient combinations for DVS. For example, the whole sample (2A) identified five combinations for DVS. The first row in 2A “~mh * ~pov * ~ins * ~sub” is read as follows: A person with “no mental health disorder AND not experiencing poverty AND has insurance AND has no substance use disorder” likely achieved DVS (i.e., at least 85% of the group with this combination achieved DVS).
4To facilitate visual comparison across groups and pattern identification, we converted combinations to color-coded tables. Each cell in the table represents the setting of a condition. Blue means is in a favorable setting (e.g., “~hou” or has permanent housing, “~mh” or no mental health disorder) while Red is in an unfavorable setting (e.g., “hou” or no permanent housing, “mh” or has a mental health disorder). White means the factor is not part of the combination. The first row in 2A are all favorable settings and are thus all blue. Housing was not mentioned in the combination and is set to white. The second row has three factors, two positive (“~hou” and “~sub”) and one negative (“pov”).
5The numbers in parentheses are “raw coverage for sufficiency” which is how much of the DVS is explained by the combination. This number ranges from 0 to 1 and the closer to one means the more important a combination is for the group. Since these are raw coverages, the numbers will not add up to one.
6 Sample only included complete case data. Subgroup sizes are in Supplemental Table 4, Supplemental Digital Content 2
The subgroup analyses (Figures 2B-2O) demonstrated heterogeneity in sufficient combinations across intersectional groups. Each result can be interpreted like Figure 2A in that PWH with identified combinations were likely to achieve DVS in this setting. For example, Black gay/lesbian transgender women with substance use disorder and have four favorable (“blue”) factors (third row combination, Figure 2H) were likely to achieve DVS.
f. Sensitivity analysis using the Alternative Outcome
The sensitivity analysis using the alternative DVS definition yields mostly similar results using the “main effects” model (Supplemental Table 8, SDC 2). We noted some significant interactions not identified in the main analysis (Supplemental Table 9, SDC 2) The disparities analysis also showed results like the main analysis (Supplemental Table 10, SDC 2). For QCA, different patterns emerged but the observation seen in the main QCA that different groups having different combinations remained. (SDC 3)
IV. Discussion
In this analysis of data from an LGBTQ+ focused health center in Chicago, we demonstrated how SDOH variables could combine in complex ways and contribute to DVS disparities. While we replicated single factor associations previously noted in other studies[9,10,12,42], our intersectionality-informed analyses highlighted the nuanced associations that might be missed by relying only on “main effects” regression. First, we found a possible heterogenous associations between insurance and DVS by race & ethnicity. Second, LCA revealed that disparities exist based on the combination of race, gender, and class. We observed that historically marginalized groups have lower odds of DVS compared to the group comprised of mostly white cisgender gay men, stressing the continued need to examine equity efforts within health systems.[43] While “main effects” only models can demonstrate disparities, this portrayed an incomplete picture. We demonstrate how LCA can be an alternative to using interactions for creating groups especially in the face of data sparsity. Finally, QCA showed that combinations (and not single factors) were associated with achievement of DVS and that these combinations differ across intersectional subgroups. Prior work has shown that a higher number of adverse social conditions lowers DVS achievement.[44] We show, however, that it is not just that total number: The combination and the person’s social position likely influences DVS achievement.
Our work extends the literature in two ways. First, we focused on DVS instead of one-time achievement unlike prior studies.[28,42] This is important since benefits are only achieved if suppression is durable,[28] and it’s known that suppression is not always sustained.[42] While the DVS achievement in our sample seems high, this number is comparable to 90% DVS rate reported by Ryan White Clinics which primarily serve un/under-insured PWH.[45]
The second advance is towards analysis of social factors. Unlike prior work, we utilized multiple intersectional approaches.[10,46] Thus, we identified perils of studying disparities only through singular and non-intersecting axes of identity. “Main effects” regression can potentially lead to designing programs that address only single issues (e.g., focusing only on racial disparities) or failure to recognize potential differential impact of interventions (e.g., how insurance play a different role according to racial group). This prevents addressing the differing needs of various intersectional groups. For example, in QCA, Black gay/lesbian transgender women needed four out of five conditions to be favorable to achieve DVS, while some white cisgender gay men needed only a few. This diversity of needs implies that HIV services need to be comprehensive and offer a minimum set of social interventions (e.g., social risk screening and referral, mental health services). Programs that focus on single issues (e.g., housing alone) may be inadequate for eliminating DVS disparities. Implementing social interventions undeniably presents challenges but are feasible: Ryan White clinics demonstrated how programs can be implemented and funded through existing US policies.[47]
Interestingly, the QCA results showed that unfavorable conditions can still lead to DVS, in contrast to “main effects” only regression. While having a mental health or substance use disorder can affect adherence, in the HBH context, the diagnosis may have facilitated linkage to wraparound services.[48] Wraparound services have been associated with better outcomes including PWH.[49,50] Similarly, the presence of multiple problems could lead to more intense case management. Being poor, was also seen in several combinations which could be due to the existing policies and programs targeting poor PWH. Many HIV drug access programs are accessible only to low-income individuals; this threshold varies across states and have been shown to affect ART coverage rate.[51] Finally, QCA could also be reflecting the common combinations in the subgroup. In an ideal system, we should see that any combination should be associated with DVS achievement because the health and HIV safety net system should be able support them adequately.[52,53]
While our main predictors of DVS are measured at the individual level, these must be interpreted through the lens of social systems rather than individual behaviors. Since these are present at baseline, addressing these SDOH even before a person gets the infection may translate to downstream outcomes like DVS. Importantly, plans for eliminating the HIV epidemic should include interventions for SDOH like homelessness and poverty. Fortunately, US policy documents related to HIV elimination recognize these issues.[6,23] However, these policies could still improve by putting more in the forefront that addressing social issues helps in improving HIV prevention and DVS outcomes.
Our study has several limitations. First, our findings are based on a single health system in Chicago with a specific care context. Our findings may extend to urban settings in the US but not necessarily to other contexts like rural US and non-US settings. However, our intersectional approach can be utilized to conduct similar analysis in all settings. Second, we excluded individuals with insufficient number of visits. This sub-population were not retained in care and likely failed to achieve DVS. The emergence of health information exchanges may allow studies on this important sub-population.[54] Third, outputs of latent class methods carry uncertainty. Several authors have proposed the use of correction methods to adjust for this.[55,56] Unfortunately, we had limited tools due to use of two latent class methods. However, the LTA model had good separation and correction is less important.[57] Fourth, we adjusted only for common conditions seen in primary care. Future work on the role of multimorbidity in DVS is warranted.
Our approach had to balance the principles of intersectionality with feasibility of implementation. Aside from the common issues with EHR data like misclassification and missingness, EHR does not allow direct measurement of core constructs relevant to mechanisms that link the individual experience and the outcome such as stigma and racism. We relied on demographic measures of identity rather than directly asking about stress or discrimination and imperfect measures of structural forces.[58] We also collapsed some groups which is needed to implement modeling but assumes homogeneity of experience. Second, a fully intersectional approach would center historically marginalized groups in the modeling and interpretation. However, to improve statistical stability, we opted to use larger groups as reference and these categories often are the most privileged. Third, while we were able to show QCA combinations that appeared sufficient to achieve the outcome, one must note that these were baseline conditions. We are unable to speak to the impact of intervening on these social risks on DVS but recommend future work on this. Finally, we also acknowledge that quantitative intersectionality is rapidly changing and techniques like QCA continue to be refined.[59]
We show in this work the various ways of how SDOH – including intersectional stigma – could be associated with DVS. Through multiple methods consistent with intersectionality, we described the various aspects of the complexity underlying the DVS achievement and population-level disparities. Quantitative intersectional approaches are useful for investigating the role of social factors in health disparities and are necessary for better evidence-based policymaking in HIV. While we focused on PWH experiences, our methods are potentially generalizable to studies that engage with issues faced by people of color, sexual minorities, and people facing adverse socioeconomic conditions.
Supplementary Material
Acknowledgements
We want to acknowledge the HBH staff who have helped curate the data source and the REACH and EDIT research teams at Northwestern for their inputs on preliminary results of this research. The final contents of this paper remain the responsibility of the authors.
This research was supported in part through the computational resources and staff contributions provided for the Quest high performance computing facility at Northwestern University which is jointly supported by the Office of the Provost, the Office for Research, and Northwestern University Information Technology.
Sources of Funding:
ASR was supported by the American Heart Association (AHA) Predoctoral Fellowship (825793). JS was partially supported by NHLBI R01HL158963. LBB was partially supported by K12 HL143959 (Ardehali/Schneider). Data pull funding for NM was through MF (AHA 16FTF31200010) for NM. The HBH data pull was possible with support from the Third Coast Center for AIDS Research, an NIH funded center (P30 AI117943).
References
- 1.Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP, Klein DB, et al. Narrowing the Gap in Life Expectancy between HIV-Infected and HIV-Uninfected Individuals with Access to Care. J Acquir Immune Defic Syndr 2016; 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living with HIV: A Scientific Statement from the American Heart Association. Circulation 2019; 140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: Exploratory analyses from the SMART trial. Antivir Ther 2008; 13:177–187. [DOI] [PubMed] [Google Scholar]
- 4.Martin EG, MacDonald RH, Gordon DE, Swain CA, O’Donnell T, Helmeset J, et al. Simulating the End of AIDS in New York: Using Participatory Dynamic Modeling to Improve Implementation of the Ending the Epidemic Initiative. Public Health Rep 2020; 135:158S–171S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Yuan X, Wang J, Zhang W, Zhou Y, Liu G. Evaluation of impact of social support and care on HIV-positive and AIDS individuals’ quality of life: a nonrandomised community trial. J Clin Nurs 2017; 26:369–378. [DOI] [PubMed] [Google Scholar]
- 6.Fauci AS, Redfield RR, Sigousnas G, Weahkee MD, Giroir BP. Ending the HIV Epidemic A Plan for the United States. JAMA 2019; 321:844–845. [DOI] [PubMed] [Google Scholar]
- 7.Granich R, Gupta S, Hersh B, Williams B, Montaner J, Young B, et al. Trends in AIDS deaths, new infections and ART coverage in the top 30 countries with the highest AIDS mortality burden; 1990-2013. PLoS One 2015; 10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers For Disease Control And Prevention Division of HIV Prevention. Treatment Pillar. End. HIV Epidemic United States Monit. Prog 2022.https://www.cdc.gov/endhiv/indicators/treat.html (accessed 29 Jan2022).
- 9.Pellowski JA, Kalichman SC, Matthews KA, Adler N. A pandemic of the poor: social disadvantage and the US HIV epidemic. Am Psychol 2013; 68:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nance RM, Chris Delaney JA, Simoni JM, Wilson IB, Mayer KH, Whitney BM, et al. HIV viral suppression trends over time among HIV-infected patients receiving care in the United States, 1997 to 2015 a cohort study. Ann Intern Med 2018; 169:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberhart MG, Yehia BR, Hillier A, Voytek CD, Fiore DJ, Blank M. Individual and community factors associated with geographic clusters of poor HIV care retention and poor viral suppression. J Acquir Immune Defic Syndr 2015; 69:S37–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palar K, Wong MD, Cunningham WE. Competing susbsistence needs are associated with retention in care and detectable viral load among people living with HIV. J HIV AIDS Soc Serv 2018; 17:163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young R, Ayiasi RM, Shung-King M, Morgan R. Health systems of oppression: applying intersectionality in health systems to expose hidden inequities. Health Policy Plan 2020; 35:1228–1230. [DOI] [PubMed] [Google Scholar]
- 14.Bowleg L. Evolving intersectionality within public health: From analysis to action. Am J Public Health 2021; 111:88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earnshaw VA, Rendina HJ, Bauer GR, Bonett S, Bowleg L, Carter J, et al. Methods in HIV-Related Intersectional Stigma Research : Core Elements and Opportunities. Am J Public Health 2021; 112:S413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer GR. Social Science & Medicine Incorporating intersectionality theory into population health research methodology : Challenges and the potential to advance health equity. Soc Sci Med 2014; 110:10–17. [DOI] [PubMed] [Google Scholar]
- 17.Bowleg L. When Black + lesbian + woman ≠ Black lesbian woman: The methodological challenges of qualitative and quantitative intersectionality research. Sex Roles 2008; 59:312–325. [Google Scholar]
- 18.Graetz N, Boen CE, Esposito MH. Structural Racism and Quantitative Causal Inference: A Life Course Mediation Framework for Decomposing Racial Health Disparities. J Health Soc Behav Published Online First: 2022. doi: 10.1177/00221465211066108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streed CG, Beach LB, Caceres BA, Dowshen NL, Moreau KL, Mukherjee M, et al. Assessing and Addressing Cardiovascular Health in People Who Are Transgender and Gender Diverse: A Scientific Statement from the American Heart Association. Circulation 2021; :136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer GR, Scheim AI. Advancing quantitative intersectionality research methods : Intracategorical and intercategorical approaches to shared and differential constructs. Soc Sci Med 2019; 226:260–262. [DOI] [PubMed] [Google Scholar]
- 21.Hancock A. Empirical Intersectionality : A Tale of Two Approaches A Tale of Two Approaches. UC Irvine L Rev 2013; 3:259–296. [Google Scholar]
- 22.Turan JM, Elafros MA, Logie CH, Banik S, Turan B, Crockett KB, et al. Challenges and opportunities in examining and addressing intersectional stigma and health. BMC Med 2019; 17:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown RN. Ending the HIV Epidemic Plan for Cook County 2021-2025. 2020.https://www.chicago.gov/content/dam/city/depts/cdph/HIV_STI/Ending the HIV Epidemic Plan for Cook County 2021-2025 FINAL.pdf [Google Scholar]
- 24.Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob Heal 2016; 1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enns EA, Reilly CS, Horvath KJ, Baker-James K, Henry K. HIV Care Trajectories as a Novel Longitudinal Assessment of Retention in Care. AIDS Behav 2019; 23:2532–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ocampo JMF, Plankey M, Zou K, Collmann J, Wang C, Young MA, et al. Trajectory analyses of virologic outcomes reflecting community-based HIV treatment in Washington DC 1994-2012. BMC Public Health 2015; 15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powers KA, Samoff E, Weaver MA, Lynne A, Miller WC, Leone PA. Longitudinal Trajectories of HIV Care Retention in North Carolina. J Acquir Immune Defic Syndr 2017; 74:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diepstra K, Lu H, McManus KA, Rogawski McQuade ET, Rhodes AG, Westreich D. What we talk about when we talk about durable viral suppression. AIDS 2020; 34:1683–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins L, Lanza S. Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences. Vol 718. John Wiley & Sons; 2009. [Google Scholar]
- 30.Lanza ST, Patrick ME, Maggs JL. Latent Transition Analysis: Benefits of a Latent Variable Approach to Modeling Transitions in Substance Use. J Drug Issues 2010; 40:93–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowleg L. The Problem With the Phrase Women and Minorities : Intersectionality — an Important Theoretical Framework for Public Health. Am J Public Health 2012; 102:1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Studio Team. RStudio: Integrated Development for R. 2019.
- 33.R Core Team. R: A Language and Environment for Statistical Computing. 2022.
- 34.Proust-Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: The R package lcmm. J Stat Softw 2017; 78. doi: 10.18637/jss.v078.i02 [DOI] [Google Scholar]
- 35.Linzer DA, Lewis JB. poLCA: An R Package for Polytomous Variable Latent Class Analysis. J Stat Software1 2011; 42:1–18. [Google Scholar]
- 36.Duşa A. QCA with R: A comprehensive resource. Springer; 2018. [Google Scholar]
- 37.Stekhoven DJ, Bühlmann P. Missforest-Non-parametric missing value imputation for mixed-type data. Bioinformatics 2012; 28:112–118. [DOI] [PubMed] [Google Scholar]
- 38.Hatzenbuehler ML, Link BG. Introduction to the special issue on structural stigma and health. Soc Sci Med 2014; 103:1–6. [DOI] [PubMed] [Google Scholar]
- 39.Kane H, Lewis MA, Williams PA, Kahwati LC. Using qualitative comparative analysis to understand and quantify translation and implementation. Transl Behav Med 2014; 4:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanckel B, Petticrew M, Thomas J, Green J. The use of Qualitative Comparative Analysis (QCA) to address causality in complex systems: a systematic review of research on public health interventions. BMC Public Health 2021; 21. doi: 10.1186/s12889-021-10926-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiem A. Conducting Configurational Comparative Research With Qualitative Comparative Analysis: A Hands-On Tutorial for Applied Evaluation Scholars and Practitioners. Am J Eval 2017; 38:420–433. [Google Scholar]
- 42.Kassaye SG, Wang C, Ocampo JMF, Wilson TE, Anastos K, Cohen M, et al. Viremia Trajectories of HIV in HIV-Positive Women in the United States, 1994-2017. JAMA Netw open 2019; 2:e193822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huebenthal J. Un/Detectability in Times of “Equality”: HIV, Queer Health, and Homonormativity. Eur J Am Stud 2017; 11. doi: 10.4000/ejas.11729 [DOI] [Google Scholar]
- 44.Menza TW, Hixson LK, Lipira L, Drach L. Social Determinants of Health and Care Outcomes among People with HIV in the United States. Open Forum Infect Dis 2021; 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Health Resources and Services Administration. Ryan White HIV/AIDS Program Annual Client-Level Data Report 2020. ; 2021. www.hab.hrsa.gov/data/data-reports
- 46.Sheehan DM, Dawit R, Gbadamosi SO, Fennie KP, Li T, Gebrezgi M, et al. Sustained HIV viral suppression among men who have sex with men in the Miami-Dade County Ryan White Program: The effect of demographic, psychosocial, provider and neighborhood factors. BMC Public Health 2020; 20:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiser J, Beer L, Frazier EL, Patel R, Dempsey A, Hauck H, et al. Service delivery and patient outcomes in Ryan White HIV/AIDS Program-funded and -nonfunded health care facilities in the United States. JAMA Intern Med 2015; 175:1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, Mellins CA. Mental health and HIV/AIDS: The need for an integrated response. Aids 2019; 33:1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brennan-Ing M, Seidel L, Rodgers L, Ernst J, Wirth D, Tietz D, et al. The impact of comprehensive case management on HIV client outcomes. PLoS One 2016; 11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vest JR, Harris LE, Haut DP, Halverson PK, Menachemi N. Indianapolis provider’s use of wraparound services associated with reduced hospitalizations and emergency department visits. Health Aff 2018; 37:1555–1561. [DOI] [PubMed] [Google Scholar]
- 51.Snider JT, Goldman DP, Rosenblatt L, Seekins D, Juday T, Sanchez Y, et al. The impact of state AIDS drug assistance policies on clinical and economic outcomes of people with HIV. Med Care Res Rev 2016; 73:329–348. [DOI] [PubMed] [Google Scholar]
- 52.Doshi RK, Milberg J, Jumento T, Matthews T, Dempsey A, Cheever LW. For many served by the ryan white HIV/AIDS program, disparities in viral suppression decreased, 2010-14. Health Aff 2017; 36:116–123. [DOI] [PubMed] [Google Scholar]
- 53.Watkins-Hayes C. Remaking a life: How women living with HIV/AIDS confront inequities. University of California Press; 2019. [Google Scholar]
- 54.Sharp J, Angert CD, McConnell T, Wortley P, Pennisi E, Roland L, et al. Health Information Exchange: A Novel Re-linkage Intervention in an Urban Health System. Open Forum Infect Dis 2019; 6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakk Z, Tekle FB, Vermunt JK. Estimating the Association between Latent Class Membership and External Variables Using Bias-adjusted Three-step Approaches. ; 2013. doi: 10.1177/0081175012470644 [DOI] [Google Scholar]
- 56.Vermunt JK. Latent class modeling with covariates: Two improved three-step approaches. Polit Anal 2010; 18:450–469. [Google Scholar]
- 57.Elliott MR, Zhao Z, Mukherjee B, Kanaya A, Needham BL. Methods to Account for Uncertainty in Latent Class Assignments When Using Latent Classes as Predictors in Regression Models, with Application to Acculturation Strategy Measures. Epidemiology 2020; 31:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adkins-Jackson PB, Chantarat T, Bailey ZD, Ponce NA. Measuring Structural Racism: A Guide for Epidemiologists and Other Health Researchers. Am J Epidemiol 2021; 00:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skaaning SE. Assessing the robustness of crisp-set and fuzzy-set QCA results. Sociol Methods Res 2011; 40:391–408. [Google Scholar]
- 60.Mahendran M, Lizotte D, Bauer GR. Quantitative methods for descriptive intersectional analysis with binary health outcomes. SSM - Popul Heal 2022; 17:101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.